Abstract
Diamine oxydase and peroxidase have been co-immobilized onto layered double hydroxide (LDH) thin films for the development of real-time histamine biosensors. The chosen LDH materials are Mg2AlCO3, Mg4FeCl and Ca2AlCl. Prepared bi-enzymatic hybrid nanomaterials are capable of detecting histamine through the electrochemical oxidation of H2O2 and are used as the sensitive membrane for potentiometric microelectrode. Histamine biosensors developed in this work have fast response of less than 20 s, are sensitive and selective, with a large dynamic range of 10–8–10–3 M and a limit of detection of less than 10–8 M. The detection limit of the developed bi-enzymatic biosensors is relatively higher than those corresponding with gas and liquid chromatography, which are still considered as the reference methods. Finally, the reproducibility, the specificity and the storage stability of the biosensors were studied.
Keywords: Potentiometric biosensor, Histamine, Hybrid nanomaterial, Immobilization, Layer double hydroxide, Specificity
Introduction
Biogenic amines (histamine, putrescine, cadaverine) are low-molecular weight organic compounds present in various foods particularly in fish and fish products, cheese, meat and wine (Razavi-Rohani et al. 2013; Biji et al. 2016). Histamine is related to food poisoning while putrescine and cadaverine are not toxic, but they increase the toxicity of histamine (Tortorella et al. 2014; Del Rio et al. 2019). The food intoxication related to histamine consumption is due to higher activity of decarboxylase enzymes (Landete et al. 2008). As described by Taylor and Eitenmiller (1986), a high dose of histamine reduces the capacity of the intestinal tract of human to detoxify histamine that enters bloodstream and causes intoxication (Visciano et al. 2014; Maintz and Novak 2007). However, a low level of biogenic amines and especially histamine is an indicator of a high level of food quality and hygienic processing (Prester 2011). In human metabolism, histamine is a monoamine that has an important role to release stomach acid (Jumarie et al. 2017). It is also a neurotransmitter in the central nervous system (Lieberman 2009; Miyasaka et al. 2016). The official conventional method to measure histamine and other biogenic amines is HPLC. Other traditional techniques such as immunochemical methods (Lange et al. 2002; Lange and Wittmann 2002), gas or liquid chromatography (Tombelli and Mascini 1998) were used too. However, all these techniques are expensive, need trained personnel and the sample needs a pretreatment. Furthermore, these methods are not suitable for industrial food processing that needs rapid methods of analysis, with the possibility of real-time monitoring and with no pretreatment. Since 1992, studies have focused mainly on the enzymatic methods based on biosensors (Chemnitius et al. 1992; Salleres et al. 2016; Keow et al. 2012 and Apetrei and Apetrei 2016) that exhibit advantages that should help industry such as quick analysis, no treatment needed, possibility of miniaturization and development of monitoring devices, low cost and repeatability of measurement. In the last decade, a new generation of histamine biosensors based on amperometric methods (Niwa et al. 2000; Keow et al. 2007; Tsutsumi et al. 2009) and on piezoelectric measurements (Pietrzyk et al. 2009) has been developed. The principle of histamine detection was based on the histamine decarboxylation metabolism that produces ammonia and hydrogen peroxide. Then, the decreasing of oxygen concentration consumed, or the increasing of hydrogen peroxide produced was measured using oxygen or H2O2 electrode with mediator for the electron transfer (Marcus and Sutin 1985; Marcus 1997). However, the difficulty of this challenge is to choose a suitable host matrix and the technique of immobilization that permits well the immobilized enzymes conformation for high activity of enzymes and accessibility of substrates.
In this paper we describe the construction of a sensitive potentiometric histamine sensor based on a type of non-toxic synthesized anionic clays known as layered double hydroxide (LDH). We aim to study the immobilization of diamine oxidase enzyme (DAO) and the peroxidase (HRP) onto deposited Layered Double Hydroxide as label free micro-Ion Selective Electrodes (µ-ISE) histamine hybrid biomembrane. It is hoped that the performance of the biosensor will be suitable for detecting histamine at concentrations from a few nanomoles to high concentrations of 0.1 mM.
