Abstract
Wastewater-based disease surveillance is a promising approach for monitoring community outbreaks. Here we describe a nationwide campaign to monitor SARS-CoV-2 in the wastewater of 159 counties in 40 U.S. states, covering 13% of the U.S. population from February 18 to June 2, 2020. Out of 1,751 total samples analyzed, 846 samples were positive for SARS-CoV-2 RNA, with overall viral concentrations declining from April to May. Wastewater viral titers were consistent with, and appeared to precede, clinical COVID-19 surveillance indicators, including daily new cases. Wastewater surveillance had a high detection rate (>80%) of SARS-CoV-2 when the daily incidence exceeded 13 per 100,000 people. Detection rates were positively associated with wastewater treatment plant catchment size. To our knowledge, this work represents the largest-scale wastewater-based SARS-CoV-2 monitoring campaign to date, encompassing a wide diversity of wastewater treatment facilities and geographic locations. Our findings demonstrate that a national wastewater-based approach to disease surveillance may be feasible and effective.
Keywords: Wastewater surveillance, SARS-CoV-2, United States, Detection rate, Spatiotemporal dynamics
Graphical Abstract
1. Introduction
COVID-19 was first reported in the United States on January 20, 2020, and spread to all 50 states and the District of Columbia by mid-March (Holshue et al., 2020; Johns Hopkins University Center for Systems Science and Engineering, 2020). As of February 1, 2021, over 26 million confirmed cases and over 440,000 deaths have been reported in the U.S. (Johns Hopkins Coronavirus Resource Center, 2021). Establishing a national COVID-19 surveillance system, like those for viral hepatitis (Division of Viral Hepatitis | CDC, 2021) and influenza (U.S. Influenza Surveillance System, 2020), would be helpful for long-term monitoring of SARS-CoV-2, allowing healthcare officials to recognize and respond to new outbreaks efficiently. However, COVID-19 poses specific challenges to clinical surveillance systems, with its long infectious incubation time (up to 14 days; median: 4-5 days) greatly increasing the risk of viral transmission and infection among the population before clinical reporting, contact tracing, and containment can occur (CDC, 2020a; Lauer et al., 2020). “Test and trace” systems were rapidly overwhelmed in many countries early in the pandemic, and are often ineffective once a disease reaches exponential community spread (Contreras et al., 2021; Kretzschmar et al., 2020). The emergence of more infectious variants may exacerbate this problem (Galloway, 2021; Grubaugh et al., 2021).
As a complementary approach to clinical disease surveillance, wastewater monitoring can help detect the presence of pathogens like the coronavirus SARS-CoV-2 across municipalities, and estimate disease incidence independent of individual testing (Orive et al., 2020; Wu et al., 2020a, 2020b). Wastewater surveillance is less resource intensive than large-scale clinical testing, making it an optimal tool for unobtrusive, long-term monitoring as well as early identification of viral circulation in the population (Thompson et al., 2020). Our recent findings (Wu et al., 2020a, 2020b) along with work from other groups have described reliable detection of SARS-CoV-2 gene fragments in wastewater samples across the world, including Australia (Ahmed et al., 2020a), Brazil (Prado et al., 2020), France (Wurtzer et al., 2020), the Netherlands (Medema et al., 2020), Italy (La Rosa et al., 2020), Spain (Chavarria-Miró et al., 2020; Orive et al., 2020; Randazzo et al., 2020b), and the U.S. (Peccia et al., 2020; Wu et al., 2020a). Furthermore, longitudinal wastewater viral titers correlate with clinically diagnosed new COVID-19 cases, and trends in wastewater precede those in clinical reports by 4-10 days, suggesting that wastewater data could be used as an early warning of impending outbreaks to define public health and hospital planning (Wu et al., 2020b). The potential value of wastewater surveillance is gaining recognition, with the Centers for Disease Control and Prevention and several state and local health agencies initiating wastewater-based monitoring programs to supplement their COVID-19 responses (CDC, 2020b).
In this study, a nationwide COVID-19 surveillance campaign was implemented to measure viral concentrations of SARS-CoV-2 in the wastewater of 159 counties in 40 U.S. states, from February to June 2020. We investigated the detection rate and accuracy of wastewater surveillance of SARS-CoV-2 by comparing wastewater data to clinically reported case counts from state and local health agencies. Our results demonstrated the feasibility of utilizing wastewater surveillance as a supplement to national SARS-CoV-2 clinical reporting data to understand important past, current and future trends in viral dynamics.
