Abstract
Periodontal disease has been reported to be associated with diabetes mellitus. However, the direction of the association and the influence of bias are not clear. Thus, the aim of this systematic review and meta-analysis was to summarize the existing evidence on the bidirectional prospective association between periodontal disease and diabetes mellitus by accounting for the risk of bias of the original studies. The literature search was conducted on the electronic data sources PubMed and Web of Science up to February 9th, 2021. We included observational studies, which investigated the prospective association between diabetes mellitus and periodontal disease or vice versa. The risk of bias of the primary studies was evaluated by applying the Quality in Prognosis Studies (QUIPS) tool. Random effects models were used to calculate summary relative risk (SRR) with 95% CI. Subgroup analyses were applied to investigate heterogeneity and the robustness of the finding. In total, 15 studies were included . The SRR for incident diabetes mellitus was 1.26 (95% CI 1.12, 1.41; I2: 71%, n = 10; participants = 427,620; identified cases = 114,361), when comparing individuals with periodontitis to individuals without periodontitis. The SRR for incident periodontitis was 1.24 (95% CI 1.13, 1.37; I2: 92%, n = 7; participants = 295,804; identified cases: > 22,500), comparing individuals with diabetes to individuals without diabetes. There were no significant differences between subgroups after stratification for risk of bias. The findings show a positive bidirectional association between periodontal disease and diabetes mellitus, and thus, underline the need for screening of patients with periodontitis regarding diabetes mellitus and vice versa. The main limitation of the study is the high unexplained heterogeneity between the studies including the different assessment methods of the disease diagnosis.
Subject terms: Dental diseases, Endocrine system and metabolic diseases
Introduction
Diabetes mellitus is one of the most common chronic diseases worldwide and current estimates assume that 463 million adults were affected in 2019. The prevalence is projected to increase by almost 50% over the next years, and it is estimated that there will be about 700 million people living with diabetes by 20451. Individuals with diabetes are at higher risk of developing further health-related complications and disorders, including cardiovascular disease2, retinopathy3, nephropathy4 and neuropathy5. In addition, there is indication, that patients with diabetes suffer more often from dental disease (e.g. periodontitis) compared to individuals without diabetes6. Periodontitis is a widespread disease, whose most severe form afflicted about 743 million people worldwide in 2010, but an underestimation is expected7. The disease is characterized by a chronic inflammation of the entire periodontium that can irreparably destruct the tooth-surrounding tissue and result in the resorption of the alveolar bone. Consequences such as gingival bleeding, increased tooth mobility and tooth loss can be expected8. Recently, a meta-analysis summarized findings on glucose disturbance, including diabetes, and periodontal disease and indicated a positive association between these two factors9. However, in this meta-analysis, studies with different exposures and outcomes were mixed. For example, the authors combined studies on diabetes, prediabetes and diabetes severity10,11. In addition, the outcome was a mixture of periodontal disease and progression of the disease12. Moreover, there is indication that periodontal disease is a risk factor for diabetes mellitus13. Both conditions are driven by inflammatory processes, which might be a possible explanation for this bidirectional association14. To draw clear conclusions on these associations, a systematic review and meta-analysis is needed that considers methodological challenges when combining the existing data from primary studies. First, the time sequence of exposure and outcome needs to be taken into account to obtain the direction of the association. Second, the measurement of periodontal disease differs between the studies. In observational studies, periodontal disease has been assessed as self-reported periodontitis, clinical measurements attained from oral examinations (e.g. clinical attachment loss (CAL), periodontal pocket depth (PPD)), or established scores, such as the Community Periodontal Index (CPI; based on components including gingival bleeding, dental calculus and periodontal pocket depths), or Russell’s Periodontal Index (PI; including signs of periodontal disease as inflammation, pocket formation and breakdown of function), respectively. Further, the risk of bias of the primary studies, including selection bias, information bias and confounding, should be considered by applying an appropriate tool15 when interpreting the data.
Thus, our aim was to conduct a systematic review and meta-analyses of the existing evidence of observational studies, which investigate prospectively the bidirectional association between periodontal disease and diabetes mellitus. In addition, we accounted for the risk of bias of the original studies, especially the assessment of periodontal disease in our meta-analyses.
Research design and methods
The systematic literature search was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) guidelines16. A protocol has been registered in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=118829).