Material and methods
The ISE device
The microelectrodes (µ-ISEs) developed by Zine et al. (2003) were purchased from Centro National de Microelectronicà de Barcelona (CNM), Spain.
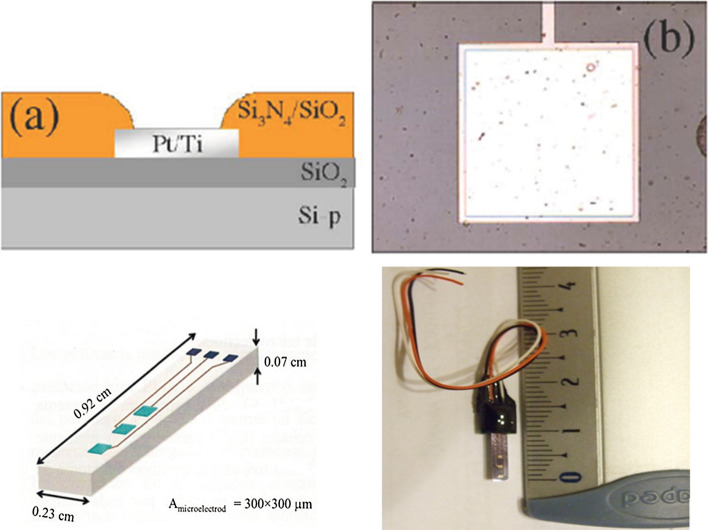
Figure 1 shows the design of microelectrodes. Three platinum µ-ISEs, each measuring 300 nm by 300 nm were fabricated from silicon needle. The area of µ-electrode is 300 × 300 nm. A conducting layer of Polypyrrole (PPy) was deposited over the Pt substrate to make it sensitive to hydrogen ion. Before first use of the electrode, the active microelectrode area was cleaned with ethanol then rinsed thoroughly with milli-Q ultrapure water and dried with nitrogen.
Fig. 1.
The design and the schematic representation of the µ-ISEs fabricated by CNM of Barcelona, Spain (Marques de Oliveira et al. 2006)
Reagents and materials
Diamine oxidase from porcine kidney (DAO, EC: 1.4.3.6), peroxidase from horseradish type II (HRP EC: 1.11.1.7), histamine, putrescine, cadaverine and L-histidine and glutaraldehyde (grade II, 25% aqueous solution) were purchased from Sigma-Aldrich. All chemical reagents were of analytical grade and used as received. DAO and HRP enzymes solutions were prepared in 0.1 M sodium phosphate buffer solution at pH 7.2. All other solutions were prepared using Milli-Q water.
Enzyme immobilization on the gate of µ-ISEs
LDH were synthesized by the co-precipitation method at constant pH and temperature. As described in Baccar et al. (2011), the identification of LDH phases and structures are studied using the XRD measurements. Then, solutions of LDHs were prepared by dissolving 0.2 g/mL in Milli-Q.
DAO and HRP solutions were freshly prepared before immobilization. A mixture of the 2 enzymes solution of (DAO:HRP) was prepared at a DAO: HRP volume ratio of 1:2.
The gate of the transducer was activated by depositing a thin layer of LDH followed by spinning at 1000 rpm for 10 s then continuously at 3000 rpm for 30 s. Next, a 10-μl drop of the mixed enzyme solution was deposited, dried at room temperature for 60 min then reticulated in saturated glutaraldehyde (GA) atmosphere for 10 min (Baccar et al. 2009). Finally, the µ-electrode was stored at 4 °C overnight in phosphate buffer solution (PBS).