2. Material and methods
2.1. Sample collection and viral inactivation
We initiated a national call for wastewater samples to quantify SARS-CoV-2 viral load in wastewater catchment areas from mid-February to early June 2020. Eligible sites included public works and wastewater authorities across the continental United States, with catchment areas spanning 65 people to 5.3 million people, with a median size of 31,745 people. Average daily influent flow rates at these sites ranged from 0.005-300 million gallons per day. Samples were collected by each wastewater treatment facility on a voluntary basis, generally on a weekly basis (for more detailed sampling frequency, see Supplemental Table S1). Raw wastewater samples were collected from the wastewater treatment plants or catchments in 40 U.S. states and stored at 4 °C before being mailed overnight to the laboratory for analysis. Samples were processed within 1-3 days of receipt using the method as previously described (Wu et al., 2020a, 2020b). Briefly, UV light was used to sterilize the exterior of the sample's container (20 min) before handling, and pasteurization (heat treatment in a 60 °C water bath for 90 min) was used to inactivate the pathogens in sewage. Pasteurized samples were vacuum filtered with a 0.22-μm polyethersulfone membrane to remove cell debris and solid materials. Supernatant was used to concentrate the viral particles as described below, and the rest was stored in 4 °C.
2.2. Viral precipitation, RNA extraction, reverse transcription and quantitative PCR (RT-qPCR)
Samples were processed starting from viral enrichment to quantitative PCR using two comparable methods as previously described (Wu et al., 2020b). We processed 60 samples from Deer Island wastewater treatment plant with both two methods, and no significant difference between viral titers was observed (Wu et al., 2020b). Briefly, viral particles in 40-ml filtrate were precipitated with polyethylene glycol 8000 (10% w/v, Millipore sigma) and NaCl (0.3M, Millipore sigma) in Method I. Viral pellet was resuspended in 1.5 ml Trizol reagent (Cat# 15596026, Thermo Fisher Scientific), and mixed thoroughly with 300 μl chloroform (Cat# C2432, Sigma-Aldrich) for 1 min. The mixture was incubated for 5 min at room temperature before centrifugation for 15 min at 16,000 g at 4 °C. Aqueous phase (600 μl) containing the RNA was transferred to a new 1.5-ml tube, and 600 μl isopropanol (Cat# 470157-450, VWR) was added into the aqueous phase. After 10 min incubation at room temperature, samples were centrifuged for 10 min at 16,000 g at 4 °C. The supernatant was discarded and pellet was washed twice with 75% ethanol, followed by air dry for 5–10 min. 30 µl of DEPC water was used to resuspend the RNA for cDNA synthesis. cDNA was synthesized by reverse transcription (RT) based on the manufacturer's protocol (M0368, New England Biosciences), followed by real-time PCR with the TaqMan® Fast Advanced Master Mix and U.S. CDC N1, N2 primer/probes. The qPCR reaction was carried out for 48 cycles using Bio-Rad CFX96 Real-Time PCR Detection System with following program: polymerase activation (95 °C for 2 min), PCR (48 cycles, denature at 95 °C for 1 s, and anneal/extend at 55 °C for 30 s).
15 ml of filtrate in Method II were first concentrated with 10 kDa Amicon Ultra Centrifugal Filter (Sigma, Cat# UFC9010) to 150 ~ 200 μl, which is further lysed with 600 μl AVL buffer (Qiagen, Cat# 19073) for RNA extraction (Qiagen RNeasy kit, Cat# 74182). The eluted RNA was used for one-step RT-PCR with TaqMan™ Fast Virus 1-Step Master Mix (Thermofisher, Cat# 4444436), based on the following protocol: 50 °C 10 min for reverse transcription, 95 °C 20 s for RT inactivation and initial denaturation, and 48 cycles of denature (95 °C 1 s) and anneal/extend (55 °C 30 s).
In total, 1023 samples were processed with Method I, and 729 samples were processed with Method II.
Evaluation of the viral concentration in Methods I and II using Murine Hepatitis Virus, a widely used SARS-CoV-2 surrogate, indicated recoveries of 58.09 ± 20.21% and 31.42 ± 2.59%, respectively, which agree well with other published studies (Ahmed et al., 2020b; Dumke et al., 2021; Jafferali et al., 2021; La Rosa et al., 2021; Torii et al., 2021; Ye et al., 2016) (Table S2). Briefly, MHV (MHV, ATCC® VR-764) was spiked into the wastewater and then concentrated by PEG8000 and Amicon filter in Method I and Method II, respectively. In parallel, the same amount of virus was directly lysed using Trizol reagent or AVL buffer in Method I and Method II. After that, viral RNA extraction, RT, and qPCR were performed by following the steps as described above in each method, except using MHV-specific primers and probe (Ahmed et al., 2020b).
Ct values for N1 or N2 primer sets were first converted to viral gene copies in the cDNA sample (copies per μl of cDNA) based on the standard curves established with the positive control plasmid (Method I) or Twist SARS-CoV-2 RNA (Method II) (Wu et al., 2020b). The concentration was further converted to viral gene copies per microliter of the wastewater sample by multiplying the dilution factor. For Method I, the dilution factor is: the volume of total cDNA * the total volume of RNA / (the volume of RNA used for reverse transcription * the starting volume of filtered wastewater sample). For Method II, the dilution factor is: The total volume of RNA / the starting volume of filtered wastewater sample). Two or three replicates were performed for each primer set, averaged within each primer set and then across primers to derive the concentration values. Pepper mild mottle virus (PMMoV), a stable and persistent indicator of fecal concentration in wastewater (Kitajima et al., 2018, 2014; Wu et al., 2020a), was also measured as an internal reference for wastewater samples. Each sample had two technical replicates, and the mean Ct values were converted to relative concentrations of viral particles based on the standard curve (Wu et al., 2020a) and sample's dilution factor.
