Abstract
This study was conducted to investigate the microbial, chemical, and sensory quality of chicken drumsticks vacuum-packaged at 4 °C, using chitosan (CH) coating containing ethanol extracts and the essential oils (EO) of Elettaria Cardamomum. The treatments were stored for 16 days in cold conditions and investigated in three-day intervals. Total volatile base nitrogen analysis showed that, on the 6th day, the uncoated treatment showed unacceptable values, while treatments containing the EO and extracts stayed below the specified level even on the 16th day. In addition, during storage, the Peroxide values for the uncoated sample were higher than the documented for the coated groups. Results of the Thiobarbituric acid reactive substances index revealed that the sample containing the EO of E. Cardamomum is the best treatment. Regarding to pH on the 16th day, the microbial growth in the mixed sample was 0.46 units lower than the control group. Microbial analysis showed that coating significantly reduce the growth of all five groups of bacteria at 4 ± 1 °C; thus, on the 6th day, the differences between mesophiles, Enterobacteriaceae, psychrotrophic, and H2S-producing bacteria with the control group were 4.5, 4.5, 2.5, and 2 logs Cfu/g, respectively (p < 0.05). Furthermore, the lactic acid bacteria growth was completely stopped. Finally, it was found that adding EO and extracts could significantly preserve the sensory quality of the samples. Thus, it was concluded that vacuum-packaged CH coatings enriched with the extract and EO of E. Cardamomum can preserve the quality of chicken drumsticks during storage in refrigerators.
Keywords: Chicken drumsticks, Chitosan, Coating, Elettaria cardamomum, EO, Extract
Introduction
Chicken is more widely consumed than any other meat in most countries. It is nutritious, affordable and relative includes low fat in the meat itself in comparison to other animals (Fu and Chen 2019). However, chicken has been classified as highly perishable and is almost a complete environment for microbial (both spoilage and pathogenic microorganisms) growth. Thus, the poultry industry and interested researchers have focused on the development of new methods in order to reduce the rate of microbial growth in fresh chicken. Nowadays, because of the consumers’ increasing concerns regarding the side effects of chemical preservatives and the excessive demands for the use of natural preservatives in foods, the use of natural preservatives has gained popularity (Zhang et al. 2016).
Recently, studies on edible coatings for extending the shelf-life of foods has increased significantly and among the many methods applied, the use of bio-based films and coatings has shown to be a promising area. For increasing the efficiency of edible films applied on foods, the use of natural herbal extracts, particularly EOs that have antimicrobial and antioxidant activity, has been proposed by researchers (Raeisi et al. 2016).
The fruit of Green Cardamom (Elettaria Cardamomum) belongs to the ginger family and is also known as the Queen of Spices. The fruit of cardamom, particularly its seed, oil, and extract, contains a significant amount of phenolic compounds and antioxidant flavonoids such as quercetin, kaempferol, pelargonidin, and luteolin. Because of containing significant amounts of phenolic compounds, the plant can be used as a natural antioxidant and a preservative in foods (Martins et al. 2001).
Chitosan is a non-poisonous polymer that can be decomposed in nature, is compatible with the environment, and can be naturally found in the shell of crabs, shrimps, and the cell walls of mushrooms. In addition to being bio-based, it has other advantages such as its antimicrobial activity against a wide spectrum of microorganisms such as fungi, algae, and some bacteria (Cuero 1999). Chitosan can be applied directly on the surface of foods through methods such as sprinkling or immersing that can result in the creation of solid, transparent, and flexible films. The mixing of EOs and extracts with the inner sides of edible films and coatings such as CH not only enhances their antimicrobial and antioxidant features, but also reduces the permeability of water vapor and postpones the oxidation of fat within the covered product (Vatavali et al. 2013).
Therefore, this study was undertaken to evaluate the effect of CH coating enriched with EO and extract of green cardamom, as well as their combination, on the shelf-life of vacuum-packaged chicken drumsticks in refrigerators.
Materials and methods
Preparation of extracts and EO
The green cardamom (Elettaria Caramomum) was purchased in its green and dried forms from the Traditional market of Urmia, Iran. After being ground, the cardamom was immersed in water and EO extracted by the hydro distillation technique using a Clevenger-type apparatus (Azmiran Co, Iran). It was dewatered and stored in a low temperature (Raeisi et al. 2016).
In order to produce the herbal extract of cardamom, the dried fruit was made into powder by the use of a grinder in ambient temperature in the shade. Then, the powder was put through a 60-mesh sieve. Next, 200 g of the powder was dissolved inside 1 L pure ethanol (70–30 V/V) (Merck Millipore, Burlington, USA) and was placed for 24 h inside a rotary shaker (KS 4000i control; IKA Werke GmbH & Co. KG, Staufen, Germany) with the speed of 150 rpm in 42 °C. Next, the solution was filtered through Watman Filter Paper. The ethanol extract was at first condensed using the Rotary Evaporator (Heidolph, Schwabach, Germany) in 50 °C until the extraction of at least 90 percent of the solvent. Then, it was placed in the oven (D-645, Heraeus, Hanau, Germany) in 37 °C for the purpose of final condensation and in the end, with a vacuum freeze-dryer (Ariyan vacuum, Iran)) in − 50 °C for 24 h, it was dried and stored in 4 °C in a refrigerator until conducting the analysis (Lorenzo et al. 2013).