Measurements
The µISE instrument used a data acquisition system homemade in the Scientific Park of Barcelona (PCB) by Errachid–taff (Marques de Oliveira et al. 2008). It monitors simultaneously four channels microelectrode and four ion-sensitive field-effect transistors (ISFETs) connected and controlled by a computer. LabView software (National Instruments, Austin, TX, USA) was used for data acquisition and for recording variation of potential after stabilization.
Measurements were performed at room temperature and carried out in 10 mL of phosphate buffer solution. The electrochemical cell was a bi-enzymatic membrane-LDH/µ-ISE/analyte/AgCl reference Electrode. To control the proton diffusion from the inside of the biomembrane to the outside, measurements are carried out versus shifted + 0.75 V of Ag/AgCl double junction electrode under stirring (Prodromidis and Karayannis 2002). Dynamic potentiometric response of the modified µ-ISEs was studied from 2 × 10–8 to 10–3 M of analyte in different concentrations of PBS.
Results and discussions
Effect of buffer concentration
Figure 2 shows of the effect of phosphate buffer concentration on the response of the histamine biosensor. The activity of the enzyme system depends on the ionic strength of the analyte. In fact, for ionic strength higher than 10 mM, a linear response was obtained from 10–8 to 10–4 M of histamine solution. The optimum linear response was obtained in analyte for a concentration of 50 mM phosphate buffer with a variation of 90.29 mV with 0.1 mM histamine added substrate. Then, it decreases to 69.68 mV in 100 mM phosphate buffer. This decreasing for highest ionic strength of analyte is due to the increase in diffusional resistances exerted by the substrate and the products reducing their accessibility to the sensitive membrane (Marconi 1989; Onnerfjord et al. 1995) especially for low molecular weight substrates like biogenic amine and reducing the enzymatic reaction.
