Abstract
Physical and chemical properties of Asian sea bass (Lates calcarifer) backbone subjected to high pressure heating (autoclaving, 121ºC) for different times (30 min; AT-30; 60 min; AT-60; 90 min; AT-90) were studied. As heating time augmented, the increases in protein, α-amino group and hydroxyproline contents in liquor were attained, while hardness was declined indicating that more organic compounds were leached out from the bone matrix. More pronounced XRD pattern was observed in backbone autoclaved for 90 min as a consequence of nano-hydroxyapatite crystals agglomeration. Extension of high-pressure heating time reduced the organic matters as elucidated by TGA and DTA data. Asian sea bass backbone heated at 121ºC for 90 min using autoclave had the lowered protein and lipid contents but increased ash content with highest calcium (29.65 ± 0.69%), phosphorus (15.80 ± 0.07%) and iron (15.10 ± 0.61 mg/kg) contents, compared to those with shorter heating time. Therefore, pre-heat treatment could reduce the organic compounds and decrease bone hardness for further process, especially for bio-calcium production.
Keywords: Asian sea bass, Frame, Bone, Autoclave, Chemical composition, Physical property
Introduction
Calcium is very important for human since it is essential for developing and maintaining bone health (Fischer et al. 2018). Calcium is involved in several biological functions, e.g. muscle functions, vascular contraction, nerve transmission, intracellular signaling, hormonal secretion, and vasodilation (Beto 2015). Therefore, calcium deficiency negatively impacts on bone health and human metabolism. For human, calcium can be obtained through food, food fortification or supplements. Fish bone, especially in form of bio-calcium, has been used for food fortification (Benjakul and Karnjanapratum 2018; Idowu et al. 2019a). Fish bone contains calcium phosphate that is important not only for food but also for pharmaceutical uses such as bio-ceramics material which has good bioactivity, osteoconductivity and biocompatibility with grafted tissue (Corrêa and Holanda, 2019). Calcium in fish bone was able to activate tilapia indigenous transglutaminase (Hemung 2013). In industrial field, calcium oxide from fish bone could be used as catalyst on biodiesel production (Sulaiman et al. 2019).
Thailand has become one of the world’s largest fishery exporters, producing around 20 percent of total export of Thai foods. Total value of fishery exports increased from 5.6 billion US dollars in 2016 to 5.8 billion US dollars in 2017. Thai fishery industries manufacture and export a variety of fishery products such as frozen products, semi-processed and value-added products (Ngamprasertkit and Nicely 2018). Those industries generate the leftovers consisting of head, gill, frame meat, skin, scale, bone and viscera. Bones, which constitute 8–10% of whole fish, have been known to comprise protein, chondroitin and minerals (Binsi 2018). Fish bone calcium is beneficial and applicable since it is cheap and easy to obtain from fish industry waste. Moreover, the utilization of bones can reduce environmental problem and increase the value of these by-products.
Fish bone is biologically formed as hard tissues, known as biominerals. Recently, it has gained interest for production of bio-calcium (Benjakul et al. 2017; Idowu et al. 2019b). Production of calcium (bio-calcium, hydroxyapatite, calcium oxide, etc.) from fish bone is governed by several factors including pre-treatment using alkaline (Benjakul et al. 2017), temperature and time for calcination (Piccirillo et al. 2013; Chen et al. 2019), etc. High mineral content causes bones to have high strength and stiffness, which is needed to provide skeletal support. With fragile ceramic particles in the vicinity as well as a flexible collagen matrix, bones therefore have extraordinary toughness (Gower 2008). Because of its chemical compound and strong structure, fish bone is hard to reduce in size via grinding before further production of several products, particularly bio-calcium. Reduction in the size of bone directly increases the surface area and escalates the homogeneity. Bone hardness could be reduced via weakening the matrix by pre-treatment using high pressure heating. Heating should be sufficient to get the softened bone. Nevertheless, characteristics of fish backbone after high pressure heating has never been documented. This study aimed to examine chemical composition and physical properties of Asian sea bass backbone treated with high pressure heating for different times.
Material and methods
Material
Asian sea bass (Lates calcariver) frame was obtained from fish fillet processing factory in Songkhla, Thailand. Frozen samples were carried on ice, which was packaged in insulated box to International Center of Excellence in Seafood Science and Innovation, Prince of Songkla University within 2 h. Upon arrival, the samples were kept in a freezer (−20 °C) and used within 1 month.
Preparation of backbones
Backbones were firstly cut longitudinally into half and further cut into the length of 4–5 cm with a sawing machine and fish bone marrow was removed using running water. The cleaned fish bone was boiled using boiling water (100 °C) at a ratio of 1:5 (w/v) to remove adherent fish meat. Thereafter, cleaned boiled bones (BB) (100 g) were put in the Erlenmeyer flask, added with 4 volumes of distilled water and covered with aluminum foil. The samples were placed in an autoclave and heated at 121 °C with a pressure of 0.11 MPa (1.0856 atm) for different times (30, 60, and 90 min). After high pressure heating, the bones were separated from the liquor in the flask using sieve and the filtrates were collected. The bones autoclaved for 30, 60 and 90 were dried at 45º for 18 h. The dried bone samples were ground using high speed multifunctional grinder (Model 1000A, Qingdao, China). They were named as ‘AT-30′, ‘AT-60′, and ‘AT-90′, respectively. Both obtained bones and filtrates were analyzed.