Search strategy
The literature search was conducted on PubMed and Web of Science up to February 9th, 2021 to identify studies analyzing the association either between diabetes mellitus and periodontal disease, or the other direction, respectively. We used a combination of predefined search terms developed for each database without applying filters or restriction of language and calendar date (Table S1). Furthermore, we checked the reference lists of identified related reviews and included articles if eligible. Four investigators (JS, JB, MN, SS) independently screened the titles and abstracts of the identified studies in EndnoteX8 and read the full-text, if the study seemed to be relevant. Disagreements were discussed and resolved by consensus.
Selection of studies
Studies were included, if: (1) they investigated the association between either periodontal disease and diabetes mellitus, or the other direction, respectively, (2) the association was investigated prospectively (this included prospective and retrospective cohort studies, nested case–control studies, case-cohort studies), (3) reported risk ratios (relative risk (RR), odds ratio (OR), hazard ratio (HR)) with corresponding 95% confidence intervals (CI) and 4) the papers were published following a peer-reviewed process.
Studies were excluded, if (1) they were animal studies, cross-sectional studies, case–control studies, conference abstracts or reviews, (2) they only reported crude estimates, (3) the study population was not relevant (including pregnant women or children and adolescents), (4) they focused on hyperglycemia (Fasting plasma glucose (FPG) > 110 ml/dg or haemoglobin A1c (HbA1c) > 6%) as exposure or outcome, (5) they focused on the progression of the particular disease and (6) when studies investigated potential indicators for periodontal diseases, such as tooth loss. In more detail, several studies investigated tooth loss as an indicator of periodontal disease,because assessing the number of missing teeth is less difficult and less time-consuming. However, tooth loss is not a reliable marker for periodontitis17, as caries is another leading cause for tooth loss18. Thus, we decided to exclude tooth loss as exposure/outcome, to minimize bias.
If different studies reported on similar data (same exposure and outcome), we selected the study with the largest number of participants and cases.
Data extraction and risk of bias assessment
The extraction of the data from the studies was conducted by one investigator (JS or SS) and checked by two other investigators (JB or MN). Each inconsistency was debated until agreement was reached. The following data were extracted from each eligible study: the last name of the first author, the name of the study and the country in which it was conducted, the study design (prospective and retrospective cohort studies), the publication year, follow-up time, number of participants and cases, definition and assessment of exposure and outcome, the exposure categories and number of cases and non-cases for each category, the RRs and 95% (CI) and the variables adjusted for in the analysis.
The risk of bias of the primary studies was evaluated by applying the Quality In Prognosis Studies (QUIPS) tool15 by at least two independent investigators (JB, JS, MN) and discrepancies were discussed with another investigator (SS) and resolved by discussion. It includes the following 6 domains: study participation, study attrition, prognostic factor measurements, outcome measurements, study confounding and statistical analysis, and reporting. In each domain, the studies were evaluated for their reliability and eligibility and each domain was rated as low, moderate or high risk of bias. Finally, an overall risk of bias for each primary study was determined, whereby the domains prognostic factor and outcome measurements were assigned a higher weight, as we considered these domains to be of decisive importance. Thus, if a study was rated with high risk in one of these domains, the overall risk of bias was also judged as high. In this context, assessment of periodontal disease was judged as low risk of bias if clinical measurements of CAL or PPD were obtained, or ICD-codes based on the previously valid classification system for periodontal disease by Armitage (according to CAL) were used19. Further, we considered the CPI, introduced by the World Health Organization (WHO), as appropriate method, since it is faster and more reproducible in large epidemiological studies. The extend of PPD is the main factor for establishing the code and thus provides an indication for CAL20. A self-reported diagnosis that is validated by a dentist, classification by the PI and ICD-codes from a health insurance database, without further specification, were judged with moderate risk of bias. Self-reported diagnosis without validation is rated with high risk. The assessment of the diabetes mellitus diagnosis was classified with low risk of bias, if one of the following diagnostic tests was conducted, in accordance with the criteria of the American Diabetes Association: values of HbA1c or of plasma glucose (FPG or 2-h FPG after an oral glucose tolerance test)21. If a study included a self-reported diagnosis, validated by a physician or on ICD-codes from a health insurance database, it was considered to be at moderate risk. Diagnosis based on self-reports without validation were rated with high risk. For the domain study confounding, we defined the following confounders as important, for which a study should have at least been adjusted: age, sex, body mass index (BMI)/overweight, smoking status and socio-economic status. This domain could not be rated higher than moderate risk of bias, because residual confounding cannot be completely excluded in observational studies. In detail, the signaling questions for the QUIPS tool can be found in Table S2.