Fig. 2.
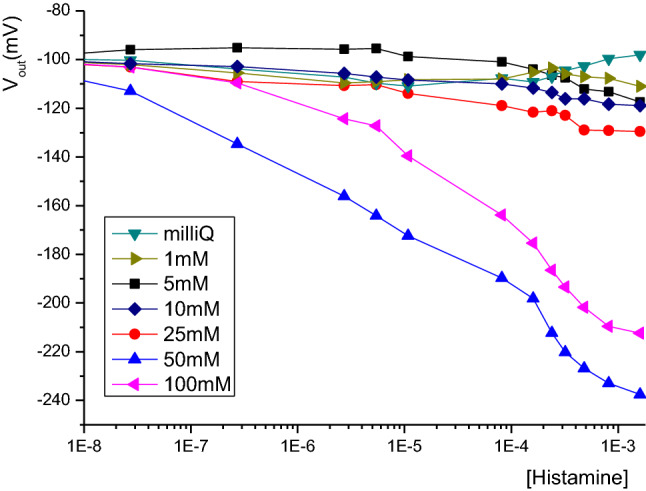
The effect of phosphate buffer concentration on the sensitivity of histamine microelectrodes based on Mg4FeCl
Histamine calibration of electrodes:
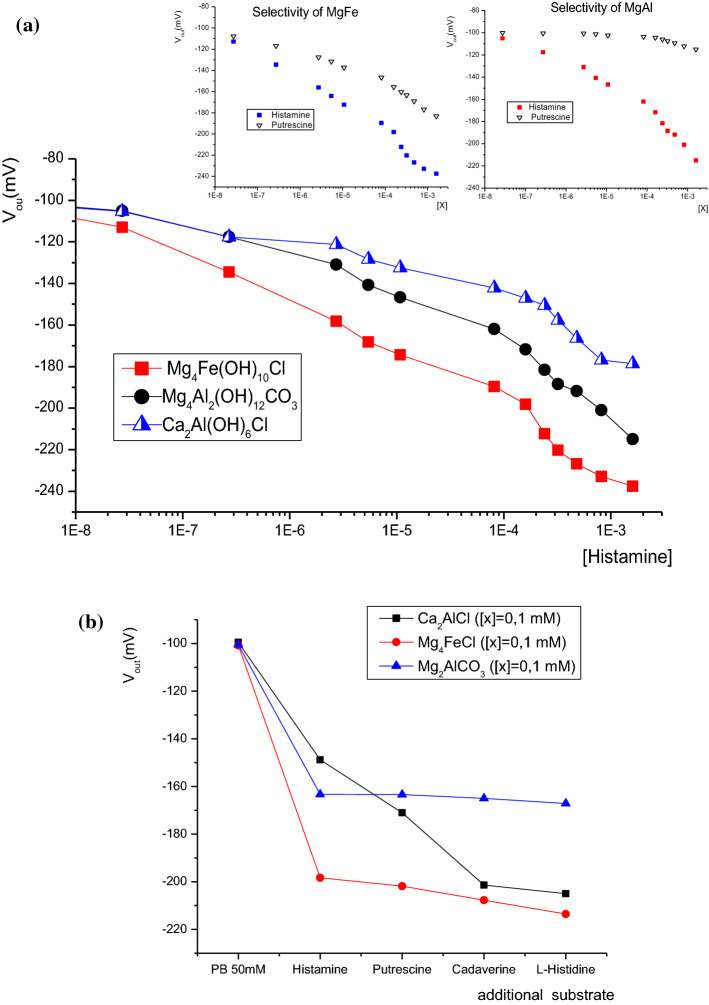
Based on the results of the effect of varying the phosphate buffer concentration, we studied the response of modified µelectrodes to histamine in a buffered solution of 50 mM at room temperature. As shown in Fig. 3, the calibration curves of the different bio-hybrid membranes have two linear responses from 2 × 10–8 to 10–4 M, then from 0.1 to 1 mM with an increasing slope that can be attributed to the ionic strength effect.
Fig. 3.
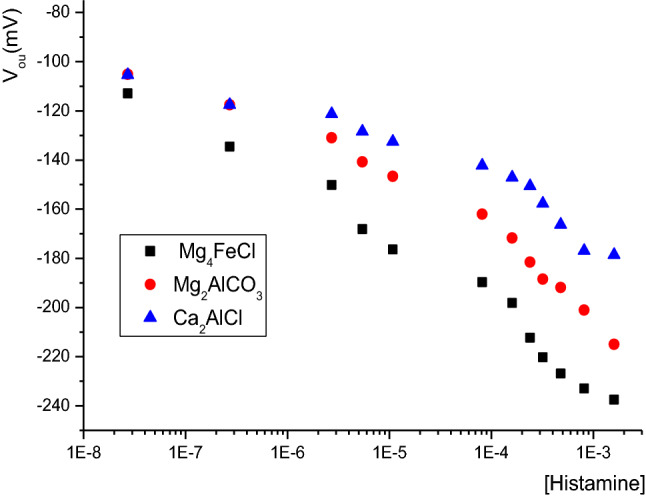
The effect on histamine concentration of the output of microelectrodes based on different LDH host matrices
In the dynamic range of 2 × 10–8 to 10–4 M, the response obtained with immobilized enzymes for Mg4FeCl (Iowaite), Mg2AlCO3 (hydrotalcite) and Ca2AlCl (hydrocalumite) host matrices are were − 22.8 ± 2.0 mV/decade (with a linear correlation of R2 = 0.982 and Y = − 284.83 − 22.84x), − 17.6 ± 2.0 mV/decade (R2 = 0.980 and Y = − 234.24 − 17.58x) and ( − 11.5 ± 2.0)mV/decade (R2 = 0.997 and Y = − 189.76 − 11.5x) respectively. The limit of detection of all these µ-ISEs was less than 10 nM. This value was 10 times less than traditional chromatography methods such as HPLC (Lange et al. 2002) or a similar amperometric biosensor (Keow et al. 2007). Finally, the response times of all biosensors were less than 20 s. This relative long response time reflected the time necessary for diffusion of the substrate, decarboxylation of histamine by the DAO producing ammonia and hydrogen peroxide (metabolism reaction of histamine) and the oxidizing reaction of hydrogen peroxide by peroxidase.