Analyses
Proximate analysis
Protein content of liquors was analyzed using the Biuret method (Layne 1957). The value was expressed as g/100 g BB. Proximate compositions of backbone samples were analyzed according to AOAC method, using analytical Nos. 950.46, 920.153, 960.39, and 928.08 (AOAC 2000) for protein, fat, ash and moisture contents, respectively.
Hydroxyproline analysis
Hydroxyproline content of liquor was analyzed using the method of Bergman and Loxley (1963). The content was reported as mg/g BB.
Determination of α-amino group content (AAGC)
α-Amino group content was measured as per the procedure of Benjakul and Morrissey (1997). Liquor sample (62.5 µL) was mixed thoroughly with 1.0 mL of 2.125 mM phosphate buffer, pH 8.2, and 1.0 mL of 0.01% 2,4,6-trinitrobenzene sulfonic acid (TNBS) solution. The mixture was incubated in water bath (Model W350, Memmert, Schwabach, Germany) at 50ºC in dark for 30 min. The reaction was ended by incorporation of 1.0 mL of 0.1 M sodium sulfite, followed by cooling for 15 min at room temperature. The absorbance was read at 420 nm using a spectrophotometer UV–Vis (UV-1800, Shimadzu, Kyoto, Japan) and AAGC was expressed in term of l-leucine equivalent/g BB.
Texture analysis
The hardness of BB and autoclaved bones for different times, was determined by texture analyzer (TA.XT Plus Texture Analyzer, Stable Micro Systems, Surrey, United Kingdom). A piece of bone (the length of 1.5 cm and thickness of 1 cm) was placed at the center of texture analyzer plate. Then, a cylinder probe with a 5 cm diameter was used for hardness analysis. The distance of analysis was 5 mm at a speed 2 mm/s. The measurement was replicated for 5 times.
X-ray diffraction analysis
The phase composition of bone samples was analyzed by X-ray diffraction (XRD) as explained by Benjakul et al. (2017) using an X-ray Diffractometer (WI-RES-XRD EMPREYAN-001, Panalytical, Netherlands), with wavelength of 0.154 nm (Cu K-α radiation). The powder samples were swept at 2θ angle ranging from 5° to 90° with the time/step of 0.27 s and the step size of 0.026° at 40 kV and 30 mA. Phase identification was carried out using a peak profile matching to the standard powder diffraction data file from the Joint Committee on Powder Diffraction Standards (JCPDS).
Thermo gravimetric analysis
Thermo gravimetric analysis was performed using Thermo Gravimetric analyzer (TGA7, Perkin Elmer, Waltham, MA, USA). The powder samples were scanned from 50 to 1000 ºC using a heating rate of 10 °C/min under nitrogen atmosphere.
Differential thermal analysis (DTA)
Differential thermal analysis was conducted using Differential Thermal Analyzer (DTA7, Perkin Elmer, Waltham, MA, USA). The powder samples were heated from 50 to 1200 ºC using a heating rate of 10 °C/min in nitrogen atmosphere.
Determination of mineral
Mineral (Ca, P and Fe) contents were determined using an inductively coupled plasma optical emission spectrometer (ICP-OES) (Model Optima 4300 DV, Perkin Elmer, Shelton, CT, USA) as explained by Feist and Mikula (2014). The wavelength of 317.933, 213.617 and 238.204 nm were used for detection of Ca, P and Fe, respectively.
Scanning electron microscopic (SEM) analysis
Scanning electron microscopic analysis was used to analyze the morphology of the backbone. Raw, BB and autoclaved bone with heating time of 90 min (AT-90) were cut into cross-section with the dimension of 0.7 × 0.7 cm. The samples were fixed by soaking in 2.0% glutaldehyde in 0.1 M phosphate buffer pH 7 for 2 h at 5ºC. Subsequently, the fixed samples were dehydrated using a series of 25%, 50%, 75%, 95% and 100% ethanol. Dehydrated samples were critical point dried. The dried specimens were placed on a bronze stub and sputter-coated with gold (Sputter coater SPI-Module, West Chester, PA, USA). The specimens were visualized using a scanning electron microscope (Model JEOL JSM-5800 LV, Tokyo, Japan) with an accelerating voltage of 20 kV and magnification of 500x (bone surface) and 130x (inner portion of bone) (Benjakul et al. 2017).
Experimental design and statistical analysis
Experimental design for entire study was Completely Randomized Design (CRD). The experiment was done in triplicate. Data were analyzed by ANOVA. Duncan’s multiple range test was used for mean comparison. Statistical analysis was performed using the Statistical Package for Social Science (SPSS 17.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Chemical compositions of liquor obtained from bone after autoclaving for different times
After boiled bone (BB) was autoclaved, compositions of liquor were determined (Table 1). Autoclaving time had the pronounced effect on chemical composition of liquor. Longer autoclaving time increased protein, AAGC and hydroxyproline contents (P < 0.05), except for protein contents, in which no differences were observed between AT-60 and AT-90 (P > 0.05). Thermal degradation of protein in the bones mainly occurred, thus releasing proteins or peptides from bone matrix into liquor. The escalation of AAGC in liquor might be mainly due to thermal degradation of proteins, in which solute such as small peptide or free amino acid could be liberated. Shimosaka et al. (1996) reported that protein content of horse mackerel bone was declined when heated in cooking water with increasing cooking time. Proteins were generally leached out into the water during cooking. Jiang et al. (2018) reported that prolonged heating times more likely increased protein solubility of bighead carp muscle, reflecting the degradation of proteins as confirmed by SDS-PAGE. In general, protein has a specific three-dimensional structure to maintain biological functions. When protein is heated, the protein structure is destroyed and known as thermal denaturation (Ichimura 1991).
