Abstract
The Bacillus subtilis natto fermented soy protein isolate (FSPI) exhibited concentration-dependent scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS+·) and hydroxyl (·OH) free-radicals. In addition, FSPI administration significantly increased superoxide dismutase activity in mice liver and serum by 20.2% and 86.2%, and suppressed the production of malondialdehyde by 51.3% and 35.1%, respectively, compared to high-fat control (HFC) group. Notably, the movement of mice treated with FSPI was livelier and more active, and its weight gain was significantly lower than that of both NC and HFC groups. The production and accumulation of perirenal fat was also significantly inhibited by FSPI, however, no significant difference in TG and TC levels were observed between FSPI and HFC groups. The results revealed the great potential of FSPI applying in the development of health food or sports food.
Keywords: Soy protein isolate, Natto, Fermentation, Weight loss, Movement
Introduction
B. subtilis var. natto was usually used to ferment soybean as a traditional Japanese food, natto. A similar soybean food fermented by B. subtilis was also consumed in many countries, such as shuidouchi of China. Exception of ordinary consumption, the health-promoting properties was specially focused on such foods. For example, it is rich in vitamin K2, which is associated with bone health by increasing bone mass and slowing bone loss (Weng et al. 2019). Another active substance is nattokinase, a thrombolytic enzyme, which has been shown to help prevent blood clots, promote blood flow and improve heart health (Wu et al. 2019). On the other hand, oxidative stress is essentially an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects (Abbasi et al. 2018). In most case, unreasonable dietary structure or excessive caloric intake acutely causes oxidative stress (Boden et al. 2015). Foods rich in healthy diets characterized by a high intake of fruit, vegetables, and whole grains as well as a low intake of saturated fat are aggregate sources of bioactive compounds. These compounds work as components in antioxidant systems, such as superoxide dismutases or glutathione peroxidase, and may break free radical chain reactions (Fang et al. 2002; Zia-Ul-Haq et al. 2013; Senica et al. 2019; Gecer et al. 2020). Therefore, it is important to find food ingredients for improving dietary-induced oxidative stress. Researches have showed that extract of soybean fermentation product fermented by Aspergillus oryzae possessed higher antioxidant activities, and suggested that the antioxidant action was concerned to the soybean-peptides (Wang et al. 2018). In this experiment, an antioxidant property of B. subtilis natto fermented soy protein isolate was investigated, in order to create a health food for the protection of oxidative damage.
Materials and methods
Materials
Soy protein isolate (Shanwei, China) dissolved in water (final concentration, 1%) was fermented by B. subtilis natto with shaking at 37 °C for 3 days. The fermentation broth was recovered by centrifugation and the supernatant was freeze-dried for use. All other chemical reagents used were of analytical grade.
In vitro radical scavenging assay
The in vitro free-radical scavenging activity of FSPI was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS+·) and hydroxyl (·OH) free-radical assays.
The DPPH radical scavenging activity was measured according to a method described previously (Li et al. 2014a, b). In brief, 0.5 ml of FSPI (0.1–10 mg ml−1) was mixed with 2.0 ml DPPH (0.2 mmol l−1). After incubation at 25 °C in dark for 30 min, the absorbance was recorded at 517 nm. The 0.2 mmol l−1 DPPH solution without FSPI was used as a control and 60% ethanol was used as blank. The inhibition radio of DPPH free radical was used as the index of radical scavenging activity and was counted as follow:
ABTS+· radical scavenging activity was determined as described in previous report with a slight modification (Floegel et al. 2011). The ABTS radical solution was prepared by reacting 7.4 mmol l−1 ABTS stock solution with 2.6 mmol l−1 potassium persulfate in equal quantities. The mixture was allowed to stand in the dark at room temperature for 12 h before use. FSPI (0.2 ml) was mixed with the color reaction mixture of ABTS (0.8 ml) and reacted for 6 min at room temperature. The absorbance was immediately measured at 734 nm using the reaction solution without FSPI as a control. Inhibition radio was calculated by comparing the results of control and tested samples (same equation as for DPPH). Radical scavenging activity was defined as the inhibition percentage of ABTS+· radical.