Selectivity study
The selectivity of the different µ-ISEs was studied by measuring the response to similar biogenic amine such as putrescine and cadaverine which are structural analogs of histamine.
For all developed biosensors, the selectivity to cadaverine was tested in the concentration range of 2 × 10–8–10–4 M. As shown in Fig. 4, the membrane based on hydrotalcite host matrix was not sensitive to cadaverine for concentrations lower than 0.1 mM. However, responses of 2 and 4 mV/decade were obtained with biomembranes based on Mg4FeCl and Ca2AlCl respectively. Regardless, the response to histamine and cadaverine interferon is quite similar for biomembrane based on hydrocalumite host matrix. We can conclude that this hybrid membrane cannot be considered for histamine detection. In this case only hydrotalcite and Iowaite can be considered as sensitive and highly selective biomembrane to histamine.
Fig. 4.
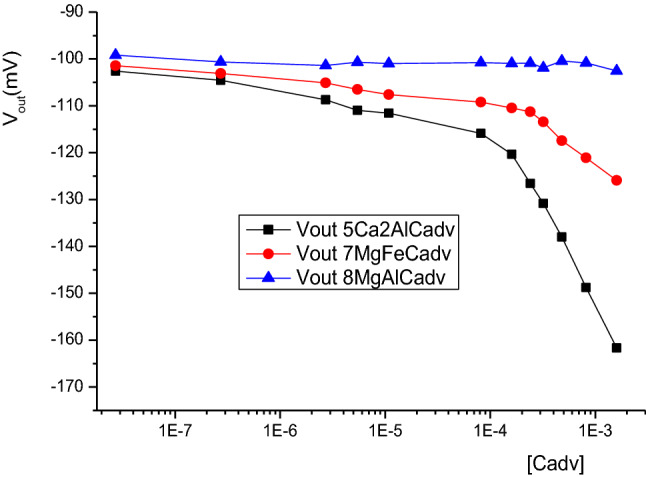
Effect of cadaverine concentration of the output of microelectrodes based on different LDH host matrices
In this order we studied the response of the two most sensitive sensors (Mg4FeCl and Mg2AlCO3) to putrescine. As shown in Fig. 5, biosensor based on Mg4FeCl present a response of 11.3 ± 2.0 mV/decade from 10 nM to 0.1 mM. However, no significant response less than 1 mV/decade to putrescine was obtained with the modified µelectrode by the hybrid hydrotalcite (Mg2AlCO3) membrane. This result suggests that the hydrotalcite hybrid biomembrane is more selective than others studied and should be considered as a sensitive and selective histamine biosensor.
Fig. 5.
a Putrescine response of the selected hybrid membrane: Left plot: Iowaite (Mg4FeCl) LDH, right plot: hydrotalcite (Mg2AlCO3), b Specificity response curve of the different hybrid membrane for addition of 10–4 M of successively histamine, putrescine, cadaverine and L-histidine to an initial blank of 50 mM of phosphate buffer
Specificity measurement
The specificity of the biosensors responses was determined to verify the effect of structural analogue-molecules and their competitiveness with histamine. The specificity of the response was carried out in a 50 mM phosphate buffer solution containing 100 µM of histamine, then to the same concentration of each structural analogue substrate: putrescine, cadaverine and L-histidine was added.
Table 1 show the variation of potential electrode as function of compound of analyte. As presented in Table 1, the Mg4FeCl calibration is only affected in presence of putrescine, a shift of − 7 mV.decade−1 was observed when 100 µM of putrescine was in competition with histamine. No significant potential variation was caused by the presence of L-histidine and cadaverine.
Table 1.