Preparation of the coatings and the treatment of the samples
For the purpose of preparing the CH-based coating, the CH powder with medium molecular weight (190,000–310,000) (Fluka, Sigma-Aldrich, 70–85% deacetylated) was dissolved in the 1% v/v acetic acid (Merck, Darmstadt, Germany) aqueous solution to reach a final concentration of 2% v/v, while stirring on a magnetic stirrer/hot plate at 40 °C for 6 h. In the next stage, by mixing 0.75 mL Glycerol (Merck, Darmstadt, Germany) per gram of CH as plasticizer, the solution was once again stirred in magnetic stirrer/hot plate for another 30 min. For determining what concentrations have to be added to the coatings, previous studies and experiences were taken into consideration. In addition, a preliminary experiment was conducted using various concentrations on the sample and the highest concentration that did not show any undesirable organoleptic effects on the smell and color of the chicken drumsticks was selected. The samples were taken from the drumstick of different chicken and then sampled randomly for proximate analysis after that whole drumstick coated separately for each sensorial, chemical and microbiological and for every test day analyses. The samples were immersed in the prepared solutions for 1 min and after a minute of dripping, they were dried and vacuum-packaged in low-density polyethylene bags (0.1 mm in thickness having an oxygen permeability of 80 cm3/day. bar. m2 at 52% RH/25 °C). In the end, the samples were stored in a refrigerator at 4 °C. On days 0, 3, 6, 9, 12, and 16 of the study, the chemical, microbial, and sensory tests were conducted. Treatments in the current study included (1) Uncoated vacuum-packaged chicken drumstick (control), (2) Coated vacuum-packaged chicken drumsticks, (CH), (3) Coated, containing 2% extract and vacuum-packaged chicken drumsticks, (CE), (4) Coated, containing 1% EO and vacuum-packaged chicken drumsticks, (CEO) and 5. Coated, containing a combination of 1% extract and 0.5% EO and vacuum-packaged chicken drumsticks (CM).
Chemical analyses
Proximate analysis
The samples was minced and homogenized. In order to determine ashes, an electrical stove (BS OV-160; Gallenkamb, London, UK) at 500–550 °C was used, while to determine moisture an oven (Heraeus, D-645, Germany) was used at 103 °C for 18 h. The assessment of fats was conducted by using the Soxhlet Method using n-hexane (Merck Millipore) as the solvent. (AOAC, 2005). Measuring the crude protein was performed with the Kjeldahl Method); the nitrogen percentage was multiplied by 6.25 and considered as protein percentage (Bakhshi, V40, Tehran, Iran).
Measuring the pH of the meat samples
For the purpose of measuring the pH, 5 g of the chicken samples as well as 10 mL distilled water were homogenized for 30 s within Falcon 50 mL tubes at 13,500 rpm. Then, using a pH-meter (E520, Metrohm, Herisau, Switzerland) standardized at 4 and 7, the pH of the samples was measured (Jonaidi Jafari et al. 2018).
Determining the peroxide value
For this purpose, the standardized IDF method No. 74A:1991 was applied (Egan 1997).
Assessing the total volatile nitrogen (TVN)
This method was conducted through using method proposed by Goulas and Kontominas (2005).
Measuring the thiobarbituric acid reactive substance value (TBARS)
The test of TBA was conducted based on the method proposed by Wrolstad et al. (2005).
The TBARS values were calculated from a calibration curve using known concentrations of MDA (from TEP solution) and expressed as mg MDA/kg sample.
Microbial analyses conducted on the chicken carcass
For this purpose, 10 g of the chicken sample and 90 mL Buffered Peptone Water (Merck Millipore) were put in sterilized zipper bags and were mixed for 2 min at 250 rpm in the Stomacher instrument (Seward, UK). Later, the consequent dilations were prepared in tubes containing Buffered Peptone Water 0.1% and then, were cultivated in plates containing the Plate Count Agar (PCA), Violet Red Bile Glucose Agar, MRS (de Man Rogosa Sharp agar) agar and Iron Agar (Merck, Darmstadt, Germany) for aerobic mesophilic, psychrotrophic, Enterobacteriaceae, lactic acid bacteria and H2S-producing bacteria respectively. After inoculation, the plates were incubated aerobically at 37 °C for 48 h for aerobic mesophilic, at 21 °C for 48 h for psychrotrophic bacteria count, at 25 °C for 5 days for H2S-producing bacteria, at 30 °C 48 h for lactic acid bacteria and at 37 °C for 24 h for Enterobacteriaceae.
Sensory analysis
For conducting the sensory analysis on the samples, a 10-member semi trained panel was assigned. For this purpose, the sensory analysis was conducted in two stages; in the first stage, the analysis was conducted every three days during the storage days until the 16th day, while in the second stage, the analysis was only conducted in the first day by cooking the samples. The analysis was focused on taste, smell, color and texture for their acceptability in terms of appearance, texture, juiciness, bitterness, off-odor, and off-flavor based on a 5-point hedonic scale ranging from excellent = 5 (fresh) to 1 = extremely unacceptable and 3 = poor (the lower limit of sensory acceptability). Scores below three rendered the product spoiled and which would mean a rejection of the sample. The final results were calculated as the mean of all sensory analyzes (Argyri et al. 2018).
Statistical analysis
After the measurement of related indices, the statistical analyses were conducted with IBM SPSS 20. The One-Way Analysis of Variance (ANOVA) was implemented in order to analyze the data and Duncan’s test was used in order to determine whether a significant relationship exists between the treatments.
Results and discussion
Proximate analysis
The average contents of the chicken drumsticks that had been selected randomly at the beginning of the study included moisture (63%), protein (18.48%), fat (12.51%), and ash (2.02%). These data were found to be in line with the findings of the previous studies (Bogosavljevic-Boskovic et al. 2010; Ahmed et al. 2015).
Furthermore, it was observed that a number of variables such as race, age, method of production, gender, etc. can influence the nutritional value of the samples. The significant differences in the fat contents of the samples may have arisen because of the different sampling methods applied. The variation in the content of protein can be 19–23.3%, while with regard to fat, moisture, and ash such variation would be 1–17.4%, 60–75.4%, and 0.7–3.6%, respectively.
pH
The pH of the chicken drumsticks at the beginning of the study was found to be 5.8 (Fig. 1) which is in line with the previous studies (Zhang et al. 2016; Duan et al. 2017). After the 16 day storage period at 4 °C, the pH of the samples increased (6.96), which can be attributed to endogenous and microbial enzymes such as lipase or protease, or the use of amino acids by the bacteria (Manju et al. 2007). On the other hand, the pH increase for other types of treatments has been much less significant. On the 3rd and 6th days of storage, significant increases (p < 0.05) were observed in the pH of the CH (6.55–6.65) and the CE extract treatment groups (6.4–6.6). On the 9th day, the highest pH increase was observed in the control (6.78) and the CE treatment groups (6.6), while the lowest increase was observed in CM treatment (6.5). On the 12th day, the treatment containing the CH coating showed a lower rate of pH reduction (6.4) in comparison to other treatment groups. This may be related to the pH of chitosan coating and the sensitivity of the bacteria to the coating used. On the 16th day, the pH of the CM group was reduced (6.5); though the reduction was low in scale, it was significant and may be due to the antioxidant and antibacterial activity of ethanol extract. and the EO of green cardamoms.