2.3. Clinical data collection
County-level clinical data including cumulative COVID-19 cases and deaths were downloaded from USAFACTS, the largest source for standardized, publicly available US government data (https://usafacts.org/visualizations/coronavirus-covid-19-spread-map/). Daily new cases or deaths were generated through using the cumulative data on one day to subtract the data before that day. Wastewater viral titers were compared to clinical data reported for the day on which the sample was obtained. The 7-day moving average of new clinical cases (current day + 6 preceding days / 7) was also used for the detection accuracy analysis in Fig. S5c-d. Hospitalizations and positive rates of testing for each state were downloaded from The COVID Tracking Project (https://covidtracking.com/), which collects, cross-checks, and publishes COVID-19 testing, hospitalization, and patient outcome data from public health authorities in 56 US states and territories.
2.4. Detection rate analysis
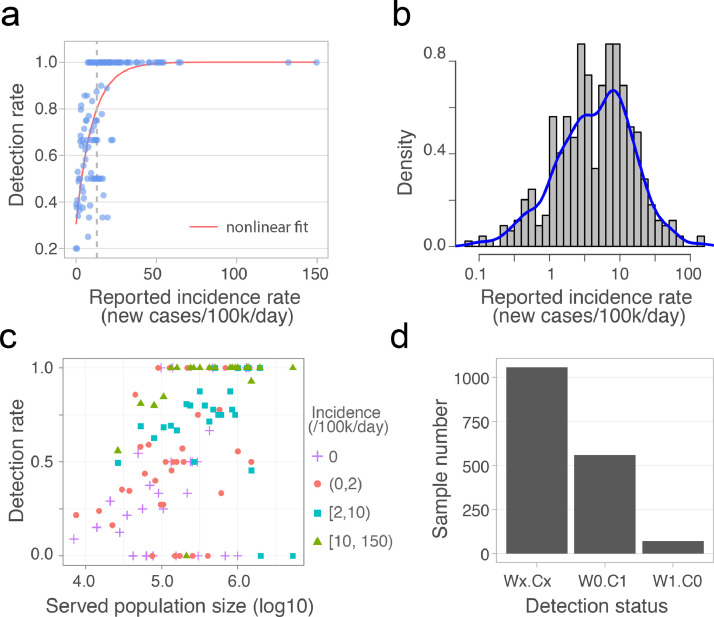
Incidence rate of daily new cases was calculated using reported daily new cases in the county divided by the county population size. In Fig. 2a, we computed the detection rates, percentage of positive wastewater samples, for a constant interval (0.2 cases per 100,000 people) of daily incidence, starting from 0 to 149.6 cases per 100,000 people (maximum daily incidence). The results were fitted using an exponential decay function with formula: y ~ k 1 + V max * (k 2 - exp(-x /τ), starting from Vmax = 10, τ = 1, and k 1 = 0.2, k 2 = 1.15. To estimate the distribution of daily incidence for all the positive samples, wastewater viral titers were aggregated for each county, since clinical cases were reported at the county level. Positive samples were then selected and plotted the histogram and Kernel density estimation for the distribution of daily incidence (Fig. 2b). Fig. 2c showed the relationship between detection rate of positive wastewater samples and the catchment population size. Samples were first separated based on four different daily incidences 0, (0,2), [2,10), and [10,150), per 100,000 people in the county where the sample was obtained. For each daily incidence, detection rate were computed within a constant interval of population size, i.e. the maximum minus minimum of population size, and divided by the number of bins (n=200). All the analysis was done with R (3.5.0).
Fig. 2.