Table 1.
Chemical compositions of liquor obtained from bone after autoclave for different times
| Liquor samples | Protein content (g/100 g BB) | α- Amino group content (g/100 g BB) | Hydroxyproline content (g/100 g BB) |
|---|---|---|---|
| BB | 2.57 ± 0.39 b | 0.15 ± 0.02 d | 0.01 ± 0.002 d |
| AT-30 | 2.54 ± 0.25 b | 0.21 ± 0.01 c | 0.05 ± 0.001 c |
| AT-60 | 5.99 ± 0.01 a | 0.47 ± 0.01 b | 0.06 ± 0.002 b |
| AT-90 | 12.84 ± 0.45 a | 0.57 ± 0.01 a | 0.07 ± 0.001 a |
BB Boiled bone, AT-30 bone autoclaved for 30 min, AT-60 bone autoclaved for 60 min, AT-90 bone autoclaved for 90 min. Four volumes of distilled water were added into BB prior to autoclaving (121 ºC)
Values are presented as mean ± SD (n = 3)
Different lowercase superscripts in the same column indicate significant differences (P < 0.05)
AAGC content of liquor increased with augmenting autoclave time. This coincided with the result of hydroxyproline content in liquor. Hydroxyproline content in liquor was elevated with prolongation of autoclave time. Hydroxyproline value in liquor indicated that collagen in fish bone matrix was degraded and leached out into the liquor. In the boiled water obtained from BB preparation, the low hydroxyproline was attained. During autoclaved for 30–90 min, hydroxyproline content in the water medium (liquor) increased sharply. However no further increase was found after 120 min of heating (data not shown). Glycine, proline and hydroxyproline are dominat amino acids in bone collagen. Muralidharan et al. (2013) reported that glycine, proline and hydroxyproline in collagen from Leather jacket bone were 356.8, 103.1, and 87.2 residue/1000 residue, respectively. Thus, autoclave with high temperature and pressure could break down proteins, including collagen in bone matrix, thus likely softening the bones.
Properties of bone after autoclaving for different times
Bone texture
Bone texture expressed as hardness after being autoclaved compared to BB is displayed in Fig. 1. The hardness of bone decreased drastically after being autoclaved. BB showed the hardness more than 57,000 g that could not be measured by a texture analyzer. BB bone tissue has high strength, rigidity and toughness since it consists of bone cell, minerals and organic mostly made up of collagen (Gower 2008). Boiling process was not sufficient to leach out the collagen in the bone matrix as indicated by low hydroxyproline content in the liquor (Table 1). Thus, matrix was still in the complex form and hard bone was still attained. During high pressure heating, organic matrix was lost and destructed, resulting in the softened bone. The softest bone was observed when Asian sea bass bone was autoclaved for 90 min (P < 0.05). Longer time up to 120 min showed no further decrease in hardness, compared to 90 min (data not shown). According to Okada et al. (1998) autoclaving at 15 psi was sufficient to soften the bones of most fish satisfactorily. AT-90 sample had the lower hardness than AT-30 and AT-60 samples by 6.26 and 4.54 folds, respectively.
Fig. 1.
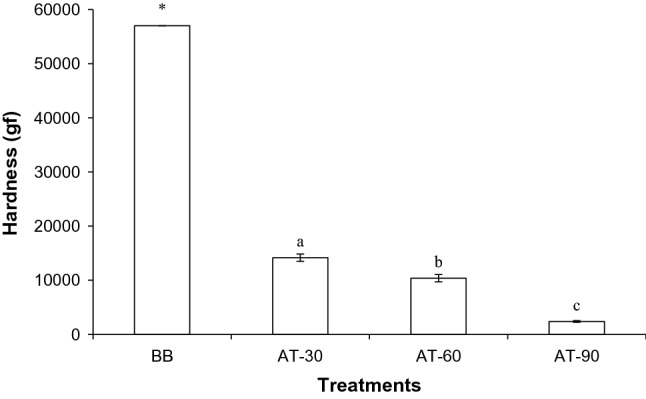
Hardness of Asian sea bass backbone subjected to high pressure heating for different times. RM: raw bone; BB: boiled bone; AT-30: bone autoclaved for 30 min; AT-60: bone autoclaved for 60 min; AT-90: bone autoclaved for 90 min. *Value was greater than 57,000 gf and the texture analyzer could not detect higher value. The bars indicate standard deviation (n = 5). Different lowercase letters on the bars indicate significant differences (P < 0.05)
X-ray diffraction (XRD)
X-ray diffraction diffractograms of raw material (RM), BB, AT-30, AT-60, AT-90 samples are depicted in Fig. 2. The lower intensity of the raw bone was observed with a wider peak since its intensity was dispersed by X-ray radiation. This might be due to the presence of extracellular matrix and other proteins, especially collagen, in the raw bone. More intense and narrower full width at half maximum (FWHM) of diffraction peaks were observed after bone was heated by boiling or autoclaving. This suggested the agglomeration of nano-hydroxyapatite to larger crystals. Boiling and autoclaving can remove some organic compounds to some extent, thus allowing crystal agglomeration to occur at higher degree ascertained by higher crystallinity. All the samples in this study showed relatively broad peaks which were the result of the combination of elastic and inelastic scattering of nano-hydroxyapatite crystals present in the bone matrix (Londoño-Restrepo et al. 2019).
Fig. 2.