The hydroxyl radical (OH·) scavenging activity was determined by the method of Halliwell et al. (1987) with some modifications (Li et al. 2014a, b). The hydroxyl radical was produced by the Fe3+/ascorbate/EDTA/H2O2 system. The reaction mixture contained 2.8 mol l−1 deoxyribose, 100 μmol l−1 FeCl3, 100 μmol l−1 EDTA, 1.0 mol l−1 H2O2, 100 μmol l−1ascorbic acid in phosphate buffer (20 mmol l−1, pH 7.4) and different concentrations of FSPI in a final 1.0 ml volume. After 1 h incubation at 37 °C, 1 ml of thiobarbituric acid (1% w/v) and 1 ml of trichloroacetic acetic acid (2.8% w/v) were added to the reaction mixture and then further heating the reaction solution to 100 °C for 15 min to develop the pink chromagen. After cooling, the absorbance was measured at 532 nm using the reaction mixture with no test sample as control. Radical scavenging activity was defined as the inhibition percentage of the hydroxyl (·OH) radical. The inhibition percentage was calculated by comparing the results of control and test samples (same equation as for DPPH).
Animals and diets
Pathogen-free male Kunming mice, aged 6 weeks and weighing 20–25 g, were obtained from HFK Bioscience (Beijing, China). The animals were fed a commercial standard diet (HFK Bioscience, China; Table 1) for 1 week after arrival. After allowing a week for acclimatization, the mice were randomly divided into 3 groups (n = 10, each), which were the normal control (NC) group was fed a standard diet, the high-fat control (HFC) group was fed a high-fat diet (HFK Bioscience, China; Table 1), and the FSPI group fed a high-fat diet supplemented with FSPI at the dose of 20 mg kg−1 body weight per day, respectively. All groups were treated by oral infusion with the same volume of water daily. Animals were housed in separate cages in natural light at 20–23 °C and on a 12 h light–dark cycle, with free access to their food and water. Food intake was recorded once a day and body weights were measured 3 times a week during the experiment. After 4 weeks of treatments, mice were fasted for 12 h, and then anesthetized. Blood samples were collected and centrifuged at 1500 g for 15 min to obtain serum. Livers were immediately excised, weighed and stored at − 80 °C for further use. Perirenal fat pads were excised and weighed. Experiments were approved by the Animal Care and Use Committee of Shenyang Pharmaceutical University (SYXK-L-2010-0009).
Table 1.
Compositions of the experimental diets
| Ingredient | Standard diet (Kcal %) | High-fat diet (Kcal %) |
|---|---|---|
| Protein | 20 | 20 |
| Carbohydrate | 70 | 35 |
| Fat | 10 | 45 |
| Total | 100 | 100 |
| g/kcal | 3.85 | 4.73 |
Biochemical analyses
Triglyceride (TG) and total cholesterol (TC) levels were determined with an enzymatic colorimetric kit (Biosino, China) in accordance with manufacturer’s instructions. The SOD activity and MDA level were evaluated by spectrophotometry using enzymatic kits according to the manufacturer’s instructions (Nanjing Jiancheng, China). The Protein content was measured using a protein quantification kit (Dingguo Bio, China). Bovine serum albumin was used as the standard (Smith et al. 1985).
Statistical analyses
All data are shown as the mean ± SD. Significant differences among the groups were determined by SPSS software (version 19.0 SPSS, Chicago, IL, USA). Statistical significance was accepted at p < 0.05 or p < 0.01.