Detection rate and accuracy of wastewater-based SARS-CoV-2 surveillance. (a) Detection rate for varying daily incidence of COVID-19 cases. Each dot represents the percentage of positive wastewater samples for a constant incidence interval, and the red line is the nonlinear fit. The vertical dashed line indicates the incidence (x = 13) above which the fitted detection rate exceeds 0.8. (b) The distribution of daily incidence for the counties where SARS-CoV-2 was detected in the wastewater samples. Blue line is the Kernel density estimation of the daily incidence's distribution. The median of the incidence is 3.7 cases per 100,000 people). (c) Relationship between the detection rate of positive wastewater samples from a given treatment plant and the population size served by that plant. Detection rate is binned by population size, and colored by the incidence intervals. (d) Detection status for all the samples (n = 1,687). Wx.Cx (x = 1 or 0): consistent results between wastewater data and clinical reports. W1.C1: SARS-CoV-2 detected in Wastewater (W1) and new Clinical cases reported (C1); W0.C0: no Wastewater detection (W0) and no new Clinical cases reported (C0); W0.C1: no Wastewater detection (W0) but new Clinical cases reported (C1); W1.C0: Wastewater detection (W1) but no new Clinical cases reported (C0). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Results
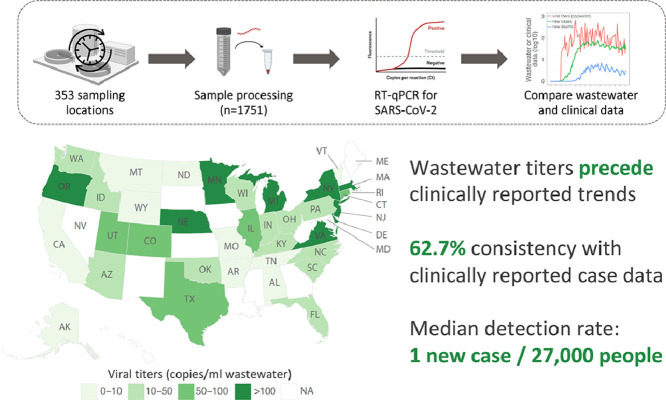
We collected and processed 1751 wastewater samples from 353 unique locations in 159 counties, in 40 U.S. states, from February 18 to June 2, 2020 (Fig. 1 a). Of these samples, 1687 were from locations that authorized the disclosure of their metadata. Individual samples represented catchments serving population sizes ranging from 65 to 5.3 million sewered individuals, with a median size of 31,745 people (Fig. S1a). In total, these wastewater samples covered 42.5 million people – approximately 13% of the U.S. population. Samples were processed as they were received in the lab and quantified by real-time quantitative PCR (see Methods). 830 samples (49.1%) were positive for SARS-CoV-2 gene fragments.
Fig. 1.
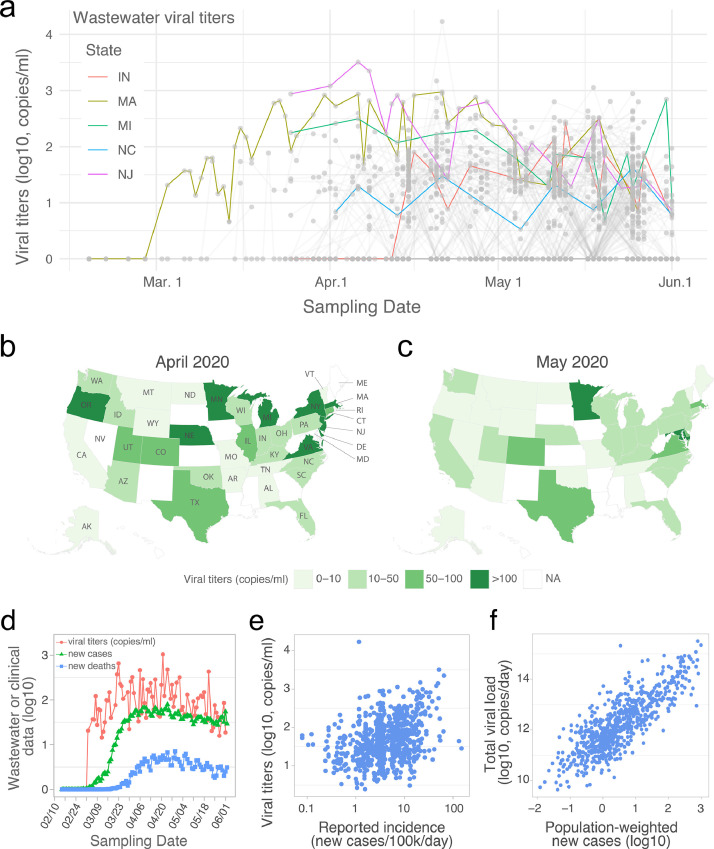
SARS-CoV-2 RNA gene copies in wastewater samples from 40 U.S. states. (a) Temporal profile of SARS-CoV-2 viral RNA gene copies (viral titers) in the wastewater samples collected from February 18 to June 2, 2020. Each grey point represents a sample, and the grey line connects samples collected from the same catchment. Temporal dynamics of mean viral titers from five U.S. states are highlighted. Negative samples (SARS-CoV-2 not detected) were assigned to ‘0’. (b-c) Mean viral titers for each state in April (b) and May (c). All the samples in April or May were aggregated by state. NA: data is not available for the state. (d) Temporal dynamics for the mean viral titers, daily new COVID-19 cases, and new deaths. Viral concentrations (red line) from positive wastewater samples were aggregated by date, and new cases (green line) and COVID-19-related new deaths (blue line) from the wastewater sample originated counties were also aggregated and averaged by date. (e) Association between viral titers in wastewater samples and the reported daily incidence rate in each sampled counties. (f) Association between the total viral load and estimated new cases in each of the catchment areas. Total viral load of SARS-CoV-2 in wastewater (copies/day) was calculated by multiplying SARS-CoV-2 concentration (copies/ml) by the daily average influent flow (ml/day) reported by the WWTP. Population weighted new cases was calculated as county new cases * catchment population / county population. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. Temporal dynamics of SARS-CoV-2 titers in wastewater samples from 159 counties in 40 states
For the month of March, there were 86 wastewater samples from 25 counties (42 individual catchments), in 10 states. We observed significant heterogeneity in results at the county level. 44 of the 86 samples (51%) (from 12 counties in 8 states) were positive for SARS-CoV-2. In California, only 3 positive samples were found from the 14 sampling locations (21%) in the 7 counties sampled in March. On the other hand, SARS-CoV-2 was consistently detected in a Massachusetts wastewater treatment plant (WWTP) starting on March 3 (93% of samples), with viral titers increasing throughout the month (Fig. 1a). Positive samples were also found in more than two counties in Colorado, Oregon, and Texas.