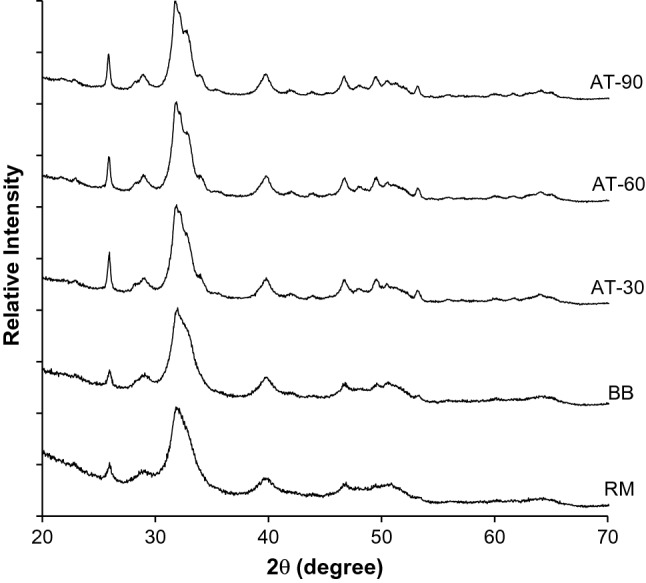
X-ray diffraction (XRD) spectra of Asian sea bass backbone subjected to high pressure heating for different times. RM: raw bone; BB: boiled bone; AT-30: bone autoclaved for 30 min; AT-60: bone autoclaved for 60 min; AT-90: bone autoclaved for 90 min
Peak positions of all XRD patterns were consistent with a reference XRD pattern data of hydroxyapatite from Joint Crystal Powder Diffraction Standard (JCPDS) No. 01-084-1998 (Benjakul et al. 2017). The results were in good agreement to the reported XRD pattern of bio-calcium from yellowfin tuna (Benjakul et al. 2017). The XRD patterns of raw material bone (RM), boiled bone (BB) and autoclaved bones for different times in this study had similar peak positions, indicating that hydroxyapatite was the main component of the fish backbone.
Thermo gravimetric analysis (TGA)
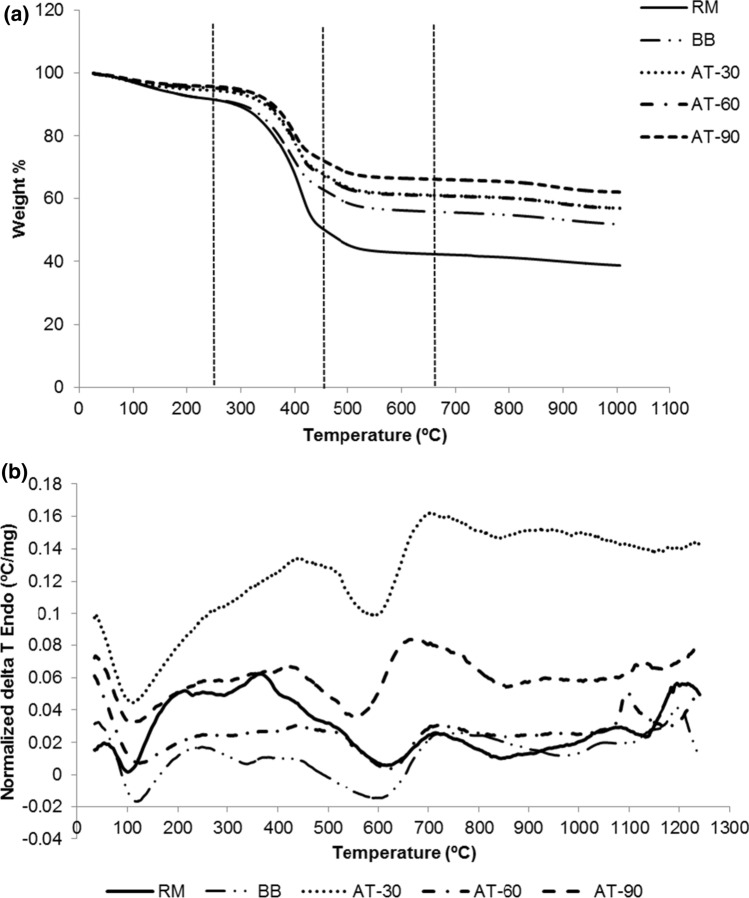
Thermo gravimetric analysis thermograms of Asian sea bass backbone (RM, BB and autoclaved bone) are shown in Fig. 3a. Four phases were observed. For the first phase (25–250 ºC), different weight losses among all the sample were found. Weight losses of RM, BB, AT-30, AT-60 and AT-90 were 8.08%, 8.30%, 5.37%, 4.62% and 4.13%, respectively. This region was related with the evaporation of free water and bound water (Da Cruz et al. 2020). The higher weight loss of RM and BB in this region indicated that those samples had higher moisture content than other samples. Those water could be evaporated during heating. For the second domain, about 250–450 ºC, the higher weight loss was detectable in all samples, compared to other regions. The highest weight loss in RM (43.23%) showed that the highest organic compounds were removed. The lowest weight reduction in this region was found in AT-90, indicating the lowest presence of organic compound such as lattice water, proteins, collagen and lipids in this sample. Piccirillo et al. (2013) reported TGA thermogram of apatite from cod fish bone, in which higher weight losses associated with the release of organic matter were attained at 200–500 ºC. The third region (450 ºC–680 ºC) loss was found around 6.31–7.8%, mostly attributed to gradual dehydroxylation of HAp, with the loss of OH-ions. The loss of CO3−2 ions was also presumed mainly due to carbonate decomposition by the removal of CO2 from the apatite network (Da Cruz et al. 2020). The fourth regions (above 680 ºC) demonstrated slight weight loss (3.66–4.04%) via continuous loss of OH ions in all samples. Goto and Sasaki (2014) reported slight differences in TGA thermogram of Hap from tuna, yellow tail, greater amberjack, and horse mackerel bone, which consist of three regions. At the first region (100–300 ºC), the removal of water molecules from fish bone was noticed, and was related to weight reduction about 30%. Furthermore 30–40% of weight was lost in the second zone (300–600 ºC) owing to decomposition of organic matters, e.g. protein and fat in bone. At the third phase (above 600 ºC), the small weight loss (1–2%) was detected, plausibly caused by de-carbonation.