Specific measurements of developed histamine sensitive electrodes
| ISE-CaAlCl-DAO/HRP | ISE-Mg2AlCO3-DAO/HRP | ISE-Mg4FeCl-DAO/HRP | |
|---|---|---|---|
| PB 50 mM (blank) | − 99.48 | − 100.44 | − 99.04 |
| Histamine | − 148.80 | − 163.29 | − 189.23 |
| Putrescine | − 171.05 | − 163.40 | − 196.51 |
| Cadaverine | − 201.43 | − 165.02 | − 202.03 |
| L-Histidine | − 204.99 | − 167.18 | − 208.95 |
In the case of biomembrane based on Mg2AlCO3, the biosensor response is highly specific to histamine substrate. In fact, the addition of 100 µM of derivative substrate does not significantly change the response of sensor and their sensitive characteristics. This result is remarkably interesting and confirms the high sensitivity and selectivity of histamine biosensors based on immobilization of the tow enzymes on hydrotalcite thin film.
Storage effects and stability
The continuous measurement of biological samples requires a stable sensor because it is difficult to calibrate the sensor during continuous measurement. Stability was tested in phosphate buffer solution containing 100 µM of histamine. Sensors did not present any change of the baseline after more than 300 min of continuous measurement.
After daily experiment, µelectrodes are stored at 4 °C in the 20 mM phosphate buffer solution, pH 7.5. The sensors are stable after storing them for more than 10 days.
Conclusion and perspective
In this work we have studied the characteristics of three hybrid membranes based on the chosen LDHs. The goal of this work consists in the development of potentiometric histamine biosensor with more competitive characteristics than the conventional techniques used today (HPLC, GC or ELISA). The main advantages required are rapid response, possibility of real time control or monitoring at least similar performance (high sensitivity and selectivity, large dynamic range with a limit detection less than 10 ppm), in-situ analysis and as possible low coast of testing. This deal was successfully assessed with modified microelectrode using bi-enzymatic (DAO: HRP) hydrotalcite hybrid films. The developed µelectrodes allow cheaper histamine analysis than with used traditional techniques, are reusable for more than 10 monitoring days. Furthermore, performances assessed are better than those obtained with HPLC that are considered as the reference techniques of histamine sensing. The detection limit for this biosensor is less than 10 nM. Their high selectivity and specificity from 10 nM to 0.1 mM can be useful and recommended for monitoring food and alcohol that contain histamine to reduce risk of histamine intolerance or toxicity for human.
Acknowledgements
The authors thank Tunisian and Spanish governments for their financial support of the Projects A/9711/07 and A/20225/08 in Spanish Agency for International Development Cooperation programs.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Apetrei IM, Apetrei C. Amperometric biosensor based on diamine oxidase/platinum nanoparticles/graphene/chitosan modified screen-printed carbon electrode for histamine detection. Sensors. 2016;16:422. doi: 10.3390/s16040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccar ZM, Hidouri S, El Bari N, Jaffrezic-Renault N, Errachid A, Zine N. Stable immobilization of anti-beta casein antibody onto layered double hydroxides materials for biosensor applications. Sens Lett. 2009;7(9):647–655. doi: 10.1166/sl.2009.1129. [DOI] [Google Scholar]
- Baccar ZM, Hidouri S, Errachid A, Ruiz-Sanchez O. Study of bi-enzyme immobilization onto layered double hydroxides nanomaterials for histamine biosensor application. J Nanosci Nanotechnol. 2011;11(10):8798–8803. doi: 10.1166/jnn.2011.3461. [DOI] [PubMed] [Google Scholar]