Results
Antioxidant activity of FSPI in vitro
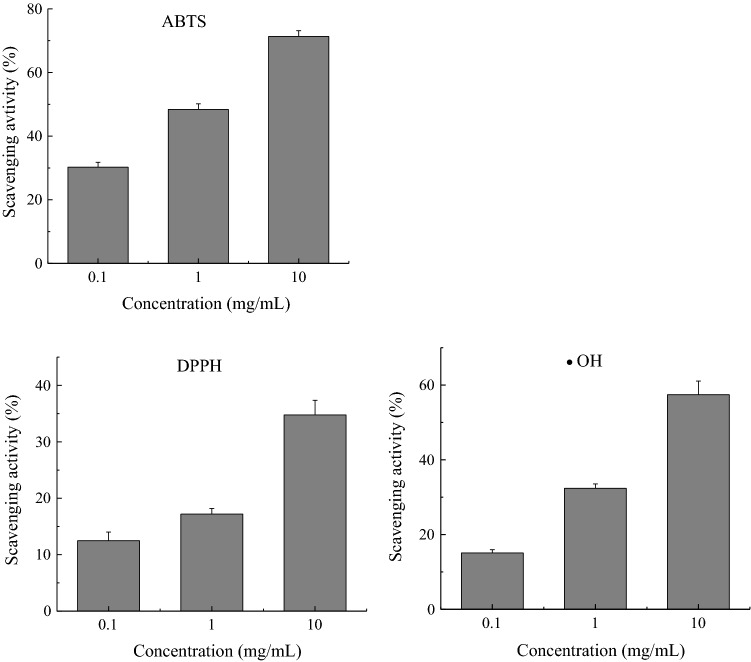
As shown in Fig. 1, FSPI exhibited concentration-dependent free-radical scavenging activity against the ABTS+·, hydroxyl radicals (·OH), and DPPH radicals (DPPH·). The scavenging response of FSPI was more sensitive for ABTS+· than for DPPH· and ·OH radicals. In contrast to the scavenging behaviors against ABTS+· and ·OH, the scavenging activity against DPPH· in higher concentration (10 mg ml−1) was much higher than that in lower concentrations (0.1–1.0 mg ml−1).
Fig. 1.
In vitro free radical scavenging activities of FSPI
In vivo antioxidant activities of FSPI
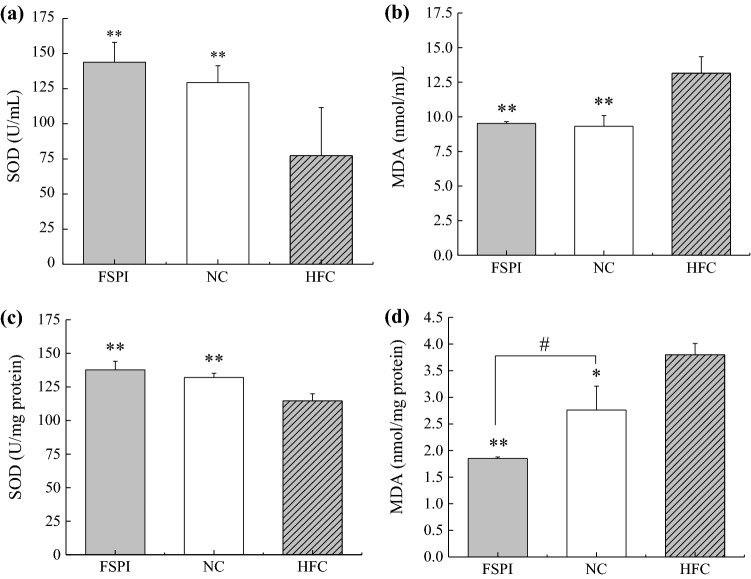
In accordance with the antioxidant action of FSPI in vitro, Fig. 2 represented antioxidant behaviors of FSPI in high-dietary-fat treated mice. A high-fat diet (HFC) significantly (p < 0.05) decreased the activity of antioxidant enzyme SOD, and increased the MDA level, an oxidative index, in both liver and serum of mice, compared to the standard-diet group (NC). FSPI administration significantly increased the hepatic and serum superoxide dismutase (SOD) activity by 20.2% and 86.2%, respectively, compared to HFC. While, the production of malondialdehyde (MDA) in mice liver and serum were inhibited significantly (p < 0.01) by FSPI administration with the suppressive percentage of 51.3% and 35.1%, respectively, compared to HFC. Interestingly, whether in liver or serum, SOD activity with treatment of FSPI was also somewhat higher than that of NC, and the hepatic MDA level of FSPI was even significantly (p < 0.05) lower than that of NC (Fig. 2d).
Fig. 2.