Fig. 3.
a Thermal gravimetric analysis (TGA) and b differential thermal analysis (DTA) thermogram of Asian sea bass backbone subjected to high pressure heating for different times. RM: raw bone; BB: boiled bone; AT-30: bone autoclaved for 30 min; AT-60: bone autoclaved for 60 min; AT-90: bone autoclaved for 90 min
TGA thermograms reconfirmed that RM had higher content of organic matters than BB and autoclaved bones. Boiling and autoclaving therefore reduced the protein, lipid and other organic compounds. Autoclaving for 30 or 60 min still retained protein or collagen in bone matrix as evidenced by the low protein and hydroxyproline contents in the liquor (Table 1). Among all samples, AT-90 showed the least weight loss, indicating the least amount of organic matters in the bones. This coincided with the highest contents of proteins or hydroxyproline in the liquor (Table 1).
Differential thermal analysis (DTA)
Differential thermal analysis thermograms of RM, BB and autoclaved bones are shown in Fig. 3b. DTA thermograms were different among the samples. All samples showed three endothermic peaks with slightly different patterns. The first peak was varied among the samples (110–115 ºC), representing dehydration of free water or entrapped water molecule in the Asian sea bass backbone. Kalantar and Fazel (2014) documented that endothermic reaction in raw bovine bone was corresponding to the evaporation of water molecules at temperature of 110–200 ºC. At 560–623 ºC, the second endothermic peak was observed as the decomposition of organic material such as protein, lipid, etc. occurred. The third endothermic peak was quite small and found at 804–963 ºC, indicating the decomposition of carbon. Bovine bone hydroxyapatite showed the third endothermic peak at 800 ºC due to the dissociation of inorganic compounds, especially the complex between carbonate and phosphate groups (Zambrano, et al. 2017).
Exothermic peak was observed in RM at 365ºC. This might be owing to some oxidation products from proteins and lipids. Exothermic peaks at high temperatures (1100 ºC and 1115 ºC) were found in AT-60 and AT-90, respectively. Common endothermic processes may be related with sublimation and melting of solid substances. Endothermic transitions also correspond to loss of water in the sample, decomposition of hydrates, carbonates and dehydroxylation of surface (Zambrano et al 2017). Exothermic peak is mainly identified by crystallization, crystalline transformations, formation of new phases, oxidation and combustion reactions (Zambrano et al. 2017). The result was in line with the higher crystallinity of AT-60 and AT-90 than others.
Microstructures of Asian sea bass backbone
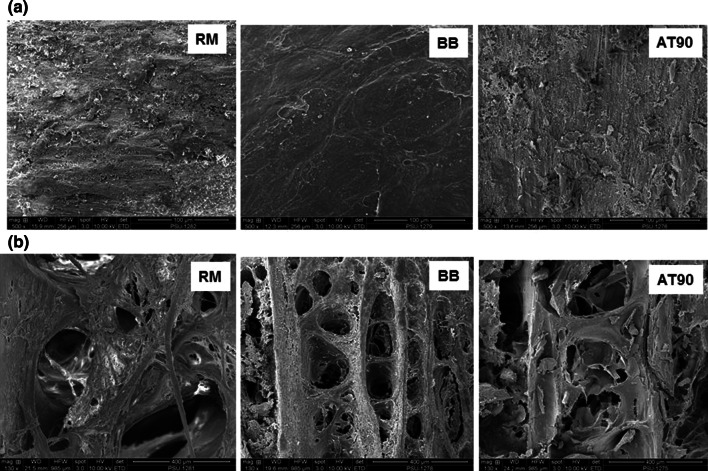
Microstructures of Asian sea bass backbone, boiled bone (BB) and autoclaved for 90 min (AT-90) are displayed in Fig. 4. According to Gower (2008), bone is organized into trabecular (spongy) and is surrounded by more compact cortical bone. Based on microstructure, the bone showed compact cortical bone on the surface (a) and was spongy (b) inside the bone structure. RM had a rough surface, indicating that organic matters such as meat were still remained on the surface of the backbone. Smoother surface was observed on the boiled backbone (BB) since adherent meat had been removed. However, the rough surface was also found in autoclaved backbone (AT-90). Disruption of surface might take place by high pressure heating. There was no difference in inner structure between RM and BB, in which no fracture was found in the bone matrix. On the other hand, autoclaved backbone (AT-90) exhibited several broken fragments inside the matrix. Under the high pressure with high temperature, collagen and other cementing materials were destroyed. This directly caused the collapsed or loosened network of inner part of bone.
Fig. 4.