- Biji KB, Ravishankar CN, Venkateswarlu R, Mohan CO, Srinivasa Gopal TK. Biogenic amines in seafood: a review. J Food Sci Technol. 2016;53(5):2210–2218. doi: 10.1007/s13197-016-2224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitius GC, Suzuki M, Isobe K, Kimura J, Karube I, Schmid RD. Thin-film polyamine biosensor: substrate specificity and application of fish freshness determination. Anal Chim Acta. 1992;263:93–100. doi: 10.1016/0003-2670(92)85430-E. [DOI] [Google Scholar]
- Del Rio B, Redruello B, Linares DM, Ladero V, Ruas-Madiedo P, Fernandez M, Cruz Martin M, Alvarez MA. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci Rep. 2019;9:120. doi: 10.1038/s41598-018-36239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumarie C, Séïde M, Marcocci L, et al. Diamine oxidase from white pea (Lathyrus sativus) combined with catalase protects the human intestinal Caco-2 cell line from histamine damage. Appl Biochem Biotechnol. 2017;182:1171–1181. doi: 10.1007/s12010-016-2390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keow CM, Abu BF, Salleh AB, Yook HL, Wagiran R, Sim BL. An amperometric biosensor for the rapid assessment of histamine level in tiger prawn (Penaeus monodon) spoilage. Food Chem. 2007;105:1636–1641. doi: 10.1016/j.foodchem.2007.04.027. [DOI] [Google Scholar]
- Keow CM, Abu BF, Abu BS, Yook HL, Wagiran R, Siddiquee S. Screen-printed histamine biosensors fabricated from the entrapment of diamine oxidase in a photocured poly(HEMA) film int. J Electrochem Sci. 2012;7:4702–4715. [Google Scholar]
- Landete JM, De las Rivas B, Marcobal A, Muñoz R. Updated Molecular Knowledge about Histamine Biosynthesis by Bacteria. Crit Rev Food Sci Nutr. 2008;48(8):697–714. doi: 10.1080/10408390701639041. [DOI] [PubMed] [Google Scholar]
- Lange J, Wittmann C. Enzyme sensor array for the determination of biogenic amines in food samples. Anal Bioanal Chem. 2002;372:276–283. doi: 10.1007/s00216-001-1130-9. [DOI] [PubMed] [Google Scholar]
- Lange J, Thomas K, Wittmann C. Comparison of a capillary electrophoresis method with high-performance liquid chromatography for the determination of biogenic amines in various food samples. J Chromatogr B Anal Technol Biomed Life Sci. 2002;779(2):229–239. doi: 10.1016/s1570-0232(02)00372-0. [DOI] [PubMed] [Google Scholar]
- Lieberman P. Histamine, antihistamines, and the central nervous system. Allergy Asthma Proc. 2009;30(5):482–486. doi: 10.2500/aap.2009.30.3264. [DOI] [PubMed] [Google Scholar]
- Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85(5):1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- Marconi W. Immobilized enzymes: their catalytic behaviour and their industrial and analytical applications. React Polym. 1989;11:1–19. doi: 10.1016/0923-1137(89)90078-X. [DOI] [Google Scholar]
- Marcus RA. Electron transfer reactions in chemistry. Theory and experiment. Pure Appl Chem. 1997;69(1):13–29. doi: 10.1351/pac199769010013. [DOI] [Google Scholar]
- Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim Biophys Acta. 1985;811:265–322. doi: 10.1016/0304-4173(85)90014-X. [DOI] [Google Scholar]
- Marques de Oliveira IA, Pla-Roca M, Escriche L, Casab J, Zine N, Bausells J, Teixidor F, Crespo E, Errachid A, Samitier J. Novel all-solid-state copper(II) microelectrode basedon a dithiomacrocycle as a neutral carrier. Electrochim Acta. 2006;51:5070–5074. doi: 10.1016/j.electacta.2006.03.042. [DOI] [Google Scholar]