Effects of FSPI on SOD activities and MDA production in serum (a, b) and liver (c, d) of mice. * p < 0.05, ** p < 0.01, compared with the HFC. # p < 0.05, the difference between FSPI and NC
Effect of FSPI on body weight, tissue fat, serum TG and TC of mice
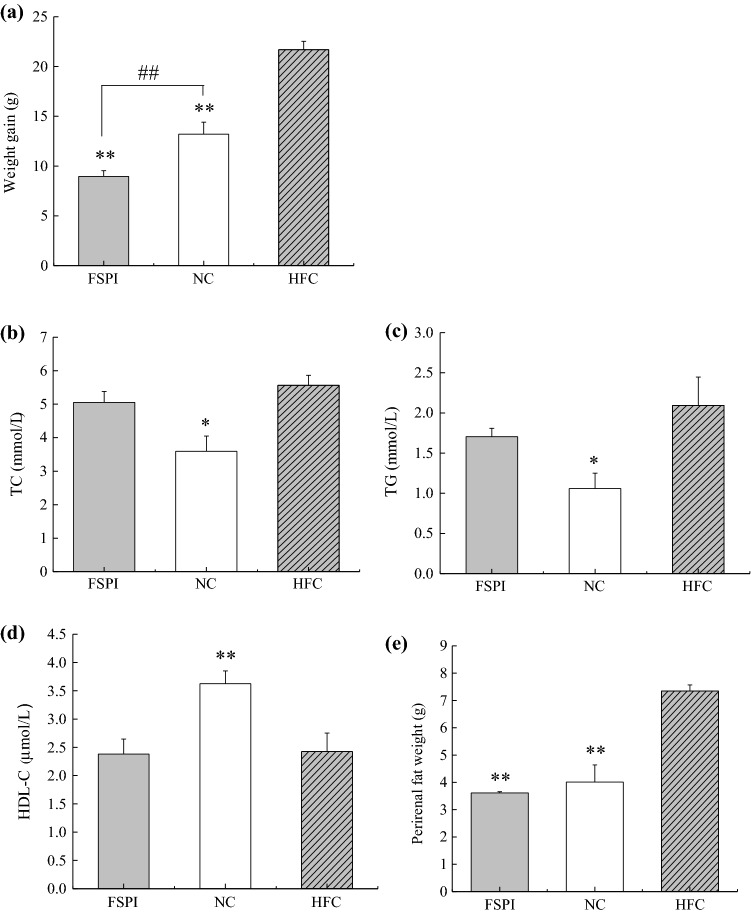
In all experiment periods, there were no significant difference in average food intake (6.4 ± 0.7–7.1 ± 0.8 g day−1) among NC, HFC, and FSPI treated groups. However, the difference in movement behaviors of mice were observed among three groups in all experiment periods, that the mice in FSPI group looks more livelier and more active than that of NC and HFC groups. After 4 weeks experiment, increase of body mass in the HFC group was significantly (p < 0.05) higher than that in the NC group. FSPI remarkably suppressed the increase in body mass compared to the HFC, and the body weight gain of FSPI was even significantly (p < 0.01) lower than that of NC (Fig. 3a). Serum TG and TC levels in NC were lower than those of HFC (Fig. 3b, c). However, no significant difference in TG, TC and HLD-C levels were found between FSPI and HFC groups (Fig. 3b–d). The accumulation of perirenal fat (Fig. 3e) was significantly (p < 0.05) suppressed by administration of FSPI, and its level was also somewhat lower than that of NC.
Fig. 3.