Microstructure of Asian sea bass backbone with different heat treatments. a surface bone (500x magnification), b inside bone (130x magnification). RM: raw bone; BB: boiled bone; AT-90: bone autoclaved for 90 min
Proximate compositions and minerals in Asian sea bass backbone as affected by boiling and autoclaving
Proximate composition
Moisture content of RM was higher, compared to those of BB and AT-90 (P < 0.05) (Table 2). The high moisture content of RM was due to the presence of water in bone and water in attached meat. Moisture content of Asian sea bass frame was similar to that of tilapia frame reported by Chomnawang and Yongsawatdigul (2013) (64.23%). Vlieg and Murray (1988) documented that moisture content of albacore tuna frame was 63.8%. During boiling and removing the adherent meat of fish frame, moisture content was decreased in BB by 30%. Boiling resulted in the cleavage of several peptide bonds and induced thermal denaturation. As a result, the meat was easy to remove from bone. The moisture content of AT-90 was higher than that of BB. During high pressure heating, bone was soaked in 4 volumes of water with increased porosity of the bone caused by autoclave. As a result, water was more absorbed in cavity or pore.
Table 2.
Proximate compositions and mineral contents of Asian sea bass backbone subjected to different heating processes
| Parameters | RM | BB | AT-90 |
|---|---|---|---|
| Moisture (%) | 64.20 ± 0.57 a | 35.83 ± 0.51 c | 43.44 ± 0.06 b |
| Protein* (%) | 44.89 ± 0.45 a | 28.17 ± 0.22 b | 12.93 ± 0.29 c |
| Lipid* (%) | 32.05 ± 0.78 a | 12.19 ± 0.16 b | 10.49 ± 0.25 c |
| Ash* (%) | 24.32 ± 0.67 a | 57.53 ± 0.21 b | 74.37 ± 0.35 c |
| Calcium* (%) | 11.96 ± 0.50 c | 23.63 ± 0.19 b | 29.65 ± 0.69 a |
| Phosphorus* (%) | 6.60 ± 0.11 c | 11.97 ± 0.10 b | 15.80 ± 0.07 a |
| Iron* (mg/kg) | 19.48 ± 0.81 a | 12.43 ± 0.97 c | 15.10 ± 0.61 b |
| Ca/P mole ratio | 1.40 | 1.52 | 1.45 |
RM raw bone, BB boiled bone, AT-90 bone autoclaved for 90 min
Values are presented as mean ± SD (n = 3)
Different lowercase superscripts in the same row indicate significant differences (P < 0.05)
*Dry weight basis
The highest protein content of Asian sea bass backbone was found in RM, due to the attached meat rich in proteins. Protein content (dry weight basis) of RM was higher than that of tilapia frame (40.03%) (Chomnawang and Yongsawatdigul 2013). Other species such as albacore tuna, Alaska pollock, Pacific cod and pink salmon showed higher protein content in their frame 63.26% (Vlieg and Murray 1988), 85.3%, 55.3% and 77.17% (Bechtel 2003), respectively. This was more likely due to different filleting process used and varying anatomies of different fish. Protein content of BB decreased since the meat was removed from the bone during boiling. However, some collagenous protein was still in the bone. Autoclaved bone (AT-90) had lowest protein content, compared to others (P < 0.05). High pressure heating could reduce the protein content by inducing the removal of protein, especially collagen in the bone matrix. This result coincided with the increased protein and hydroxyproline contents in the liquor (Table 1). AT-90 showed the highest protein content in liquor, indicating that more protein and hydroxyproline were leach out in the bone matrix as a result of the high pressure heating.
Lipid content of RM was higher, compared to those of BB and AT-90 (P < 0.05). Lipids were mostly from the meat retained on the bone and from bone marrow inside the backbone. Bone marrow, localized in medullary cavity of long bones, is a gel-like soft tissue that contains fat cells, apart from nerve fibers, blood vessels, and myeloid tissue (Kubo et al. 2011). Toppe et al. (2007) reported that lipid content of fish bone varied, dependent on species (1.14%-50.9%) (% dry matter). BB sample had lower lipid content than RM since heating could liberate fat cells and removed meat during washing process with ease. The lowest lipid content was obtained in AT-90 (P < 0.05). Thus high pressure heating could effectively reduce lipid content of the bone.
The highest ash value was noted for AT-90 (P < 0.05). High pressure heating removed organic compounds in the bone such as protein and lipid, resulting in higher concentration of inorganic matters in bone matrix. Ash content of RM was the lowest among all the samples (P < 0.05). This was in line with the highest content of protein and lipid contents. Ash contents of fish bones varied by species and size from 21.24 to 59.0% (Chomnawang and Yongsawatdigul 2013; Toppe et al. 2007).
Calcium, phosphorus and iron contents
Calcium, phosphorus and iron contents of Asian sea bass backbone as affected by boiling and autoclave are shown in Table 2. Mineral (Ca, P and Fe) contents of Asian sea bass backbone were different among samples (P < 0.05). Autoclaved bone (AT-90) had highest calcium, phosphorus and iron contents than another two samples (P < 0.05). This result was consistent with the highest ash content of AT-90 (Table 2).
The lowest calcium, phosphorus and iron contents of Asian sea bass frame were found in RM (without heating). Mineral contents of this frame were lower than those reported by Toppe et al. (2007) who found that calcium, phosphorus and iron contents of fish bones were 13.5%-23.3%, 8.1%-11.3% and 32-135 mg/kg, respectively. Since Asian sea bass frame still had meat retained on the bone at high extent, this resulted in the lower composition in minerals but higher content of protein. BB had higher content of calcium, phosphorus and iron than RM (P < 0.05) since boiling caused the removing and washing of the organic compounds, while increased inorganic substances. The highest mineral (Ca, P and Fe) contents were found in AT-90 sample. High pressure heating could break peptide bond in the matrix of bone and released collagenous proteins and other organic compounds. This resulted in concentrating minerals in bone. Inorganic compounds such as minerals in the bone have low sensitivity toward high temperature compared to organic compounds such as protein and lipid. Shimosaka et al. (1996) reported that only small amounts of calcium, phosphorus and magnesium were detected in the cooking water, indicating that less minerals were released from the bone matrix during boiling. Stability of calcium at high temperature of boiled bone (Shimosaka et al. 1996) and rainbow trout (Khosroshahi et al. 2015) was reported.