Fig. 1.
Comparing the pH changes in chicken drumsticks during storage at 4 different letters (a, b, c, d) indicate significant differences (p < 0.05)
Total volatile nitrogen
Figure 2 presents the TVN values obtained for all the samples stored in a refrigerator for 16 days. As an important index, TVN makes it possible to assess the quality and freshness of chicken samples. In the current study, it was observed that the TVN values in the treatment group’s increase with a much slower pace compared to the control group; thus, the TVN observed on the 12th day in the CH treatment group was equivalent to day 0 for the control group. In addition, it was observed that the TVN values in the control group have significant differences with the treatment groups and from the 6th day onward, the TVN of the control group became unacceptable (p < 0.05).
Fig. 2.
a Changes in the TVN values. b Changes in the PV index within different treatments of chicken drumsticks stored at 4 °C for 16 days
From day zero to the 6th day, no significant difference was observed between the treatment groups; in addition, until the 9th day, no significant difference was observed among the three treatments that contained EO and extracts (p < 0.05). However, on the 9th day, significant differences (p < 0.05) were observed among the three treatment groups (Contains different amounts of EO and extracts and the CH coating alone). Studies such as Fan et al. (2009) show that CH (alone or in combination with other materials) can significantly reduce the TVN values. This can be attributed to the reduction in the number of bacteria and the oxidative capability of bacteria to separate TVN from amine compounds (Fan et al. 2009). Therefore, the use of ethanol extracts and the EO of E. Cardamom had significant effects on the values obtained for TVN in the current study and is in line with the findings obtained in Giatrakou et al. (2010b) regarding the effects of CH coating plus the EO of thyme.
Peroxide index
Lipid peroxidation is one of the most important chemical reactions that occur during storage at cold conditions. Due to the presence of higher percentages of unsaturated fats compared to meat, chicken is more sensitive to oxidations. In general, oxygen and CO2 penetrate in very small amounts through edible coatings. Therefore, the coatings on chicken samples minimize contact with oxygen and consequently, reduce the speed of early oxidation and the formation of hydroperoxides. Hydroperoxides are the products of early oxidation that determine the rate of fat oxidation in the early stages and have significant impacts on the acceptability of products. This oxidation product are discussed in terms of the PV index. Peroxides are unstable and are dissolved into such products as aldehydes, ketones, and alcohols and result in the creation of unfavorable taste and smell (Przysiężna 2005). Changes of the PV index within the various treatment groups of the chicken drumsticks stored at 4 °C are presented in Fig. 2. The values obtained for the oxidative rancidity in the four groups including the control, CH, CE, and CM did not show any significant difference until the 9th day, while on the 9th day, a significant increase was observed in the PV index. This might be attributed to the increased pace of peroxide formation at this stage of storage. The increase was probably due to the faster rate of formation of peroxides during 9 days of storage than degradation of hydroperoxides into secondary oxidation products. After day 9, the decomposition of hydroperoxides into secondary products increased at a higher rate as compared to the formation of new hydroperoxides, and resulting in decreased PV index (Fig. 2b).
Similar patterns were found by other researchers in chicken breast meat (Bazargani-Gilani et al. 2015), salted minced chicken breast meat (Teets and Were 2008), chicken breast meat (Soyer et al. 2010) and in deep-fried chicken nuggets (Hwang et al. 2011).
Furthermore, the highest rate of reduction in the PV index was observed in the CE group (p < 0.05). The study showed that the EO of Elettaria Cardamom are capable of delaying the early oxidation in chicken drumsticks. In addition, it was found that the 1% EO of Elettaria Cardamom enhances the antioxidative features of CH.
Measurement of TBARS value
The oxidation of fat has negative effects on the sensory, functional, and nutritional quality of meats. Malondialdehyde is the second and most important product of fat oxidation and is an indicator of the degree of oxidation and rancidity. The TBARS value is widely used to determine the rate of fat oxidation and the amount of malondialdehyde in meat (Fan et al. 2009).
The results of analyzes performed on chicken drumstick samples with respect to the amount of TBA value during the storage in a refrigerator (i.e., 4 °C) have been presented in Table 1. According to all the results in the table, the amount of TBA value in the treatment groups was reduced significantly compared to the control (p < 0.05).
Table 2.