Statistical analysis
Meta-analyses were conducted for the following associations: (i) periodontal disease as exposure and incidence of diabetes mellitus as outcome, (ii) diabetes mellitus as exposure and incidence of periodontal disease as outcome. Summary relative risks (SRR) and 95% CIs for these associations were calculated by using random effects meta-analysis by DerSimonian and Laird22. In addition, we conducted dose–response meta-analyses for exposure on periodontitis, if it was measured as continuous variables (CPI or PPD), and studies provided findings (RRs and 95% CIs) for at least three quantified categories. For this, we calculated the study-specific slopes and corresponding 95% CIs from the natural logarithm of the reported RRs and 95% CIs across the exposure categories23. The shapes of the relationships were evaluated by using restricted cubic spline regression models, and a likelihood ratio test was used to test for non-linearity24.
Heterogeneity was evaluated by applying I2, tau2, and 95% prediction intervals (95% PIs), and was investigated in subgroups analyses. Subgroup meta-analyses were conducted by total risk of bias, risk of bias for the exposure and outcome domains (high, moderate, low), sex, geographic locations, type of diabetes, duration of follow-up, number of cases, smoking status, and by adjustment of the original studies for potentials confounders: education, smoking status, overweight, fruit and vegetable intake, alcohol intake, physical activity, other chronic diseases. Differences were tested by using meta-regression25. Small study effects and publication bias were assessed by using Egger’s test and by visual inspection of funnel plots26, if more than 10 studies were included in the meta-analysis27. Potential publication bias was indicated by asymmetry of the funnel plot and a p value < 0.01 for Egger’s test. All statistical analyses were conducted using Stata version 15.1 software (StataCorp, texas, US).
Certainty of evidence assessment
We evaluated the certainty of evidence for each association using the updated Grading of Recommendations Assessment, Development and Evaluations (GRADE). The GRADE tool covers the following aspects: the study design, the risk of bias of the primary studies, imprecision of the findings, inconsistency between the primary studies, indirectness in the primary studies, publication bias, the magnitude of effect of the pooled findings, indication for dose–response relations and the impact of residual confounding. The certainty of evidence can be evaluated as high, moderate, low or very low. A high certainty of evidence means that it is very likely that the effect estimate lies close to the true effect, whereas a very low certainty of evidence means that it is very likely that the inclusion of future studies will change the estimate.
Results
In total, 9384 studies were initially identified, and after removing 2427 duplicates and excluding 6411 articles by title and abstract, 546 were investigated in a full-text analysis (Figure S1). Finally, 15 cohort studies were included in our meta-analysis, the characteristics of all included studies are presented in the Supplemental Material (Table S4). A list of the excluded studies and the corresponding reasons are shown in the supplement (Table S3).
Periodontal disease and incidence of diabetes mellitus
We identified 10 studies that investigated the association between periodontal disease and incidence of diabetes mellitus with a total of 427,620 participants and 114,361 identified cases of diabetes mellitus over a mean follow-up period of 9.9 years (range 5–17 years)13,28–35. Four studies achieved a low, three of them a moderate, and three a high overall risk of bias (Table S5). The assessment of periodontal disease was evaluated with low risk of bias in seven studies, moderate in one study, and high in two studies. The assessment of diabetes was judged as low risk of bias in seven, moderate in two studies, and high in one study. Eight studies focused on type 2 diabetes13,28,31–35, one on both types30, and for one it remains unclear which type of diabetes was investigated29.
