Abstract
The effects of PMF (5–7 T, 5–30 pulses) on enzyme activity, pH, titratable acidity, soluble solids, color, ascorbic acid, total phenols and antioxidant activity (DPPH radical scavenging activity) of cloudy apple juice were evaluated. PMF inhibited activities of polyphenoloxidase (PPO), peroxidase (POD) and pectinmethylesterase (PME), but PPO was more sensitive to PMF than POD and PME. At the intensity of 6 T with 15 pulses, PPO and POD both exhibited the lowest residual activity (53.22 and 92.96%), while PME showed the lowest residual activity (83.01%) at 7 T with 30 pulses. No significant effect on soluble solids was found under all processing parameters, whereas significant decreases of ascorbic acid were observed at the intensity of 7 T with 5–30 pulses. PMF did not change pH, titratable acidity, color, total phenols and DPPH radical scavenging activity severely. These results suggest PMF can be a potential technology for enzymatic inactivation in apple juice with high retention of quality.
Keywords: Pulsed magnetic field, Apple juice, Enzymatic inactivation, Quality
Introduction
Apple juice is a very popular juice in the world, with high nutritional and medicinal value. Cloudy apple juice has a better market value than clarified juice since it is more beneficial to human health. It is a good resource of nutrients such as vitamins, minerals and sugars. It also contains several polyphenols which can prevent many diseases owing to its antioxidant ability. However, enzymatic browning has been addressed as the main challenge for apple juice that changes the color of fresh apple juice to brown, inducing the decline in quality (Sun et al. 2015). It is well known that enzymatic browning depends on oxidative enzymes including polyphenoloxidase (PPO) and peroxidase (POD), which cause browning by catalyzing oxidation of phenolic compounds. Other challenge is cloudy loss in apple juice, which is related to pectinmethylesterase (PME).
PPO is an enzyme containing copper and can hydroxylate monophenols to o-diphenols which polymerize with endogenous amino acids or proteins and form brown or dark compounds (Gong et al. 2015). POD that carries a ‘b’-type haem is another oxidoreductases and catalyzes many kinds of reactions (Marszałek et al. 2017). POD participates in enzymatic browning reaction when diphenols act as reducing substrate (Chisari et al. 2007). The contribution of POD to browning is not only influenced by hydrogen peroxide, the electron acceptor of POD, but also by lipid peroxidase and superoxide free radicals. PME is involved in the deesterification of methoxylated pectin, during which calcium ions react with demethylated pectin to form insoluble pectin, resulting in the lose of cloudy appearance in fruit juice. Therefore, development of effective strategies to inactivate these enzymes is essential for retaining the quality of apple juice.
Thermal processing used for inactivation of pathogens microorganism and enzymes can lead to the loss of polyphenols, vitamins and other nutrients in fruit juices. In addition, thermal treatment can cause chemical and physical changes, affecting color, flavor and texture of fruit juice (Rawson et al. 2013). Consequently, non-thermal techniques have been developed and applied to food processing, which can minimize changes in quality and preserve the nutritional characteristics of food. Non-thermal technologies including high hydrostatic pressure (HHP), high pressure carbon dioxide (HPCD), cold plasma, pulsed electric field (PEF) and ultrasound (US), are commonly used to process fruit juices. HPP has been proposed as a useful method for preservation of apple products and decreased the activities of tissue oxidoreductive enzymes (PPO and POD) in cloudy apple juice (Marszałek et al. 2019). HPCD has been reported as a more effective technology for PPO inactivation in cloudy apple juice than mild thermal treatment at the same temperature (Illera et al. 2018a). Cold plasma, emerged as an industrial surface decontamination method, has been employed to PPO inactivation and quality preservation of cloudy apple juice (Illera et al. 2019a). High intensity PEF has been applied to cloudy apple juice to evaluate the effect on PPO and POD inactivation and changes in quality attributes (Wibowo et al. 2019). The effects of ultrasound combined with mild heating (TS) on PPO and PME inactivation and the physicochemical properties of cloudy apple juice have been investigated (Illera et al. 2018b). Inactivation mechanisms of enzyme under non-thermal technologies treatment are still not very clear. However, the different enzyme inactivation mechanisms would lead to different inactivation degree owing to different structural changes induced by non-thermal technologies (Illera et al. 2019b).
Pulsed magnetic field (PMF) has been considered as an alternative to thermal treatment and can inactivate microorganism in liquid. The effect of PMF on microorganism inactivation is related to magnetic field parameters, including intensity and pulse number (Qian et al. 2016a). Other factors such as species of microorganism, composition of liquid and environmental temperature, also affect the inactivation. The mechanisms of microbial inactivation induced by PMF are due to the destruction of cell membranes, leakage of contents and disorder of metabolism (Qian et al. 2016b, Qian et al. 2020). Although the inactivation of microorganisms by PMF has been studied, there are lack of some reports on inactivation of enzymes by PMF. In addition, few studies are available on the application of PMF in fruit juice.
Therefore, the aim of this research was to investigate the effects of PMF on enzyme activties and quality characteristices of cloudy apple juice. PPO, POD and PME activities, physicochemical properties (pH, titratable acidity, soluble solids, color, total phenols, ascorbic acid) and antioxidant activity (DPPH radical scavenging activity) in cloudy apple juice after PMF treatment were evaluated.