Microbial counts (log Cfu/g) in chicken drumsticks during storage days (at 4 ± 1 °C)
| Treatments | 0 | 3 | 6 | 9 | 12 | 16 | |
|---|---|---|---|---|---|---|---|
| Storage time (days) | |||||||
| Mesophilic bacteria | Control | 5.06 ± 0.10a | 6.56 ± 0.16a | 7.25 ± 0.08a | 7.42 ± 0.02a | 9.44 ± 0.10a | D** |
| CH | 3.05 ± 0.05b | 3.03 ± 0.08b | 2.60 ± 0.07b | 5.30 ± 0.70b | 5.60 ± 0.05b | 6.04 ± 0.1a | |
| CE | 2.51 ± 0.07c | 3.07 ± 0.14b | 2.47 ± .10b | 5.36 ± 0.09b | 5.68 ± 0.10b | 6.32 ± 0.04b | |
| CEO | 4.03 ± 0.02d | 5.13 ± 0.15c | 5.04 ± 0.03c | 6.34 ± 0.10c | 6.17 ± 0.05c | 6.30 ± 0.12b | |
| CM | 1.90 ± 0.11e | 2.91 ± 0.02b | 2.60 ± 0.05b | 5.41 ± 0.12b | 5.57 ± 0.13b | 6.01 ± 0.13c | |
| H2S producing bacteria | Control | 3.30 ± 0.08a | 4.10 ± 0.06a | 5.16 ± 0.12a | 5.55 ± 0.07a | 6.78 ± 0.06a | 7.91 ± 0.05a |
| CH | 2.24 ± 0.07b | 3.90 ± 0.04b | 4.17 ± 0.04b | 4.02 ± 0.03b | 5.20 ± 0.04b | 5.10 ± 0.11b | |
| CE | 1.52 ± 0.06c | 2.25 ± 0.03c | 2.39 ± 0.06c | 3.79 ± 0.05c | 4.30 ± 0.07c | 4.68 ± 0.04c | |
| CEO | 2.25 ± 0.06b | 3.90 ± 0.08b | 5.0 ± 0.08a | 5.47 ± 0.09a | 6.0 ± 0.11d | 5.90 ± 0.05d | |
| CM | 1.90 ± 0.09d | 2.30 ± 0.10c | 3.32 ± 0.06d | 4.30 ± 0.07a | 4.77 ± 0.06e | 5.14 ± 0.07b | |
| Enterobacteriaceae | Control | 3.11 ± 0.10a | 4.12 ± 0.07a | 6.52 ± 0.02a | 7.18 ± 0.05a | 7.35 ± 0.06a | 7.80 ± 0.05a |
| CH | 0.45 ± 0.05b | 0.69 ± 0.04b | 1.30 ± 0.18b | 3.86 ± 0.05b | 4.77 ± 0.13b | 4.87 ± 0.17b | |
| CE | 0.41 ± 0.03b | 0.69 ± 0.09b | 3.87 ± 0.06c | 3.74 ± 0.08b | 4.60 ± 0.12b | 4.78 ± 0.15b | |
| CEO | 1.21 ± 0.04c | 2.47 ± 0.07d | 3.87 ± 0.06c | 4.83 ± 0.10c | 4.83 ± 0.15b | 4.96 ± 0.18b | |
| CM | 1.05 ± 0.05c | 1.14 ± 0.09e | 2.03 ± 0.06d | 4.09 ± 0.07d | 4.30 ± 0.14c | 4.69 ± 0.13b | |
| Psychotropic bacteria | Control | 4.15 ± 0.08a | 6.69 ± 0.06a | 8.21 ± 0.12a | 8.74 ± 0.07a | 9.06 ± 0.06a | 9.24 ± 0.05a |
| CH | 4.0 ± 0.07a | 6.44 ± 0.04b | 6.17 ± 0.06b | 7.17 ± 0.03b | 7.14 ± 0.10b | 7.04 ± 0.06b | |
| CE | 3.24 ± 0.06b | 4.95 ± 0.11c | 6.01 ± 0.06c | 6.39 ± 0.05c | 6.60 ± 0.08c | 4.14 ± 0.04b | |
| CEO | 4.02 ± 0.16a | 6.38 ± 0.05b | 6.13 ± 0.08b | 6.77 ± 0.08d | 6.84 ± 0.04d | 7.11 ± 0.05b | |
| CM | 3.75 ± 0.09d | 5.70 ± 0.05d | 5.84 ± 0.08d | 6.55 ± 0.09e | 6.69 ± 0.06c | 7.95 ± 0.04c | |
| Lactic Acid bacteria | Control | 3.86 ± 0.05a | 5.16 ± 0.06a | 6.25 ± 0.08a | 6.42 ± 0.02a | 7.04 ± 0.07a | D** |
| CH | 2.55 ± 0.05b | 3.69 ± 0.05b | 2.60 ± 0.07b | 2.80 ± 0.07b | 3.32 ± 0.06b | 3.79 ± 0.10a | |
| CE | – | – | 0.39 ± 0.02c | 1.07 ± 0.08c | 2.47 ± 0.09c | 3.25 ± 0.04b | |
| CEO | 1.63 ± 0.02c | 2.60 ± 0.10c | 2.39 ± 0.03d | 3.13 ± 0.11d | 3.38 ± 0.05d | 3.60 ± 0.08a | |
| CM | – | – | – | 0.30 ± 0.02e | 1.57 ± 0.03e | 2.95 ± 0.03e | |
Treatment: Control, Uncoating; CH, Chitosan Coating; CE, Chitosan Coating with Extract; CEO, Chitosan Coating with EO; CM, Chitosan Coating with Extract and EO. *Different letters indicate significant difference (p < 0.05), **Discarded
The amount of TBA value in the control sample stored for 16 days at 4 °C showed an increasing trend; in a way that the TBA value in day 0 (0.44 ± 0.30 mg MDA/kg) reached a significant amount on the 16th day of the study (1.92 ± 0.10 mg MDA/kg). The amount of TBA value was determinate lower in coated samples as compared to uncoated sample (0 day). Many researchers reported that chitosan coating could effectively prevent lipid oxidation which was completely consistent with results of the present study (Fan et al. 2009; Petrou et al. 2012).
According to the literature, when the TBARS value is above 1 mg/kg malondialdehyde, it is considered as the threshold for the organoleptic perception of fat oxidation. The slow rate of oxidation in the early stages of storage of control sample is due to the fact that although chicken samples contained peroxiding elements such as iron, potassium, etc., the samples in the early stages contain vitamin E and antioxidant systems such as glutathione and antioxidant enzymes that reduced the speed of fat oxidation (Lorenzo et al. 2013).
Among the treatment groups, an increasing–decreasing trend was observed until the 16th day; in a way that on the day 0, the lowest amount of TBA value (22 ± 0.13 mg MDA/kg) belonged to the mixed treatment group, while the highest amount (0.31 ± 0.70 mg MDA/kg) belonged to the CH treatment group. On the 3rd day of the study, a significant difference was observed between the treatment groups, in a way that the CE group showed the lowest amount of malondialdehyde (0.16 ± 0.70 mg MDA/kg), while the other three treatment groups showed a low-scale increasing trend (p < 0.05). On the 6th day of storage, the amount of malondialdehyde in the CE group was the lowest (0.18 ± 0.80 mg MDA/kg) among the treatment groups.