In April, viral titers stopped increasing and became relatively stable for most sampling locations (Fig. 1a and Fig. S1). Of the samples tested in April, 52.9% (255 of 482) were SARS-CoV-2 positive: 173 samples from 44 counties had viral titers between 10-100 copies per ml of wastewater; 78 samples from 24 counties had viral titers higher than 100 copies per ml (Fig. 1a). 1092 samples from 154 counties were processed in May. Of these, 69.5% (358/515) had positive SARS-CoV-2 titers of 10-100 copies per ml of wastewater, and 18.8% (97/515) had titers of >100 copies per ml (Fig. 1a). Analysis of April and May samples also showed the heterogeneity in viral titers at the county and catchment levels (Fig. S2).
Different dynamics in viral titers were observed at the state level (Fig. 1a). Viral titers in New Jersey (NJ) were high in March samples, but started to decrease after April 8. Similar temporal dynamics were also observed in Michigan (MI), but with a smaller magnitude. Comparatively, viral titers in Indiana (IN) and North Carolina (NC) varied little over the sampling period (April and May). Nine states (Virginia, Delaware, Michigan, Minnesota, Massachusetts, Oregon, New York, Nebraska, and New Jersey) had titers higher than 100 copies per ml of wastewater in April (Fig. 1b). This number dropped to two states (Maryland and Minnesota) in May (Fig. 1c). Averaged across all states, the mean viral concentration was significantly higher in April than in May (Fig. S1b). Together, these data highlight that wastewater surveillance can be implemented to explore viral transmission at different geographic and temporal scales.
3.2. Wastewater viral dynamics are consistent with clinical COVID-19 surveillance indicators
Next, we compared the wastewater viral titers with clinical surveillance data of COVID-19 across the U.S. Aggregating the positive wastewater data and daily new COVID-19 cases and new deaths by date, the mean viral titers increased from early March and became relatively stable between late March to late April, followed by a small downward trend until June 2 (Fig. 1d). This temporal profile mirrors the trends of clinical new cases and deaths at the national level, and precedes clinical data. Wastewater viral titers also reflected, and seemed to precede, the rise and fall of hospitalization and intensive care unit admissions (Fig. S3).
We also investigated the relationship between wastewater viral titers and daily COVID-19 incidence rates. Viral concentration in the wastewater is determined by the number of new infections, shedding rates from infected individuals, as well as total influent flow at the wastewater treatment plant (Wu et al., 2020b), which is linearly correlated with catchment population size (Fig. S4). A weak positive correlation was found between the incidence of daily new cases at the county level, and the wastewater viral titers at the catchment (wastewater treatment plant) level (Fig. 1e). We then compared total daily viral load for each catchment (viral concentrations detected at wastewater treatment plant, multiplied by the plant's daily influent flow rate), and the estimated number of new cases in that catchment (county-level incidence rates multiplied by the catchment population). A linear relationship was observed between the total viral load and catchment size-normalized daily new cases (Fig. 1f), consistent with the hypothesis that average shedding rates are similar across catchments.
3.3. Estimation of detection rate and accuracy of wastewater surveillance
Detection rate of wastewater surveillance were also examined by comparing wastewater titers to new clinical cases on the sampling day (Material and methods). Using the reported daily incidence COVID-19 cases (i.e. daily new cases divided by the county population size), we calculated the percentage of positive wastewater samples for different incidence rates. Wastewater-based detections increased exponentially with the clinical incidence rate, reaching an 80% rate of detection at a clinical incidence of 13 cases per 100,000 people (Fig. 2 a). For all positive wastewater samples at the county level, the associated incidence rates of daily new cases ranged from 0 – 149.6 cases per 100,000 people (median: 3.7 cases per 100,000 people) (Fig. 2b). In other words, wastewater-based surveillance was capable of detecting SARS-CoV-2 for one new reported case out of ~27,000 people. However, this new case rate does not consider unreported infections in the population, which would lower the estimated detection limit.
To evaluate whether catchment size influences the probability of SARS-CoV-2 detection in wastewater samples, we analyzed the detection rate of positive samples from counties with equal daily incidence. As shown in Fig. 2c, detection rate is positively associated with the population size of wastewater treatment plant catchments for the majority of samples. 100% detection rates were disproportionately represented among samples with high incidence (>10 cases per 100,000 people) and large population sizes (>100,000 people). This result is consistent with our previous model simulations that the probability of SARS-CoV-2 detection in the wastewater increases with population size in communities with equal incidence (Wu et al., 2020b).