- Marques de Oliveira IA, Risco D, Vocanson F, Crespo E, Teixidor F, Zine N, Bausells J, Samitier J, Errachid A. Sodium ion sensitive microelectrode based on a p-tert-butylcalix[4] arene ethyl ester. Sens Actuators B-Chem. 2008;130:295–299. doi: 10.1016/j.snb.2007.08.026.2008. [DOI] [Google Scholar]
- Miyasaka T, Okuyama-Dobashi K, Masuda C, Iwami S, Sato M, Mizoguchi H, Kawano T, Ohkawara Y, Sakurada S, Takayanagi M, Ohno I. The involvement of central nervous system histamine receptors in psychological stress-induced exacerbation of allergic airway inflammation in mice. Allergol Int. 2016;65:S38–S44. doi: 10.1016/j.alit.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Niwa O, Kurita R, Hayashi K, Horiuchi T, Torimitsu K, Maeyama K, Tanizawa K. Continuous measurement of histamine from rat basophilic leukemia cells (RBL-2H3) with an on-line sensor using histamine oxidase. Sens Actuators B. 2000;67:43–51. doi: 10.1016/S0925-4005(00)00339-7. [DOI] [Google Scholar]
- Onnerfjord P, Emneus J, Marko-Varga G, Gorton L, Ortega F, Dominguez E. Tyrosinase graphite-epoxy based composite electrodes for detection of phenols. Biosens Bioelectron. 1995;10:607–619. doi: 10.1016/0956-5663(95)96937-T. [DOI] [Google Scholar]
- Pietrzyk A, Suriyanarayanan S, Kutner W, Chitta R, D’Souza F. Selective histamine piezoelectric chemosensor using a recognition film of the molecularly imprinted polymer of bis(bithiophene) derivatives. Anal Chem. 2009;81(7):2633–2643. doi: 10.1021/ac8025652. [DOI] [PubMed] [Google Scholar]
- Prester L. Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam Part A. 2011;28(11):1547–2156. doi: 10.1080/19440049.2011.600728. [DOI] [PubMed] [Google Scholar]
- Prodromidis MI, Karayannis MI. Enzyme based amperometric biosensors for food analysis. Electroanalysis. 2002;14(4):241–261. doi: 10.1002/1521-4109. [DOI] [Google Scholar]
- Razavi Rohani SM, Aliakbarlu J, Ehsani A, Hassanzadazar H. Biogenic amines determination in some traditional cheeses in West Azerbaijan province of Iran. Vet Res Forum. 2013;4(2):115–118. [PMC free article] [PubMed] [Google Scholar]
- Salleres S, González I, Arantzamendi A, González R, Maza S, Jaureguibeitia A, Hungerford JM, DeWitt CA, Benner RA. Validation of the biofish-300 HIS enzymatic biosensor for the detection of histamine in fishery products. J AOAC Int. 2016;99(5):1338–1355. doi: 10.5740/jaoacint.16-0180. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Eitenmiller RR. Histamine food poisoning: toxicology and clinical aspects. Crit Rev Toxicol. 1986;17(2):91–128. doi: 10.3109/10408448609023767. [DOI] [PubMed] [Google Scholar]
- Tombelli S, Mascini M. Electrochemical biosensors for biogenic amines: a comparison between different approaches. Anal Chim Acta. 1998;358:277–284. doi: 10.1016/S0003-2670(97)00606-5. [DOI] [Google Scholar]
- Tortorella V, Masciari P, Pezzi M, Mola A, Tiburzi SP, Zinzi MC, Scozzafava A, Verre M. Histamine poisoning from ingestion of fish or scombroid syndrome. Case Rep Emerg Med. 2014;2014:482531. doi: 10.1155/2014/482531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Tsujimura S, Shirai O, Kano K. Direct electrochemistry of histamine dehydrogenase from Nocardioides simplex. J Electroanal Chem. 2009;625:144–148. doi: 10.1016/j.jelechem.2008.10.021. [DOI] [Google Scholar]
- Visciano P, Schirone M, Tofalo R, Suzzi G. Histamine poisoning and control measures in fish and fishery products. Front Microbiol. 2014;5:500. doi: 10.3389/fmicb.2014.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine N, Bausells J, Ivorra A, Aguilo J, Zabala M, Teixidor F, Masalles C, Viñas C, Errachid A. Hydrogen-selective microelectrodes based on silicon needles. Sens Actuators B Chem. 2003;91:76–82. doi: 10.1016/S0925-4005(03)00069-8. [DOI] [Google Scholar]