A significant decrease was observed in the TBARS value in 6 and 9 days storage period. This results was similar to that reported in chicken breast meat (Chouliara et al. 2008), in refrigerated and frozen salted raw minced chicken breasts (Teets and Were 2008) and (Bazargani-Gilani et al. 2015) which might be attributed to initial formation of malondialdehyde and its possible decomposition during the later days of storage. On the 9th and 12th day of the study, no significant difference was observed between the treatment groups (p < 0.05). On the 16th day, the CEO showed the lowest amount of TBA value (0.49 ± 0.07 mg MDA/kg). Reduction for TBA during the storage period arises due to the formation of the secondary products of oxidation that do not react with the TBA reagent. In addition, the reduction of TBA can be attributed to the reaction of malondialdehyde with proteins through a Maillard reaction. A third explanation would be that malondialdehyde and other forms of TBARS are metabolized by chemical and microbial agents that are responsible for the degeneration of chicken (Chouliara et al. 2008). Microbial degeneration can influence the oxidation of lipids and the amount of TBARS and this has been documented on the breast muscles of vacuumed turkey stored at 1 °C (Przysiężna 2005). Therefore, the rapid increase in the values of TBARS in the current study can be attributed to the higher contents of unsaturated fatty acids in the chicken samples. Furthermore, higher rates of phospholipids in the stored meat can increase the pace of oxidation during storage periods. In addition, some chemical compounds within meat such as pigments can influence the oxidation of fats during the cold storage periods. Petrou et al. (2012) argued that the mechanism of CH in reducing the oxidation of fat in meats can be related to its role as an agent that eliminates metal ions. Some researchers have pointed out that during storage periods, the samples containing herbal extracts produce lower rates of TBA compared to uncoated samples (Giatrakou et al. 2010b); thus, in the current study, adding the EO of Green Cardamom to the CH coating may have had a synergistic effect. This line of research is similar to the studies conducted on the on the effects of CH coating containing propolis ethanol extract on chicken fillets (Jonaidi Jafari et al. 2018).
Statistical analyses showed that the addition of natural antioxidants used in the current study can reduce the speed of lipid oxidation in the chicken samples. This is in line with the findings in a number of studies (Tang et al. 2001; Bozkurt 2007). As a result, Green Cardamom can be regarded as a natural antioxidant in meat products (Sharafati-Chaleshtori and Sharafati-Chaleshtori 2017).
Microbial tests
Results of the aerobic mesophilic bacteria growth are presented in Table 1. Counting the mesophilic bacteria revealed that in all treatment groups, the microbial growth increases gradually; in addition, the lowest rate of microbial growth was observed in the CM group (6.01 ± 0.13 log CFU/g), while the highest rate was observed in control treatment.. On day 0 of storage, the logarithmic growth in the mixed treatment group (1.0 ± 9.11 log CFU/g) showed a significant difference with other treatment groups (p < 0.05). From the 3rd to the 12th day of the study, the CE group showed the highest rate of logarithmic growth among the treatment groups, while the other groups did not show any significant differences with each other (p < 0.05). On the last day of storage, the CM contained the least number of aerobic mesophilic bacteria among the treatments, which is in line with the findings of Sharafati-Chaleshtori and Sharafati-Chaleshtori (2017). It has been reported that the degeneration of chicken happens when the total number of aerobic mesophile bacteria is higher than 7 log CFU/g (Cox et al. 1998). In the current study, the total number of aerobic mesophile bacteria was below 6 log CFU/g in all treatment groups until the 6th day of storage and this trend continued until the 12th day (except for the CE).
Researchers have identified a large number of Enterobacteriaceae on chicken, beef, pork, and many other kinds of meat. The Enterobacteriaceae typically exist in foods that have been stored in cold conditions (Dave and Ghaly 2011). In the current study, the Enterobacteriaceae, which constituted a significant part of the microflora of chicken samples, reached above 7 log CFU/g and on the 16th day of storage (Table 2).
Table 1.
Changes in thiobarbituric acid reactive substance value (mg MDA/kg meat) observed in drumsticks under various treatments during storage in the refrigerator (4 ± 1 °C)
| Treatment | Storage days | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 16 | |
| Control | 0.44 ± 0.3Aa | 0.71 ± 0.08Ba | 1.07 ± 0.05Ca | 1.24 ± 0.04 Da | 1.60 ± 0.10Ea | 1.92 ± 0.10Fa |
| CH | 0.31 ± 0.07Aa | 0.41 ± 0.02Ab | 0.52 ± 0.09ABb | 0.33 ± 0.09Ab | 0.44 ± 0.04Ab | 0.86 ± 0.06Bb |
| CE | 0.27 ± 0.04Aa | 0.30 ± 0.09Ab | 0.18 ± 0.08Ac | 0.46 ± 0.10ABb | 0.51 ± 0.11ABb | 1.11 ± 0.14Cc |
| CEO | 0.28 ± 0.03Aa | 0.16 ± 0.07Bc | 0.71 ± 0.3Cd | 0.57 ± 0.11Db | 0.41 ± 0.09Db | 0.49 ± 0.07Dd |
| CM | 0.22 ± 0.13Aa | 0.25 ± 0.03Ab | 0.46 ± 0.07Bb | 0.40 ± 0.04Bb | 0.82 ± 0.02Cc | 1.00 ± 0.03Dc |
Treatment: Control, Uncoating; CH, Chitosan Coating; CE, Chitosan Coating with Extract; CEO, Chitosan Coating with EO; CM, Chitosan Coating with Extract and EO
*In each column, small letters, and in each row, capital letters, indicate the significant difference (p < 0.05)
In the current study, a significant difference was observed between the treatment and control groups (p < 0.05). It can be concluded that in the treatment groups (except the coated treatment group containing 2% extract), an increase was observed in number of the Enterobacteriaceae after the 6th day of storage and in the 12th day (p < 0.05). In the last day of storage, no significant difference was observed among the treatment groups, but the CM group showed the lowest rate of bacterial growth (4.69 ± 0.13 log CFU/g; p < 0.05).
Chitosan, as well as extracts and EO, enhance the antibacterial effects and the presence of phenolic compounds in extracts and EO can increase the rate of permeability. Thus, through attacking cell membranes and removing the cytoplasmic contents, they can influence the enzymes that exist in the cell membranes of bacteria (Wu et al. 2014).