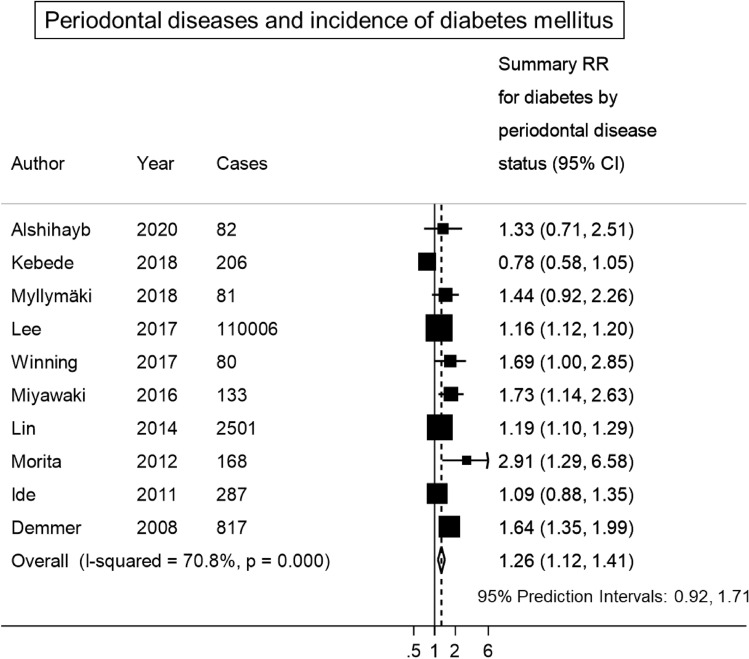
The SRR for incident diabetes mellitus was 1.26 (95% CI 1.12, 1.41; I2:71%, Tau2: 0.014, 95% PI: 0.92, 1.71), when comparing individuals with periodontitis to individuals without (Fig. 1). There was indication for a non-linear trend for the relationship between the CPI score and the incidence of diabetes. No association was observed for a CPI score ≤ 2, but an increase in the incidence of diabetes was observed after a CPI score ≥ 3 (score 3: SRR (95% CI): 1.38 (1.02, 1.87); score 4: SRR (95% CI): 2.33 (1.11, 4.87); p for non-linearity:0.090; n = 2) (Fig. 2A). The non-linear analysis for the relationship between PPD and the incidence of diabetes showed an increased relative risk for diabetes up to a PPD of 3 mm, then the graph reached a plateau with no further increase in risk (PPD 1.0 mm: SRR (95% CI): 1.15 (0.83, 1.59); PPD 2.5 mm: SRR (95% CI): 1.27 (0.83, 1.95), PPD 3,5 mm: SRR (95% CI): 1.30 (0.90, 1.89), and PPD 4,5 mm: SRR (95% CI): 1.31 (0.86, 2.01), based on n = 2; p for non-linearity: 0.653) (Fig. 2B). The certainty of evidence was judged as moderate for the association between periodontal disease and incidence of diabetes mellitus (Table S6).
Figure 1.
Meta-analysis of periodontal disease and incidence of diabetes mellitus.
Figure 2.
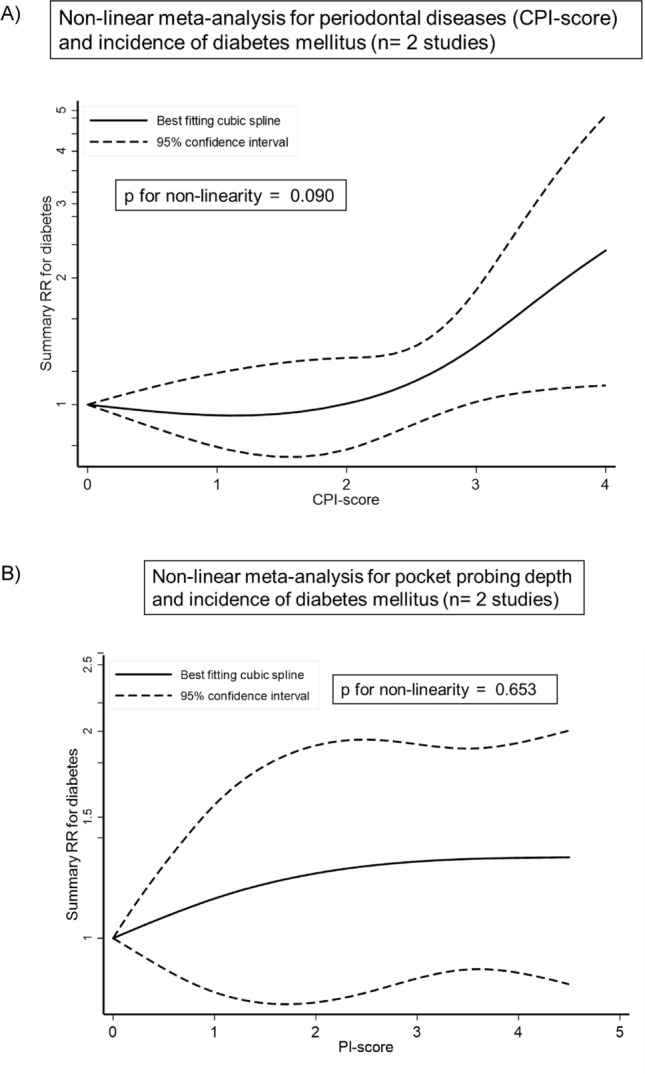
Non-linear dose–response meta-analysis for periodontal disease, defined as (A) CPI-score and (B) defined as PPD, and incidence of diabetes mellitus.