Effects of FSPI on the weight gain (a), serum lipid metabolism index (b–d) and perirenal fat accumulation (e) of mice. * p < 0.05, ** p < 0.01, compared with the HFC. ## p < 0.01, the difference between FSPI and NC
Discussion
Researches have been confirmed that high energy dietary intake, particularly a high intake of dietary fats linked to obesity (Stygar et al. 2019), and excessive accumulation of fat leads to enhance the production of reactive oxygen species in systemic tissues (Togo et al. 2018), resulting in attendant risks for human health and wellbeing. A link between high-fat diet (HFD) and oxidative stress has been recognized in many literatures (Li et al. 2010; Chung et al. 2018), and have suggested that feeding of HFD acts as an inducer of oxidative stress, since it significantly attenuates the hepatic enzyme antioxidant system, and increases the levels of lipid peroxidation products in the liver and plasma (Li et al. 2014a, b; Yang et al. 2019). The increased oxidative stress was a major pre-pathogenesis of metabolic derangements leading to obesity, non-alcoholic fatty liver, inflammation, and diabetes etc. Many food components have been reported to possess antioxidant activities. For instance, polyphenols extracted from black highland barley represented strong antioxidant activity in vitro and in vivo, and the antioxidant defense system and antioxidant gene expression were significantly improved in HFD-mice (Shen et al. 2016). As for natto, it is well known as a health food, and has been reported that its water extract exhibited an inhibitory effect on the oxidation of low-density lipoproteins (LDL) in vitro (Iwai et al. 2002a) and in vivo (Iwai et al. 2002b), but no significant effects on the activity of SOD in the liver and serum of cholesterol-fed rats was observed (Iwai et al. 2002b). Similarly, wholegrain soybean fermented by Aspergillus oryzae exhibited higher antioxidant enzyme activities in rat, however, no effect of which on the body weight was observed (Wang et al. 2018). Soy protein isolate, usually used as food emulsifier or gelling agent. In the present experiment, soy protein isolate fermented by Bacillus subtilis natto exhibited strong antioxidant activity in vitro, and significantly increased SOD activity and decreased MDA level in liver and serum of HFD-fed mice. Referring to previous researches (Kanazawa et al. 1993), the possibility of antioxidative action of FSPI might relate to its internal components involving soluble proteins and peptides formed in the fermentation process. However, the underlying mechanisms need further investigations. Other hand, it is rather remarkable that FSPI observably inhibited the body weight gain and improved the motion activity of mice in all experiment periods, compared to both HFC and NC groups. These findings might also be associated with both new bioactive components including peptides formed in the fermentation process, and more energy consumption caused by higher movement. Although the detailed justification could not be provided presently, the further investigation is valuable for the deep utilization of soybean and soy protein isolate.
Conclusion
In conclusion, soy protein isolate fermented by B. natto had significant antioxidant activities in vitro and in HF-model mice. Great weight-loss and anti-fatigue functions of FSPI were also observed in HF-mice. The results provide the evidence to support the statement that FSPI could be a good food ingredient for the protection of HFD induced oxidative damage. The observation found presently also indicated that FSPI was potential for the development of functional foods, to suppress the body weight gain and to improve the sports ability.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tuoping Li, Email: ltp0401@126.com.
Suhong Li, Email: leesuhong@126.com.
References
- Abbasi S, Gharaghani S, Benvidi A, Latif A. Identifying the novel natural antioxidants by coupling different feature selection methods with nonlinear regressions and gas chromatography–mass spectroscopy. Microchem J. 2018;139:372–379. doi: 10.1016/j.microc.2018.03.012. [DOI] [Google Scholar]