Because of having positively charged amine groups, CH reacts with anionic groups on the surface of bacterial cells and consequently, can eliminate bacteria. It has been reported that damage to the cell membrane is among the mechanisms that CH uses for the elimination of the Enterobacteriaceae, particularly E. coli (Fei Liu et al. 2001). The mechanism involved in the antibacterial activity of spices has not been discovered yet, but the destruction of cell membranes by phenols and the chelation of metals by flavonoids have been regarded as mechanisms that prevent the growth of microorganisms (Zhang et al. 2016). Roller et al. (2002) reported the effects of CH combined with sulfites on the Enterobacteriaceae in fresh pork sausages stored at 4 °C. In addition, Blaise Ouattara (2000) observed that the use of CH films containing acetic acid can prevent the growth of the Enterobacteriaceae as the microflora in meat products (e.g., Bologna sausages, beef Pastrami, and cooked Ham). It has been observed that the EO of Thyme alone or in combination with CH can result in the reduction of the Enterobacteriaceae count in chicken products stored at MAP (Giatrakou et al. 2010a).
The psychrotrophic bacteria (log CFU/g) results have been presented in Table 2. In the current study, a significant difference was observed between the treatment groups and the control group (p < 0.05). According to the findings, the CM group and the CEO were found to be the most effective treatments against the growth of psychrotrophic bacteria. In other words, the growth rate of the psychrotrophic bacteria reduced from 9 log CFU/g in the control group to 6 log CFU/g on the 12th day of storage. This might have happened because of the antimicrobial performance of CH and the EO of Green Cardamom. Furthermore, the CE group showed no significant action against the growth of the psychrotrophic bacteria, which is in line with the findings of a study on lamb that treated with CH + 1% and 2% ethanol extracts of Green Cardamom (Sharafati-Chaleshtori and Sharafati-Chaleshtori 2017). In another study, Zhang et al. (2016) found that the extract of Rosemary has a low preventive impact on the growth of Pseudomonas bacteria in raw chicken samples. The gram-negative psychrotrophic bacteria are the main group of organisms that cause aerobic degeneration in fresh chicken stored at refrigerator and the Pseudomonas bacteria are considered as the main components in the microflora responsible for the degeneration of fresh chicken stored at refrigerator conditions (Sallam 2007).
The LAB (lactic acid bacteria), as anaerobic bacteria, form a significant part of the microflora in chicken (Giatrakou et al. 2010a). In addition, these bacteria are considered as a rival to other agents of degeneration under vacuum packaging conditions (Pavelková et al. 2014). In vacuum packaging and MAP, the major bacteria responsible for degeneration are the LAB and to a lesser extent, the Enterobacteriaceae, Pseudomonas, and sometimes Brochothrix Thermosphacta (Corry 2007). The LAB are considered as the most resistant group of gram-positive bacteria against the antimicrobial agents present in EO (Kostaki et al. 2009). The higher resistance of LAB against the EO has been attributed to their ability for the production of ATP and the toleration of the osmotic pressure (Frangos et al. 2010).
According to the LAB count (Table 2), the logarithmic growth of the LAB within the treatment groups, particularly in the CM group (2.95 ± 0.03 log CFU/g), was found to be significantly lower than the control group (p < 0.05). In another study it was found that the CH coating applied in lower thicknesses (0.5–1%) is even capable of triggering microbial growth (Lee et al. 2002).
Results of counting the H2S-producing bacteria in vacuum-packaged chicken drumsticks showed that the all groups, particularly the control group, present a major increasing trend, in a way that at the end of the study (day 16), the control group had the highest count of the H2S-producing bacteria (7.0 ± 91.05 log CFU/g), while the CEO had the lowest count (5.01 ± 0.11 log CFU/g). Thus, it was seen that the treatment consisting of 1% EO can delay the bacterial growth up to nine days. Findings show that the enrichment of CH with 2% extract of Green Cardamom has not enhanced the antimicrobial effects of CH on the H2S-producing bacteria, while the addition of 1% EO of Green Cardamom makes the antimicrobial effects. Due to the higher pH of chicken drumsticks, in comparison to chicken fillets, a different degeneration mechanism applies (Jay et al. 2005). In studies conducted on chicken drumsticks stored for 16 days at 2 °C, Streptococcus putrefaciens was among the bacteria that were separated from the chicken drumsticks. In all samples in which Streptococcus Putrefaciens species were separated, a sulfur-like smell was produced that is attributed to the formation of compounds such as H2S by the microorganism. These bacteria do not play an important role in chicken fillets. Based in the previous studies, the Enterobacteriaceae can grow in vacuum-packaged meat under high pH conditions and by producing a high level of H2S, they can result in the creation of an unpleasant smell in the meat (Jay et al. 2005).
Sensory features during the storage period
In general, the reduction of scores related to the sensory factors of meat products during the storage periods is a thoroughly natural phenomenon. Typically, bad taste is regarded as an index of microbial degeneration in poultry products and usually occurs within storage periods (Petrou et al. 2012).
In the chicken drumsticks, the samples are considered as edible for human beings until the sensory scores get below 4. Changes in the scores of sensory factors (color, smell, and texture) that have been presented in Fig. 3. reveals that the samples investigated in the current study did not have any significant difference with each other until the 3rd day (p < 0.05). However, after the 6th day for the control group and the 16th day for the CEO group, the acceptable score for sensory features (i.e., 3) was given. The CH, CM, and the CE group were acceptable until the 9th day of the study, but became inedible on the 12th day. These three groups did not have any significant difference with each other on the 12th day, while the EO group was edible on the 16th day and received the highest score (p > 0.05). Due to the strong smell of the 1% EO, samples that contained this concentration did not show a high level of sensitivity. Increase in the microbial load during the storage period can be one of the reasons for the occurrence of early degeneration within the samples. In addition, the products of fat oxidation and the production of ammonia due to the decomposition of proteins by microorganisms resulted in the creation of a bad smell and the reduction of scores and the total unacceptability of the samples within the final storage days.
Fig. 3.