Diabetes mellitus and incidence of periodontal disease
The seven studies on diabetes mellitus and incidence of periodontitis included 295,804 participants and > 22,500 diagnosed cases of periodontal disease (missing information on cases for Lee et al. 36)33,36–41. The mean follow-up period was 11.1 years (range 5–20 years). The overall risk of bias was low in one study, moderate in four studies, and high in two studies (Table S5). Two studies were rated as low risk of bias regarding the exposure assessment, four as moderate and one study with high. Four studies focused on type 2 diabetes37,38,40,41, one on type 1 diabetes39, one on both types of diabetes36, and in one it was not specified33. Outcome assessment was rated as low risk of bias in four studies, moderate for two studies, and high for one study.
The SRR for incident periodontitis was 1.24 (95% CI 1.13, 1.37; I2: 92%, tau2: 0.01, 95% PI 0.94, 1.65), comparing individuals with diabetes to individuals without diabetes (Fig. 3). The certainty of evidence was evaluated as moderate for the association between diabetes mellitus and incidence of periodontal disease (Table S6).
Figure 3.
Meta-analysis of diabetes mellitus and incidence of periodontal disease.
Subgroup analyses
There was no significant heterogeneity between subgroups after stratification by overall risk of bias, risk of bias in the exposure and outcome domains, sex, geographical location, type of diabetes, duration of follow-up, number of included cases, adjustment for important confounders (e.g. smoking status, education, etc.) (Table S7–S8). Publication bias was only evaluated for the association between periodontal disease and incidence of diabetes mellitus because more than ten studies were available for these association, and no indication for publication bias was observed by visual inspection of the funnel plot (Figure S2) and Egger’s test (p = 0.194).
Discussion
This systematic review and meta-analysis of 15 cohort studies showed that there was a positive bidirectional association between both periodontal disease and diabetes mellitus with a moderate certainty of evidence. For patients with diabetes, the data indicated a 24% (95% CI 13%, 37%) increase in the incidence of periodontal disease. For patients with periodontitis, the relative risk of developing diabetes mellitus was elevated by 26% (95% CI 12%, 41%). These results coincide with those of the meta-analysis by Nascimento et al., where an 86% (95% CI 30%, 180%) increased relative risk of periodontitis for individuals with diabetes was found. The fact that the relative risk is higher than in our analysis may be due to the fact that different degrees of severity of diabetes were taken into account as exposure and the outcome was not only incidence of periodontitis but also progression of the disease and its marker9. In contrast, the meta-analysis by Ziukaite et al. focused on the other direction of the association and reported a 27% (95% CI 11%, 74%) higher prevalence of diabetes for patients with periodontitis42, which is comparable to our findings. Risk of bias was high in the previous meta-analyses because different study designs, different definitions of exposure and outcomes were combined. In our meta-analysis, we considered both directions separately, included only prospective studies and did not mix disease status with pre-disease status or progression of the diseases, respectively.
In the risk of bias assessment of the individual studies, we particularly focused on the assessment and definition of periodontal disease and diabetes. In general, the diagnosis of a disease was defined by self- reports, clinical examinations or a combination of both. While a meta-analysis showed that the prevalence for diabetes was much lower for self-reported periodontitis compared to clinical periodontal measurements42, our meta-analyses did not show a significant difference here. However, subgroup meta-analyses relied on small numbers of studies and more studies with accurate assessment methods are needed. Although it was rarely applied among the studies, assessment via CAL has been considered the gold standard for the classification of chronic periodontitis19. But with this method measurement errors may occur especially in the initial phase of periodontitis43, for example, if the manual probe is applied with incorrect force, it can be advanced into intact attachment fibers44. It should also be taken into account that these measurements are associated with a standard deviation of 0.8–1.07 mm, even for experienced investigators45. Another applied assessment method is the determination of the PPD. While the assessment is more simple than the CAL, it has been shown that this method used alone, can lead to an underestimation of cases46 because, especially with increasing age, the extent of PPD no longer correlates with CAL47. In addition, there are studies that used indices such as Russell's PI28, or the CPI13,33,37 to classify the disease. Periodontitis diagnosis based on the PI is critical because this index is only visually assessed and does not include clinical measurements, and it includes gingivitis as an early form of periodontitis48. Although the CPI is characterized by its reproducibility and simplification49, it is not considered sufficient to describe the extent of periodontal disease50. In summary, the assessment and definition of periodontal disease vary widely across studies and there are no consistent thresholds for CAL/PPD and numbers of affected teeth to determine whether the disease is present or not. The Division of Oral Health at the Centers for Disease Control and Prevention in collaboration with the American Academy of Periodontology has provided a definition that combines measurements of CAL and PPD to assess periodontal disease to avoid misinterpretation of the periodontal status48. In order to make clear statements about the association between periodontitis and diabetes mellitus, it should be ensured in the future studies that established assessment of periodontitis is applied, and thus, enables comparability between studies.