Mole ratio of calcium and phosphorus of bone was changed after boiling and high pressure heating process. The lowest calcium and phosphorus mole ratio was detected in RM and slightly increased in BB sample. Increasing calcium and phosphorus mole ratio of the bone might be due to more decrease in phosphorus during boiling than calcium. Khosroshahi et al. (2015) reported that phosphorus content of rainbow trout meat reduced after boiling process but there was no significant effect on calcium. Ca/P mole ratio of AT-90 was slightly lower than BB. However, both BB and AT-90 had higher Ca/P mole ratio than RM sample. Autoclave heating under high pressure plausibly leached out calcium from bone to some degree, thus lowering Ca/P mole ratio of AT-90 sample. Calcium and phosphorus contents indicated that hydroxyapatite is the major component in bone (Benjakul et al. 2017). Venkatesan and Kim (2010) reported that bone consisted of Ca-hydroxyapatite (Hap) around 70% and the remaining 30% was organic components. Hydroxyapatite from marine and aquatic resources had calcium and phosphorus mole ratio of 1.29–1.62 (Toppe et al. 2007).
For iron, the highest content was obtained in RM (P < 0.05), followed by AT-90 and BB, respectively. High iron content in RM might be caused by blood with hemoglobin retained. During boiling, the blood was removed by boiling water. However, when autoclave was implemented, some iron might complex with other minerals, especially Ca-hydroxyapatite, while other organic substances, were eliminated. As a consequence, iron content in AT-90 was slightly increased.
Conclusion
High pressure heating for different times had the effect on physical and chemical characteristics of Asian sea bass backbone. Heating for a longer time leached out protein and hydroxyproline into liquor, while the protein of bone was more degraded. The lowest hardness (2340 gf) of AT-90 was consistent with the highest organic compounds leached out from the bone matrix. More pronounced XRD pattern was observed in backbone autoclaved for 90 min as a consequence of nano-hydroxyapatite crystals agglomeration, while organic compounds were lowered as determined from TGA and DTA data. High pressure heating (0.11 MPa) at 121 °C for 90 min could reduce protein and lipid, but increased ash content with highest calcium (29.65%) and phosphorus (15.80%). High pressure heating for the proper time could therefore be a potential pre-treatment for reducing the organic compounds and decreasing bone hardness for the ease of size reduction to make the bio-calcium.
Acknowledgements
Prince of Songkla University (Grant No. AGR6302013N) was acknowledged for supporting the research. In addition, the authors would like to give thanks to Diponegoro University which provides Scholarship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Association of Official Analytical Chemists (AOAC) Official methods of analysis of association of chemistry. DC: AOAC Washington; 2000. [Google Scholar]
- Bechtel PJ. Properties of different fish processing by-products from pollock, cod and salmon. J Food Process Pres. 2003;27:101–116. doi: 10.1111/j.1745-4549.2003.tb00505.x. [DOI] [Google Scholar]
- Benjakul S, Karnjanapratum S. Characteristics and nutritional value of whole wheat cracker fortified with tuna bone bio-calcium powder. Food Chem. 2018;259:181–187. doi: 10.1016/j.foodchem.2018.03.124. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Mad-Ali S, Sookchoo P. Characteristics of biocalcium powders from pre-Cooked tongol (Thunnus tonggol) and yellowfin (Thunnus albacores) tuna bones. Food Biophys. 2017;12:412–421. doi: 10.1007/s11483-017-9497-0. [DOI] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963;35:1961–1965. doi: 10.1021/ac60205a053. [DOI] [Google Scholar]
- Beto JA. The role of calcium in human aging. Clin Nutr Res. 2015;4:1–8. doi: 10.7762/cnr.2015.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binsi PK (2018) Overview of waste generation in fish and shellfish processing industry. ICAR-Central Institute of Fisheries Technology, Cochin, https://krishi.icar.gov.in/jspui/bitstream/123456789/20489/1. Accessed 18th March 2020
- Chen H, Dou W, Zhu Q, Jiang D, Xia J, Wang X, Tang W, Wang S. The extraction and characterization of porous HA/β-TCP biphasic calcium phosphate from sole fish bones at different temperatures. Mater Res Express. 2019;6:125412. doi: 10.1088/2053-1591/ab5a8f. [DOI] [Google Scholar]
- Chomnawang C, Yongsawatdigul J. Protein recovery of tilapia frame by-products by ph-shift method. J Aquat Food Prod Technol. 2013;22:112–120. doi: 10.1080/10498850.2011.629077. [DOI] [Google Scholar]
- Corrêa THA, Holanda JNF. Fish bone as a source of raw material for synthesis of calcium phosphate. Mat Res. 2019;22:1–5. doi: 10.1590/1980-5373-mr-2019-0486. [DOI] [Google Scholar]
- Da Cruz JA, Weinand WR, Neto AM, Pala´cios RS, Sales AJM, Prezas PR, Costa MM, Graca MPF. Low-cost hydroxyapatite powders from tilapia fish. Min Met Mater Soc. 2020;72:1435–1442. doi: 10.1007/s11837-019-03998-4. [DOI] [Google Scholar]