Results of the sensory characteristics (color, smell, texture) in various treatments (stored at 4 ± 1 °C). Different letters indicate significant differences (p < 0.05)
Sensory features after treatment and cooking
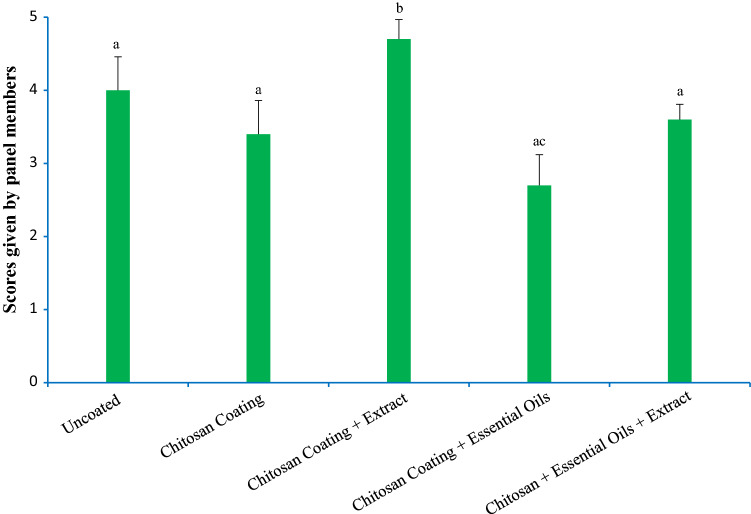
In Fig. 4, changes in the scores assigned for the acceptability of the smell of chicken drumstick samples have been illustrated. In this investigation, no significant difference exists between uncoated chicken samples, samples with CH coating, and the mixed treatment group with regard to the acceptability scores given by the panel members (p < 0.05). In addition, the treatment containing 2% extract has received higher scores of acceptability, while the treatment containing 1% EO has not received favorable scores due to the acute taste that it has given to the chicken sample.
Fig. 4.
Results of sensory characteristics (taste) in various treatments after cooking Different letters indicate significant differences (p < 0.05)
In accordance to the findings of the current study, Sharafati-Chaleshtori and Sharafati-Chaleshtori (2017) reported that the use of CH coating enriched with the 2% ethanol extract of Green Cardamom enhances the general acceptability scores of lamb in a significant way. In addition, it has been reported that CH as an antimicrobial agent with animal origins, has a usual taste and delays the bad taste typical in such products as chicken noodle soup (Karre et al. 2013), chicken breast (Petrou et al. 2012), and chicken kebab (Giatrakou et al. 2010b). In general, the mixed treatment and the treatment containing the EO were superior to other treatment groups both in microbial, chemical, and sensory tests and in preserving the quality of chicken drumsticks.
Conclusion
Results of the analysis conducted on the antioxidant capacity of Green Cardamom showed that in comparison to its EO, the ethanol extract of Green cardamom has a more effective antioxidant performance. Results of the chemical, microbial, and sensory tests conducted in the current study confirmed the superior performance of CH coating enriched with the extracts and EOs in comparison to the control group. In addition, the CH coating enriched with 2% Extract of Green Cardamom was shown to have an antioxidant effect, though it did have a significant antimicrobial effects in the food samples. Nevertheless, the mixed treatment group and the treatment containing 1% EO significantly reduced the oxidation of fats and the microbial growth in comparison to the control group, the CH coating group, and the CH coating enriched with the extract group. In the current study, coating the chicken samples with CH increased the shelf-life by 2–3 days, while adding 1% EO of Green Cardamom increased the shelf-life until the 16th day of the study. Therefore, findings of the current study showed that the use of CH coating enriched with various concentrations of EO and extracts of Green Cardamom in vacuumed packages can prevent the oxidation of fats and the microbial growth in chicken drumsticks stored at 4 ± 1 °C and enhance the products’ sensory features.
Acknowledgements
This research was made benefited from a grant from the Faculty of Veterinary Medicine, Urmia University, Iran.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed ST, Islam MM, Bostami AR, Mun HS, Kim YJ, Yang CJ. Meat composition, fatty acid profile and oxidative stability of meat from broilers supplemented with pomegranate (Punica granatum L.) by-products. Food Chem. 2015;188:481–488. doi: 10.1016/j.foodchem.2015.04.140. [DOI] [PubMed] [Google Scholar]
- Argyri AA, Papadopoulou OS, Nisiotou A, Tassou CC, Chorianopoulos N. Effect of high pressure processing on the survival of Salmonella Enteritidis and shelf-life of chicken fillets. Food Microbiol. 2018;70:55–64. doi: 10.1016/j.fm.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Bazargani-Gilani B, Aliakbarlu J, Tajik H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov Food Sci Emerg Technol. 2015;29:280–287. [Google Scholar]
- Bogosavljevic-Boskovic S, Mitrovic S, Djokovic R, Doskovic V, Djermanovic V. Chemical composition of chicken meat produced in extensive indoor and free range rearing systems. Afr J Biotech. 2010;9:9069–9075. [Google Scholar]
- Bozkurt H. Comparison of the effects of sesame and Thymbra spicata oil during the manufacturing of Turkish dry-fermented sausage. Food Control. 2007;18:149–156. [Google Scholar]
- Chouliara E, Badeka A, Savvaidis I, Kontominas MG. Combined effect of irradiation and modified atmosphere packaging on shelf-life extension of chicken breast meat: microbiological, chemical and sensory changes. Eur Food Res Technol. 2008;226:877–888. [Google Scholar]
- Corry JEL (2007) Spoilage organisms of red meat and poultry. In: Mead GC (ed) Woodhead Publishing Series in Food Science, Technology and Nutrition, Microbiological Analysis of Red Meat, Poultry and Eggs, Woodhead Publishing pp 101–122
- Cox N, Bailey J, Berrang M. Bactericidal treatment of hatching eggs I. Chemical immersion treatments and Salmonella. J Appl Poult Res. 1998;7:347–350. [Google Scholar]
- Cuero RG. Antimicrobial action of exogenous chitosan. Exs. 1999;87:315–333. doi: 10.1007/978-3-0348-8757-1_23. [DOI] [PubMed] [Google Scholar]
- Dave D, Ghaly AE. Meat spoilage mechanisms and preservation techniques: a critical review. Am J Agric Biol Sci. 2011;6:486–510. [Google Scholar]
- Duan D, Wang H, Xue S, Li M, Xu X. Application of disinfectant sprays after chilling to reduce the initial microbial load and extend the shelf-life of chilled chicken carcasses. Food Control. 2017;75:70–77. [Google Scholar]
- Egan H. Pearson's chemical analysis of food. 9. Edinburgh, UK: Churchill Livingstone; 1997. [Google Scholar]
- Fan W, Sun J, Chen Y, Qiu J, Zhang Y, Chi Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009;115:66–70. [Google Scholar]
- Fei Liu X, Lin Guan Y, Zhi Yang D, Li Z, De Yao K. Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci. 2001;79:1324–1335. [Google Scholar]