There are many possible explanations for the observed bidirectional associations between periodontitis and diabetes, which are related to inflammatory processes. For example, on the one hand, untreated diabetes mellitus, both type 1 or 2 diabetes, lead to metabolic disorders caused by hyperglycemia51. Poor glycemic control in patients with diabetes has been shown to raise the level of systemic inflammation markers, e.g. interleukin-1ß, in the gingival crevicular fluid of a periodontal pocket14, which is associated with the onset and severity of periodontal disease52. On the other hand, it has been show that gram-negative bacteria in the periodontal pockets elevate serum inflammatory markers such as c-reactive protein53. This can induce hyperinflammatory immune cells and promote the release of proinflammatory cytokines, which lead to insulin resistance54.
Strengths of our meta-analysis is the bidirectional investigation and the dose–response meta-analysis between periodontal disease and diabetes mellitus by including only prospective studies and accounting for the major risk of bias sources, namely assessment of exposures and outcomes, and other sources of bias e.g. selection of participants and confounding. However, there are also limitations in our meta-analysis. First, as already described in detail, risk of bias regarding the assessment of periodontitis could not be ruled out in all of the studies. To account for this, we conducted subgroup analysis and did not observe significant differences. However, these analyses were based on small numbers of studies, and should be treated with caution. Second, most of the included studies did not clearly differentiate between type 1 and type 2 diabetes. In the subgroup meta-analysis by type of diabetes, we did not observe substantial differences, but again, the number of studies in the subgroups were small. Third, residual confounding cannot be excluded because of the observational study design of the included studies. Fourth, the included studies did not report on repeated measurements and thus, we could not account for changes of the disease status (impairment or also improvement due to treatment). Fifth, we did not search for grey literature, because we prefer to include only peer-reviewed studies. Last, we identified high heterogeneity between studies, which may arise from different methodological aspects e.g. different assessment methods of periodontal disease and/or diabetes. However, we conducted several subgroups analyses and meta-regression for total risk of bias and risk of bias of exposure and outcome assessment, further factors such as geographic location, and methodological aspects (number of cases, duration of follow-up, adjustment for specific confounders etc.) and heterogeneity remained unchanged.
Our comprehensive meta-analysis can help investigators to plan and conduct further research regarding bidirectional associations between periodontal disease and diabetes mellitus and can help for decision making in the clinical context. Our findings support existing guidelines for physicians and dentists regarding the screening of patients with diabetes, which recommend that every new confirmed patient with diabetes mellitus, should be informed that there is an increased risk of developing periodontal disease and that glycemic control is more difficult in this case. Thus, every initial examination should include a periodontal evaluation55. It has been shown, that 40.7% of the dental patients without a diagnosis of diabetes (< 45 years) had HbA1c values around 5,7% or higher56, thus, screening for diabetes mellitus in the dental office is as important.
In conclusion, there was a bidirectional association between periodontal disease and diabetes mellitus, even after stratifying for major risk of bias. However, only few studies with low risk of bias were available. To strengthen these findings more studies with valid assessment of periodontal diseases and diabetes are needed. The findings support current guidelines that patients with periodontitis should be screened for diabetes mellitus, and that patients with diabetes mellitus should be informed about their higher risk of developing periodontal diseases.
Supplementary Information
Abbreviations
- BMI
Body mass index
- CAL
Clinical attachment loss
- CPI
Community periodontal index
- CI
Confidence interval
- FPG
Fasting plasma glucose
- Hb A1c
Haemoglobin A1c
- HR
Hazard ratio
- ICD
International classification of disease
- OR
Odds ratio
- PI
Periodontal index
- PPD
Periodontal pocket depths
- PI
Predication interval
- QUIPS
Quality in prognosis studies
- RR
Relative risk
- SRR
Summary relative risk
- WHO
World health organization
Author contributions
S.S. and J.S. designed the study question. J.S., J.B., M.N. and S.S. conducted the literature screening, and extracted the data. J.S., J.B., M.N. and S.S. assessed the risk of bias of the included studies. S.S. conducted the analyses and S.S. and J.S. interpreted the data and wrote the first draft of the manuscript. All authors critically reviewed the manuscript and approved submission of the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The German Diabetes Centre is funded by the German Federal Ministry of Health and the Ministry of Innovation, Science and Research of the State of North Rhine-Westphalia. PROSPERO Registration: CRD42018118829.