- Boden G, Homko C, Barrero CA, Stein TP, Chen X, Cheung P, Fecchio C, Koller S, Merali S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med. 2015;304(7):1–9. doi: 10.1021/jf011718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung APYS, Sunil G, Srikumar C, Mohanambal M, Palanisamy UD. Geraniin protects high-fat diet-induced oxidative stress in sprague dawley rats. Front Nutr. 2018;5:17–30. doi: 10.3389/fnut.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Floegel A, Kim D, Chung S, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal. 2011;24(7):1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- Gecer MK, Kan T, Gundogdu M, Ercisli S, Ilhan G, Sagbas HI. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet Resour Crop Evol. 2020;67:935–945. doi: 10.1007/s10722-020-00893-9. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: a simple ‘‘test tube’’ assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Iwai K, Nakaya N, Kawasaki Y, Matsue H. Antioxidative functions of Natto, a kind of fermented soybeans: effect on LDL oxidation and lipid metabolism in cholesterol-fed rats. J Agric Food Chem. 2002;50:3597–3601. doi: 10.1021/jf0117199. [DOI] [PubMed] [Google Scholar]
- Iwai K, Nakaya N, Kawasaki Y, Matsue H. The inhibitory effect of natto, a kind of fermented soybeans, on LDL oxidation in vitro. J Agric Food Chem. 2002;50:3592–3596. doi: 10.1021/jf011718g. [DOI] [PubMed] [Google Scholar]
- Kanazawa T, Tanaka M, Uemura T, Osanai T, Onodera K, Okubo K, Metoki H, Oike Y. Anti-atherogenicity of soybean protein. Ann N Y Acad Sci. 1993;676:202–214. doi: 10.1111/j.1749-6632.1993.tb38735.x. [DOI] [PubMed] [Google Scholar]
- Li TP, Li SH, Du LJ, Wang N, Guo M, Zhang JW, Yan FW, Zhang HL. Effects of haw pectic oligosaccharide on lipid metabolism and oxidative stress in experimental hyperlipidemia induced by a high-fat diet in mice. Food Chem. 2010;121:1010–1013. doi: 10.1016/j.foodchem.2010.01.039. [DOI] [Google Scholar]
- Li TP, Li SH, Dong YP, Zhu RG, Liu YH. Atioxidant activity of pentaoligosaccharide isolated from haw pectin suppresses triglyceride synthesis in high-fat fed mice. Food Chem. 2014;145:335–341. doi: 10.1016/j.foodchem.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Li TP, Liu YH, Dong YP, Li SH, Zhu RG. Anti-fat deposition and antioxidant effects of haw pectic oligosaccharide in the liver of mice fed a high fat diet. CYTA J Food. 2014;12:27–31. doi: 10.1080/19476337.2013.783625. [DOI] [Google Scholar]
- Senica M, Stampar F, Mikulic-Petkovsek M. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk J Agric For. 2019;43:576–585. doi: 10.3906/tar-1907-48. [DOI] [Google Scholar]
- Shen YB, Zhang H, Cheng LL, Wang L, Qi XG. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016;194:1003–1012. doi: 10.1016/10.1016/j.foodchem.2015.08.083. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stygar D, Skrzep-Poloczek B, Romuk E, Chełmecka E, Poloczek J, Sawczyn T, Maciarz J, Kukla M, Karcz KW, Jochem J. The influence of high-fat, high-sugar diet and bariatric surgery on hsp70 and hsp90 plasma and liver concentrations in diet-induced obese rats. Cell Stress Chaperones. 2019;24(2):427–439. doi: 10.1007/s12192-019-00976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo M, Konari N, Tsukamoto M, Kimoto R, Yamaguchi T, Takeda H, Kambayashiet I. Effects of a high-fat diet on superoxide anion generation and membrane fluidity in liver mitochondria in rats. J Int Soc Sports Nutr. 2018;15:13. doi: 10.1186/s12970-018-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang H, Xu F, Chen JP, Duan LL, Zhang FJ. Acute toxicity and genotoxicity evaluations of Nattokinase, a promising agent for cardiovascular diseases prevention. Regul Toxicol Pharmacol. 2019;103:205–209. doi: 10.1016/j.yrtph.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang LJ, Zhu FX, Zhu JY, Chen XD, Zou L, Saito M, Li LT. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food) Food Chem. 2018;107:1421–1428. doi: 10.1016/j.foodchem.2007.09.072. [DOI] [Google Scholar]
- Weng SJ, Yan DY, Gu LJ, Chen L, Xie ZJ, Wu ZY, Tang JH, Shen ZJ, Li H, Bai BL, Boodhun V, Yang L. Combined treatment with vitamin K2 and PTH enhanced bone formation in ovariectomized rats and increased differentiation of osteoblast in vitro. Chem Biol Interact. 2019;300:101–110. doi: 10.1016/j.cbi.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Yang YH, Wang YN, Sun J, Zhang JH, Guo HT, Shi YH, Cheng XG, Tang X, Le GW. Dietary methionine restriction reduces hepatic steatosis and oxidative stress in high-fat-fed mice by promoting H2S production. Food Funct. 2019;10:61–77. doi: 10.1039/C8FO01629A. [DOI] [PubMed] [Google Scholar]
- Zia-Ul-Haq M, Ahmad S, Qayum M, Ercisli S. Compositional studies and antioxidant potential of Albizia lebbeck (L.) Benth. Pods and seeds. Turk J Biol. 2013;37:25–32. [Google Scholar]