To evaluate the detection accuracy of wastewater surveillance, wastewater results were compared with the reported daily new clinical cases. For all 1687 samples for which we had access to metadata, 1057 (62.7%) exhibited results consistent with the geographically associated clinical data, meaning that SARS-CoV-2 was detected in the wastewater in areas with new clinical cases (759 samples, “W1.C1”), and not detected in areas where no new cases were reported (298 samples, “W0.C0”) (Fig. 2d). Of the remaining 630 samples, 559 had clinical cases but SARS-CoV-2 was not detected in the wastewater (“W0.C1”). Of these, 67.4% were from counties with incidence rates below the median of all wastewater samples (3.7 cases per 100,000). We also measured the concentration of PMMoV, a stable and persistent indicator of fecal concentration in wastewater (Kitajima et al., 2018; Wu et al., 2020a), and found that PMMoV copies in the W0.C1 samples were slightly but significantly lower than in other samples (Fig. S5a), suggesting that sample dilution and low incidence rates may have contributed to wastewater non-detections. Finally, there were 71 samples (“W1.C0”) for which SARS-CoV-2 was detected, but there were no new clinical cases reported (Fig. 2d). Most of these samples’ viral titers ranged from 10 to 272 copies/ml (Fig. S5b). Comparison of the wastewater data against the 7-day averages of new clinical cases did not yield substantially different results (Fig. S5c-d).
4. Discussion
In this study, we tested and quantified SARS-CoV-2 genome copies in 1751 wastewater samples, collected from 159 U.S. counties in 40 states, using RT-qPCR. This nationwide campaign covered approximately 13% of the U.S. population and demonstrated that widespread wastewater surveillance is feasible and useful across various catchment sizes. Overall, viral titers increased starting in early March, and became relatively stable until April, followed by a small decrease in May. Thirty-eight out of 40 sampled states issued stay-at-home orders or advisories between March 19 and April 7, and all 40 sampled states put statewide restrictions on activity in place between March 10 and April 6 (https://www.usatoday.com/storytelling/coronavirus-reopening-america-map/). These social distancing guidelines may have contributed to the relatively stable viral titers in April and downward trend in May.
Wastewater surveillance of SARS-CoV-2 has been widely employed in the U.S. and other countries (Ahmed et al., 2020a; Albastaki et al., 2021; Bivins et al., 2020a; Chavarria-Miró et al., 2020; Gibas et al., 2021; Medema et al., 2020; Peccia et al., 2020; Prado et al., 2020; Randazzo et al., 2020b; Singh et al., 2021; Wu et al., 2020a; Wurtzer et al., 2020). Together with those studies, our results further support that wastewater surveillance is a cost-effective tool to detect and track SARS-CoV-2 in the population, and could provide an early warning of impending outbreaks in the catchment area. However, the detection rate and limit of wastewater surveillance remain unclear. Our spatiotemporal wastewater dataset enabled us to address this question. Assuming an equal incidence rate throughout the county, our analysis showed that wastewater-based SARS-CoV-2 monitoring has a high chance (>80%) of detecting the viral RNA when the incidence of daily new cases exceeds 13 cases per 100,000 people. Considering only positive wastewater samples, the median detection limit becomes 1 case per ~27,000 people. Our analysis is based on case reports from public health agencies, which are likely underestimates of true infection rates.
This study has several limitations. First, all surveillance data are limited by sampling regimes which could introduce bias through either the frequency of sampling or the specific locations sampled, which is true for both wastewater surveillance and case counts reported by public health authorities. A more unbiased sampling strategy including representative locations and appropriate time intervals would improve our estimate about the viral transmission in the population. Second, sample numbers were biased by month, 5.1% samples were from March and 55.5% samples were from May, thus our analysis may be affected by the low sampling resolution during the early stage of the pandemic. Third, the effect of UV surface sterilization of the tubes and heat inactivation of the sewage on viral concentrations remains unclear, although UV light has poor penetration beyond the surface and previous studies have shown that pasteurization has little influence on the detection of SARS-CoV-2 RNA copies in clinical specimens (Liu et al., 2020; Pastorino et al., 2020; Wang et al., 2020). Fourth, all the samples were collected by each wastewater treatment plant, and mailed to us for analysis, and thus variation in sample collection and transport conditions may have further influenced data comparability. National implementation of a wastewater-based detection system would require standard operating procedures for sample collection, local processing, and analysis.