- Feist B, Mikula B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014;72:302–306. doi: 10.1016/j.foodchem.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Ignatius B, Haffner-Luntzer M, Amling M, Ignatius A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur Cells Mater. 2018;35:365–385. doi: 10.22203/eCM.v035a25. [DOI] [PubMed] [Google Scholar]
- Goto T, Sasaki K. Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram Int. 2014;40:10777–10785. doi: 10.1016/j.ceramint.2014.03.067. [DOI] [Google Scholar]
- Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;40:4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemung BO. Properties of tilapia bone powder and its calcium bioavailability based on transglutaminase assay. Int J Biosci Biochem Bioinform. 2013;3:306–309. doi: 10.7763/IJBBB.2013.V3.219. [DOI] [Google Scholar]
- Ichimura Y. Thermal denaturation of protein II. Appl Brief Hitachi High-Tech Sci Corporat TA. 1991;54:1–2. [Google Scholar]
- Idowu AT, Benjakul S, Sinthusamran S, Pongsetkul J, Sae-Leaw T, Sookchoo P. Whole wheat cracker fortified with biocalcium and protein hydrolysate powders from salmon frame: characteristics and nutritional value. Food Qual Safe. 2019;3:191–199. doi: 10.1093/fqsafe/fyz012. [DOI] [Google Scholar]
- Idowu AT, Benjakul S, Sae-Leaw T, Sookchoo P, Kishimura H, Suzuki N, Kitani Y (2019b) Amino acid composition,volatile compounds and bioavailability of biocalcium powders from salmon frame as affected by pretreatment. J Aquat Food Prod Technol, 1–9. 10.1080/10498850.2019.16392
- Jiang Q, Han J, Gao P, Xu Y, Xia W. Effect of heating temperature and duration on the texture and protein composition of bighead carp (Aristichthysnobilis) muscle. Int J Food Prop. 2018;3:2110–2120. doi: 10.1080/10942912.2018.1489835. [DOI] [Google Scholar]
- Kalantar M, Fazel AYA. Comparison of purity and properties of hydroxyl carbonate apatite extracted from natural thigh bone by different physiochemical methods. Int J Eng Trans A Basics. 2014;27:1619–1626. [Google Scholar]
- Khosroshahi NK, Hosseini H, Rezaei M, Khaksar R, Mahmoudzadeh M. Effect of different cooking methods on minerals, vitamins and nutritional quality indices of rainbow trout (Oncorhynchus mykiss) Int J Food Prop. 2015;19:2471–2480. doi: 10.1080/10942912.2015.1039028. [DOI] [PubMed] [Google Scholar]
- Kubo T, Fujimori K, Cazier N, Saeki T, Matsukawa M. Properties of ultrasonic waves in bovine bone marrow. Ultrasound Med Biol. 2011;37:1923–1929. doi: 10.1016/j.ultrasmedbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Layne E. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 1957;10:447–455. doi: 10.1016/s0076-6879(57)03413-8. [DOI] [Google Scholar]
- Londoño-Restrepo SM, Jeronimo-Cruz R, Millán-Malo BM, Rivera-Muñoz EM, Rodriguez-García ME. Effect of the nano crystal size on the x-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-42269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan N, Shakila RJ, Sukumar D, Jeyasekaran G. Skin, bone and muscle collagen extraction from the trash fish, leather jacket (Odonus niger) and their characterization. J Food Sci Technol. 2013;50:1106–1113. doi: 10.1007/s13197-011-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamprasertkit S, Nicely R (2018) Thailand Seafood Report,USDA Foreign Agricultural Service, Global Agricultural Information Network. https://www.fas.usda.gov/data/thailand-seafood-report. Accessed 18th March 2020.
- Okada M, Machino T, Kato S. “Bone softening” a practical way to utilize smal fish. Mar Fish Rev. 1988;50:1–7. [Google Scholar]
- Piccirillo C, Silva MF, Pullar RC, Braga da Cruz I, Jorge R, Pintado MME, Castro PML. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones. Mater Sci Eng. 2013;C33:103–110. doi: 10.1016/j.msec.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Shimosaka C, Shimomura M, Terai M. Changes in the physical properties and composition of fish bone during cooking by heating under normal pressure. J Home Econ Jpn. 1996;47:1213–1218. [Google Scholar]
- Sulaiman S, Jamaludin NFA, Kabbashi NA. Development of CaO/PVA catalyst from fish bone for biodiesel production. Bull Chem React Eng Catal. 2019;14:153–157. doi: 10.9767/bcrec.14.1.3327.153-157. [DOI] [Google Scholar]
- Toppe J, Albrektsen S, Hope B, Aksnes A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp Biochem Phys B. 2007;22:395–401. doi: 10.1016/j.cbpb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Venkatesan J, Kim SK. Effect of temperature on isolation and characterization of hydroxyapatite from tuna (Thunnus obesus) bone. Materials. 2010;22:491–496. doi: 10.3390/ma3104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vileg P, Murray T. Proximate composition of albacore tuna, Thunnus alalunga, from the temperate South Pacific and Tasman Sea. N Z J Mar Freshw. 1988;22:491–496. doi: 10.1080/00288330.1988.9516318. [DOI] [Google Scholar]
- Zambrano A, Castellar G, Valejo W, Piñeres I, Cely MM, Valencia J. Conceptual approach to thermal analysis and its main applications. Prospectiva. 2017;15:117–125. doi: 10.15665/rp.v15i2.1166. [DOI] [Google Scholar]