- Frangos L, Pyrgotou N, Giatrakou V, Ntzimani A, Savvaidis I. Combined effects of salting, oregano oil and vacuum-packaging on the shelf-life of refrigerated trout fillets. Food Microbiol. 2010;27:115–121. doi: 10.1016/j.fm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Fu X, Chen J. A review of hyperspectral imaging for chicken meat safety and quality evaluation: application, hardware, and software. Compr Rev Food Sci Food Saf. 2019;18:535–547. doi: 10.1111/1541-4337.12428. [DOI] [PubMed] [Google Scholar]
- Giatrakou V, Ntzimani A, Savvaidis IN. Combined chitosan-thyme treatments with modified atmosphere packaging on a ready-to-cook poultry product. J Food Prot. 2010;73:663–669. doi: 10.4315/0362-028x-73.4.663. [DOI] [PubMed] [Google Scholar]
- Giatrakou V, Ntzimani A, Savvaidis IN. Effect of chitosan and thyme oil on a ready to cook chicken product. Food Microbiol. 2010;27:132–136. doi: 10.1016/j.fm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food chem. 2005;93:511–520. [Google Scholar]
- Hwang KE, Choi YS, Choi JH, Kim HY, Kim HW, Lee MA, Chung HK, Kim CJ. Effect of ganghwayakssuk (Artemisia princeps Pamp) on oxidative stability of deep fried chicken nuggets. Food Sci Biotechnol. 2011;20:1381. [Google Scholar]
- Jay JM, Loessner M, Golden D. Modern food microbiology. 7. New York: Springer; 2005. [Google Scholar]
- Jonaidi Jafari N, Kargozari M, Ranjbar R, Rostami H, Hamedi H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J Food Process Preserv. 2018;42:e13336. [Google Scholar]
- Karre L, Lopez K, Getty KJ. Natural antioxidants in meat and poultry products. Meat Sci. 2013;94:220–227. doi: 10.1016/j.meatsci.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kostaki M, Giatrakou V, Savvaidis IN, Kontominas MG. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 2009;26:475–482. doi: 10.1016/j.fm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Lee HW, Park YS, Jung JS, Shin WS. Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe. 2002;8:319–324. doi: 10.1016/S1075-9964(03)00030-1. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, González-Rodríguez RM, Sánchez M, Amado IR, Franco D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “chorizo”. Food Res Int. 2013;54:611–620. [Google Scholar]
- Manju S, Jose L, Gopal TS, Ravishankar C, Lalitha K. Effects of sodium acetate dip treatment and vacuum-packaging on chemical, microbiological, textural and sensory changes of Pearlspot (Etroplus suratensis) during chill storage. Food Chem. 2007;102:27–35. [Google Scholar]
- Martins A, Salgueiro L, Goncalves M, Cunha AP, Vila R, Canigueral S, Mazzoni V, Tomi F, Casanova J. Essential oil composition and antimicrobial activity of three Zingiberaceae from S. Tome e Principe Planta Medica. 2001;67:580–584. doi: 10.1055/s-2001-16494. [DOI] [PubMed] [Google Scholar]
- Ouattara B, Simard RE, Piette G, Bégin A, Holley RA. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol. 2000;62:139–148. doi: 10.1016/s0168-1605(00)00407-4. [DOI] [PubMed] [Google Scholar]
- Pavelková A, Kačániová M, Horská E, Rovná K, Hleba L, Petrová J. The effect of vacuum packaging, EDTA, oregano and thyme oils on the microbiological quality of chicken's breast. Anaerobe. 2014;29:128–133. doi: 10.1016/j.anaerobe.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Petrou S, Tsiraki M, Giatrakou V, Savvaidis I. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int J Food Microbiol. 2012;156:264–271. doi: 10.1016/j.ijfoodmicro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Przysiężna E. Effect of chilling storage time on the proteolysis and lipid oxidation in vacuum-packed turkey breast muscles. Pol J Food Nutr Sci. 2005;14:397–402. [Google Scholar]
- Raeisi M, Tabaraei A, Hashemi M, Behnampour N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int J Food Microbiol. 2016;238:139–145. doi: 10.1016/j.ijfoodmicro.2016.08.042. [DOI] [PubMed] [Google Scholar]
- Roller S, Sagoo S, Board R, O’mahony T, Caplice E, Fitzgerald G, Fogden M, Owen M, Fletcher H. Novel combinations of chitosan, carnocin and sulphite for the preservation of chilled pork sausages. Meat Sci. 2002;62:165–177. doi: 10.1016/s0309-1740(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Sallam KI. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control. 2007;18:566–575. doi: 10.1016/j.foodcont.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafati-Chaleshtor F, Sharafati-Chaleshtori R. In vitro antibacterial and antioxidant properties of Elettaria cardamomum Maton extract and its effects, incorporated with chitosan, on storage time of lamb meat. Vet Arhiv. 2017;87:301–315. [Google Scholar]
- Soyer A, Özalp B, Dalmış Ü, Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010;120:1025–1030. [Google Scholar]
- Tang S, Kerry JP, Sheehan D, Buckley DJ, Morrissey PA. Antioxidative effect of added tea catechins on susceptibility of cooked red meat, poultry and fish patties to lipid oxidation. Food Res Int. 2001;34:651–657. [Google Scholar]
- Teets AS, Were LM. Inhibition of lipid oxidation in refrigerated and frozen salted raw minced chicken breasts with electron beam irradiated almond skin powder. Meat Sci. 2008;80:1326–1332. doi: 10.1016/j.meatsci.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Vatavali K, Karakosta L, Nathanailides C, Georgantelis D, Kontominas M. Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food Bioprocess Technol. 2013;6:3510–3521. [Google Scholar]
- Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P (2005) Handbook of food analytical chemistry, vol 1: water, proteins, enzymes, lipids, and carbohydrates, JWS, pp 547–567.
- Wu J, Ge S, Liu H, Wang S, Chen S, Wang J, Li J, Zhang Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag Shelf Life. 2014;2:7–16. [Google Scholar]
- Zhang H, Wu J, Guo X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci Hum Wellness. 2016;5:39–48. [Google Scholar]