As a summary, we describe a nationwide campaign to monitor SARS-CoV-2 in the wastewater of 159 counties in 40 U.S. states, covering 13% of the U.S. population, from February 18 to June 2, 2020. Our findings demonstrate that a national wastewater-based approach to disease surveillance may be feasible and effective. This noninvasive and cost-effective approach could be employed as a complementary tool for long-term monitoring of SARS-CoV-2 - as well as other infectious diseases and other health-relevant biomarkers - across the United States and around the world (Bivins et al., 2020b; Naughton et al., 2021; Thompson et al., 2020; Wu et al., 2020b). Furthermore, this approach could be deployed to identify unvaccinated communities and inform vaccination distribution (Smith et al., 2021), and be integrated with next-generation sequencing to investigate the emerging genomic variants circulating in the population (Crits-Christoph et al., 2021; Izquierdo-Lara et al., 2021; Nemudryi et al., 2020).
5. Conclusions
Through analyzing 1751 wastewater samples from 353 unique locations in 40 U.S. states and comparing wastewater viral titers to clinical COVID-19 surveillance data, our results showed:
-
•
Across the country, wastewater viral titers are consistent with, and precede clinical COVID-19 surveillance indicators, including daily new cases.
-
•
Wastewater surveillance has a high SARS-CoV-2 detection rate (>80%) when the local daily incidence exceeds 13 reported cases per 100,000 people.
-
•
Detection rate of SARS-CoV-2 in wastewater is positively associated with catchment size, and 100% detections are disproportionately represented among samples with high incidence and large population size.
-
•
The median wastewater detection of SARS-CoV-2 is one new reported case out of 27,000 people, and overall wastewater surveillance shows a 62.7% consistency to clinical reported data.
Declaration of competing interest
MM and NG are cofounders of Biobot Analytics. EJA is advisor to Biobot Analytics. CD, KAM, KF, and NE are employees at Biobot Analytics, and all these authors hold shares in the company. PRC and TBE have a financial interest in Biobot Analytics, a company engaged in the collection and analysis of wastewater to develop epidemiological data. PRC and TBE’s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the management and sampling teams at all the wastewater treatment facilities who worked with us in providing the samples for analysis; to Franciscus Chandra (SMART, Singapore) for sharing the MHV recovery result; to Penny Chisholm (MIT) and Allison Coe (MIT) for access to equipment and other supplies, Shandrina Burns (MIT) for logistical support; and to all healthcare professionals and first-line responders who have been caring for patients with COVID-19.
This work was supported by the Center for Microbiome Informatics and Therapeutics and Intra-CREATE Thematic Grant (Cities) grant NRF2019-THE001-0003a to JT and EJA; National Institute on Drug Abuse of the National Institutes of Health award numbers K23DA044874; and R44DA051106 to MM and PRC, Hans and Mavis Psychosocial Foundation funding, and e-ink corporation funding to PRC; funding from the Morris-Singer Foundation and NIH award R01AI106786 to WPH; funds from the Massachusetts Consortium on Pathogen Readiness and China Evergrande Group to TBE, PRC, MM, and EJA; funding from the Singapore Ministry of Education and National Research Foundation through an RCE award to Singapore Centre for Environmental Life Sciences Engineering (SCELSE) to SW and JT. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2021.117400.
Appendix. Supplementary materials
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albastaki A., Naji M., Lootah R., Almeheiri R., Almulla H., Almarri I., Alreyami A., Aden A., Alghafri R. First confirmed detection of SARS-COV-2 in untreated municipal and aircraft wastewater in Dubai, UAE: the use of wastewater based epidemiology as an early warning tool to monitor the prevalence of COVID-19. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., de los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam Md.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., van der Voorn T., Wigginton K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2020. Coronavirus Disease 2019 (COVID-19)-COVID-19 Pandemic Planning Scenarios [WWW Document] URL https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2020. National Wastewater Surveillance System.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance.html [WWW Document]URL. [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G., Pintó R., Bosch A. 2020. Sentinel Surveillance of SARS-CoV-2 in Wastewater Anticipates the Occurrence of COVID-19 Cases. medRxiv 2020.06.13.20129627. [DOI] [Google Scholar]
- Contreras S., Dehning J., Loidolt M., Zierenberg J., Spitzner F.P., Urrea-Quintero J.H., Mohr S.B., Wilczek M., Wibral M., Priesemann V. The challenges of containing SARS-CoV-2 via test-trace-and-isolate. Nat. Commun. 2021;12:378. doi: 10.1038/s41467-020-20699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crits-Christoph A., Kantor R.S., Olm M.R., Whitney O.N., Al-Shayeb B., Lou Y.C., Flamholz A., Kennedy L.C., Greenwald H., Hinkle A., Hetzel J., Spitzer S., Koble J., Tan A., Hyde F., Schroth G., Kuersten S., Banfield J.F., Nelson K.L. Genome sequencing of sewage detects regionally prevalent SARS-CoV-2 variants. mBio. 2021;12 doi: 10.1128/mBio.02703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Division of Viral Hepatitis CDC [WWW Document], 2021. URL https://www.cdc.gov/hepatitis/index.html.