Data availability
All data are available in the manuscript and its Supplement file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93062-6.
References
- 1.IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available at: https://www.diabetesatlas.org. Accessed February 22, 2021.
- 2.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/s0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 6.Bharateesh J, Ahmed M, Kokila G. Diabetes and oral health: A case–control study. Int. J. Prev. Med. 2012;3(11):806–809. [PMC free article] [PubMed] [Google Scholar]
- 7.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br. Dent. J. 2014;217(8):433–437. doi: 10.1038/sj.bdj.2014.907. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento GG, Leite FRM, Vestergaard P, Scheutz F, Lopez R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol. 2018;55(7):653–667. doi: 10.1007/s00592-018-1120-4. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Kim EK, Kim JW, Choi YH, Mechant AT, Song KB, et al. The relationship between metabolic conditions and prevalence of periodontal disease in rural Korean elderly. Arch. Gerontol. Geriatr. 2014;58(1):125–129. doi: 10.1016/j.archger.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki M, Sato M, Minagawa K, Manz MC, Yoshihara A, Miyazaki H. Longitudinal relationship between metabolic syndrome and periodontal disease among Japanese adults aged >/=70 years: The Niigata Study. J. Periodontol. 2015;86(4):491–498. doi: 10.1902/jop.2015.140398. [DOI] [PubMed] [Google Scholar]
- 12.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann. Periodontol. 1998;3(1):30–39. doi: 10.1902/annals.1998.3.1.30. [DOI] [PubMed] [Google Scholar]
- 13.Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: A seven-year study. J. Dent. Res. 2011;90(1):41–46. doi: 10.1177/0022034510381902. [DOI] [PubMed] [Google Scholar]
- 14.Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, Hsu D, Celenti RS, Grbic JT, et al. Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J. Periodontol. 2004;75(9):1203–1208. doi: 10.1902/jop.2004.75.9.1203. [DOI] [PubMed] [Google Scholar]
- 15.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmlund A, Lind L. Number of teeth is related to atherosclerotic plaque in the carotid arteries in an elderly population. J. Periodontol. 2012;83(3):287–291. doi: 10.1902/jop.2011.110100. [DOI] [PubMed] [Google Scholar]
- 18.Chauncey HH, Glass RL, Alman JE. Dental caries. Principal cause of tooth extraction in a sample of US male adults. Caries Res. 1989;23(3):200–205. doi: 10.1159/000261178. [DOI] [PubMed] [Google Scholar]
- 19.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Akazawa H. Periodontitis and diabetes mellitus: Be true to your teeth. Int. Heart J. 2018;59(4):680–682. doi: 10.1536/ihj.18-410. [DOI] [PubMed] [Google Scholar]
- 21.Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: An update 2015. Rev. Diabet. Stud. 2015;12(1–2):48–62. doi: 10.1900/RDS.2015.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 24.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). in Cochrane. www.training.cochrane.org/handbook (2019).
- 28.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: Results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31(7):1373–1379. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kebede TG, Pink C, Rathmann W, Kowall B, Volzke H, Petersmann A, et al. Does periodontitis affect diabetes incidence and haemoglobin A1c change? An 11-year follow-up study. Diabetes Metab. 2018;44(3):243–249. doi: 10.1016/j.diabet.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Oh JY, Youk TM, Jeong SN, Kim YT, Choi SH. Association between periodontal disease and non-communicable diseases: A 12-year longitudinal health-examinee cohort study in South Korea. Medicine. 2017;96(26):e7398. doi: 10.1097/md.0000000000007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SY, Lin CL, Liu JH, Wang IK, Hsu WH, Chen CJ, et al. Association between periodontitis needing surgical treatment and subsequent diabetes risk: A population-based cohort study. J. Periodontol. 2014;85(6):779–786. doi: 10.1902/jop.2013.130357. [DOI] [PubMed] [Google Scholar]
- 32.Miyawaki A, Toyokawa S, Inoue K, Miyoshi Y, Kobayashi Y. Self-reported periodontitis and incident type 2 diabetes among male workers from a 5-year follow-up to my health up study. PLoS ONE. 2016;11(4):e0153464. doi: 10.1371/journal.pone.0153464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita I, Inagaki K, Nakamura F, Noguchi T, Matsubara T, Yoshii S, et al. Relationship between periodontal status and levels of glycated hemoglobin. J. Dent. Res. 2012;91(2):161–166. doi: 10.1177/0022034511431583. [DOI] [PubMed] [Google Scholar]
- 34.Myllymaki V, Saxlin T, Knuuttila M, Rajala U, Keinanen-Kiukaanniemi S, Anttila S, et al. Association between periodontal condition and the development of type 2 diabetes mellitus—Results from a 15-year follow-up study. J. Clin. Periodontol. 2018 doi: 10.1111/jcpe.13005. [DOI] [PubMed] [Google Scholar]
- 35.Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: A prospective cohort study. J. Clin. Periodontol. 2017;44(3):266–274. doi: 10.1111/jcpe.12691. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Choi JK, Jeong SN, Choi SH. Charlson comorbidity index as a predictor of periodontal disease in elderly participants. J. Periodontal Implant Sci. 2018;48(2):92–102. doi: 10.5051/jpis.2018.48.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu SY, Lai H, Yen AM, Fann JC, Chen LS, Chen HH. Temporal sequence of the bidirectional relationship between hyperglycemia and periodontal disease: A community-based study of 5,885 Taiwanese aged 35–44 years (KCIS No. 32) Acta Diabetol. 2015;52(1):123–131. doi: 10.1007/s00592-014-0612-0. [DOI] [PubMed] [Google Scholar]
- 38.Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ. Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res. Clin. Pract. 2012;98(3):494–500. doi: 10.1016/j.diabres.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun KT, Chen SC, Lin CL, Hsu JT, Chen IA, Wu IT, et al. The association between Type 1 diabetes mellitus and periodontal diseases. J. Formosan Med. Assoc. = Taiwan yi zhi. 2018 doi: 10.1016/j.jfma.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Lee CY, Kuan YH, Tsai YF, Tai CJ, Tsai TH, Huang KH. Correlation between diabetes mellitus and periodontitis in Taiwan: A nationwide cohort study. Diabetes Res. Clin. Pract. 2019;150:245–252. doi: 10.1016/j.diabres.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Alshihayb TS, Kaye EA, Zhao Y, Leone CW, Heaton B. A quantitative bias analysis to assess the impact of unmeasured confounding on associations between diabetes and periodontitis. J. Clin. Periodontol. 2021;48(1):51–60. doi: 10.1111/jcpe.13386. [DOI] [PubMed] [Google Scholar]
- 42.Ziukaite L, Slot DE, Van der Weijden FA. Prevalence of diabetes mellitus in people clinically diagnosed with periodontitis: A systematic review and meta-analysis of epidemiologic studies. J. Clin. Periodontol. 2018;45(6):650–662. doi: 10.1111/jcpe.12839. [DOI] [PubMed] [Google Scholar]
- 43.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018;45(Suppl 20):S149–S161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 44.Robinson PJ, Vitek RM. The relationship between gingival inflammation and resistance to probe penetration. J. Periodontal Res. 1979;14(3):239–243. doi: 10.1111/j.1600-0765.1979.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 45.Haffajee AD, Socransky SS. Attachment level changes in destructive periodontal diseases. J. Clin. Periodontol. 1986;13(5):461–475. doi: 10.1111/j.1600-051x.1986.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 46.Carlos JP, Brunelle JA, Wolfe MD. Attachment loss vs. pocket depth as indicators of periodontal disease: A methodologic note. J. Periodontal Res. 1987;22(6):524–525. doi: 10.1111/j.1600-0765.1987.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 47.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J. Periodontol. 1999;70(1):13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 48.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007;78(7 Suppl):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 49.Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: The WHO approach. J. Periodontol. 2005;76(12):2187–2193. doi: 10.1902/jop.2005.76.12.2187. [DOI] [PubMed] [Google Scholar]
- 50.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89(Suppl 1):S173–S182. doi: 10.1002/jper.17-0721. [DOI] [PubMed] [Google Scholar]
- 51.Nazir MA, AlGhamdi L, AlKadi M, AlBeajan N, AlRashoudi L, AlHussan M. The burden of diabetes, its oral complications and their prevention and management. Open Access Macedonian J. Med. Sci. 2018;6(8):1545–1553. doi: 10.3889/oamjms.2018.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J. Periodontol. 1997;68(2):127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- 53.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J. Periodontol. 2001;72(9):1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 54.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 2005;76(11 Suppl):2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 55.Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018;45(2):138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 56.Genco RJ, Schifferle RE, Dunford RG, Falkner KL, Hsu WC, Balukjian J. Screening for diabetes mellitus in dental practices: A field trial. J. Am. Dent. Assoc. (1939) 2014;145(1):57–64. doi: 10.1421/jada.2013.7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript and its Supplement file.