- Dumke R., de la Cruz, Barron M., Oertel R., Helm B., Kallies R., Berendonk T.U., Dalpke A. Evaluation of two methods to concentrate SARS-CoV-2 from untreated wastewater. Pathogens. 2021;10:195. doi: 10.3390/pathogens10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway, S.E., 2021. Emergence of SARS-CoV-2 B.1.1.7 Lineage — United States, December 29, 2020–January 12, 2021. MMWR Morb. Mortal. Wkly Rep. 70. https://doi.org/10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed]
- Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Roppolo Brazell L., Hinton K., Lontai J., Stark N., Young I., Quach C., Russ M., Kauer J., Nicolosi B., Chen D., Akella S., Tang W., Schlueter J., Munir M. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh N.D., Hodcroft E.B., Fauver J.R., Phelan A.L., Cevik M. Public health actions to control new SARS-CoV-2 variants. Cell. 2021 doi: 10.1016/j.cell.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Lara R., Elsinga G., Heijnen L., Munnink B.B.O., Schapendonk C.M.E., Nieuwenhuijse D., Kon M., Lu L., Aarestrup F.M., Lycett S., Medema G., Koopmans M.P.G., Graaf M. Monitoring SARS-CoV-2 circulation and diversity through community wastewater sequencing, the Netherlands and Belgium. Emerg. Infectious Dis. J. - CDC. 2021;27 doi: 10.3201/eid2705.204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins Coronavirus Resource Center . Johns Hopkins Coronavirus Resource Center; 2021. COVID-19 Map [WWW Document]https://coronavirus.jhu.edu/map.html URL. [Google Scholar]
- Johns Hopkins University Center for Systems Science and Engineering . 2020. COVID-19 Map - Johns Hopkins Coronavirus Resource Center.https://coronavirus.jhu.edu/map.html [WWW Document]. URL. (accessed 9.11.20) [Google Scholar]
- Kitajima M., Sassi H.P., Torrey J.R. Pepper mild mottle virus as a water quality indicator. NPJ Clean Water. 2018;1:1–9. doi: 10.1038/s41545-018-0019-5. [DOI] [Google Scholar]
- Kretzschmar M.E., Rozhnova G., Bootsma M.C.J., Boven M., van, Wijgert J.H.H.M., van de, Bonten M.J.M. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5:e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa L., G. Iaconelli, M. Mancini, P., Ferraro Bonanno, G. Veneri, C. Bonadonna, L. Lucentini, L. Suffredini, E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa La, G. Mancini, P., Ferraro Bonanno, G. Veneri, C. Iaconelli, M. Bonadonna, L. Lucentini, L. Suffredini, E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Cao Z., Chen M., Zhong Y., Luo Y., Shi G., Xiang H., Luo J., Zhou H. 2020. Effect of Heat Inactivation on Real-Time Reverse Transcription PCR of the SARS-COV-2 Detection. medRxiv 2020.05.19.20101469. [DOI] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Naughton C.C., Roman F.A., Alvarado A.G.F., Tariqi A.Q., Deeming M.A., Bibby K., Bivins A., Rose J.B., Medema G., Ahmed W., Katsivelis P., Allan V., Sinclair R., Zhang Y., Kinyua M.N. 2021. Show us the Data: Global COVID-19 Wastewater Monitoring Efforts, Equity, and Gaps. medRxiv 2021.03.14.21253564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Heat inactivation of different types of SARS-CoV-2 samples: what protocols for biosafety, molecular detection and serological diagnostics? Viruses. 2020;12 doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020:1–4. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Kumar V., Kapoor D., Dhanjal D.S., Bhatia D., Jan S., Singh N., Romero R., Ramamurthy P.C., Singh J. Detection and disinfection of COVID-19 virus in wastewater. Environ. Chem. Lett. 2021;19:1917–1933. doi: 10.1007/s10311-021-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Cassell G., Bhatnagar A. Wastewater surveillance can have a second act in COVID-19 vaccine distribution. JAMA Health Forum. 2021;2 doi: 10.1001/jamahealthforum.2020.1616. [DOI] [PubMed] [Google Scholar]
- Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Furumai H., Katayama H. Applicability of polyethylene glycol precipitation followed by acid guanidinium thiocyanate-phenol-chloroform extraction for the detection of SARS-CoV-2 RNA from municipal wastewater. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. 2020. Influenza Surveillance System: Purpose and Methods | CDC [WWW Document]https://www.cdc.gov/flu/weekly/overview.html URL. [Google Scholar]
- Wang T., Lien C., Liu S., Selveraj P. 2020. Effective Heat Inactivation of SARS-CoV-2. medRxiv 2020.04.29.20085498. [DOI] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. 2020. SARS-CoV-2 Titers in Wastewater Are Higher Than Expected from Clinically Confirmed Cases. mSystems 5, 2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., Matus M., McElroy K.A., Nagler J., Rhode S.F., Santillana M., Tucker J.A., Wuertz S., Zhao S., Thompson J., Alm E.J. 2020. SARS-CoV-2 Titers in Wastewater Foreshadow Dynamics and Clinical Presentation of New COVID-19 Cases. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, PARTITIONING, AND RECOVERY OF ENVELOPED VIRUSES IN UNTREATED MUNICIPAL WASTewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.