Materials and methods
Chemicals
Pectin and DPPH were purchased from Sigma-Aldrich (Shanghai, China). Catechol, pyrogallol, hydrogen peroxide, glacial acetic acid, 2, 6-dichloroindophenol, gallic acid, Folin-Ciocalteu reagent, sodium carbonate and ethanol were acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All other chemicals and solvents were of analytical grade and water was double distilled.
Cloudy apple juice preparation
Fresh apples (Malus pumila Mill, cv. Red Fuji, Yantai, China) were purchased from local market of Zhenjiang, China. Apples were washed, peeled and disintegrated. The juice was obtained by using electrical extractor of HR1871 (Philis, Holland Royal Philips Electric Co., Holland). The squeezed apple juice was centrifuged at 10,000 g for 10 min at 4 °C (H1850R, Xiangyi Inc, Hunan, China) and 5 mL of the supernatant was exposed to PMF treatment.
PMF treatment
PMF was performed using a PMF generator (TSK-H15300, Tingjin Magnetic Components Co., Ltd, Nanjing, China) which was equipped with a DC power supply, a series of capacitances and a coil. Capacitances charged by DC power supply discharged to the coil and were charged again after discharge, generating PMF in the coil alternately. The cooling water circulation system were connected to the treatment chamber where the coil was placed to maintain the room temperature. PMF intensity was measured by Tesla meter (LZ-610H, Lianzhong Technology Co., Ltd, Hunan, China) and pulse number was recorded by manual counting. PMF treatments were carried out at the intensity of 5, 6 and 7 T with the pulse numbers from 5 to 30 and the treatment temperature was 28 °C in this experiment. The shape of PMF pulse is rectangular. Pulse duration in coil is 0.3 ms. However, time between repetitive pulses is different, depending on the intensity of PMF. Time between repetitive pulses at 5, 6 and 7 T is 4, 4.5 and 5.2 s, respectively.
Assay of enzyme residual activity
PPO
PPO activity was measured by UV absorption as reported earlier (Augustin et al. 1985). 1.5 mL of cloudy apple juice sample and 0.5 mL of 0.05 M catechol were added into 3.0 mL of 0.2 M potassium phosphate buffer (pH 6.8). The mixture was shaken and changes of absorbance were determined by a spectrophotometer (UV 1801, Ruili Analytical Instruments, Beijing, China) at 410 nm in 1 min intervals for 10 min. The residual activity of PPO was calculated as percentage according to the following equation:
| 1 |
where As and Ao are PPO specific activity of treated sample and untreated sample, respectively.
POD
POD activity was measured spetrophotometrically, as previously described by Kwak et al. (1995). 2.2 mL of cloudy apple juice sample was mixed with 0.32 mL of 0.1 M potassium phosphate buffer (pH 6) containing 0.32 mL of 5% (w/v) pyrogallol. 0.16 mL of 0.15 M H2O2 was added into mixture to start the reaction. Changes of absorbance were determined using a spectrophotometer (UV 1801, Ruili Analytical Instruments, Beijing, China) at 420 nm in 1 min intervals for 5 min. The residual activity of POD was calculated as percentage according to the following equation:
| 2 |
where As and Ao are POD specific activity of treated sample and untreated sample, respectively.
PME
PME activity was measured based on the method proposed by Ortuño et al. (2013) with slight modifications. A 1% of pectin solution was prepared as substrate by adding 10 g of pectin powder to 1 L of 0.15 M NaCl. The reaction mixture consisting of 10 mL of cloudy apple juice sample and 40 mL of substrate solution was grossly adjusted to pH 7 using 0.5 M NaOH and then finely adjusted to pH 7.5 using 0.05 M NaOH. The time required to reach pH 7.5 with 0.05 M NaOH was recorded. The unit of PME activity was expressed as μmol of H+ produced in 1 min per mL of sample. The residual activity of PME was calculated as percentage according to the following equation:
| 3 |
where As and Ao are PME specific activity of treated sample and untreated sample, respectively.
pH value
pH value was determined using a PHS-3C digital pH meter (Honggai Instrument Co., Ltd, Shanghai, China) at 25 °C. Buffer solutions of pH 4.0 and 7.0 were used to calibrate the pH meter before use, and 10 mL of cloudy apple juice was put into a beaker for pH measurement.
Soluble solids content
Soluble solids content was determined by an Abbe WAY-2 W refractometer (Yidian physico- optical Co. Ltd., Japan). Cloudy apple juice was placed on the lens of the refractometer and determined at 20 °C. Content of soluble solids was expressed as Brix.
Titratable acidity
Titratable acidity was measured based on the method of AOAC (1995). 10 g of cloudy apple juice sample was titrated to pH 8.1 using 0.1 M NaOH and the result was expressed as g of tartaric acid per 100 g of sample. Titratable acidity was calculated as percentage according to the following equation:
| 4 |
where V is titre volume of NaOH (mL), C is concentration of NaOH (0.1 M), and M is the mass of sample (g).
Color value
The color was determined by a colorimeter (Color Quest XE, Hunter Lab, USA) at 25 °C. Colorimeter was calibrated using the reference tile prior to the use. The color values were read in CIE L*a*b* color space system and expressed as lightness/darkness (L*), redness/greenness (a*) and yellow/blueness (b*). Cloudy apple juice were placed in glass tubs and measured. The total color difference (∆E) was calculated according to the following equation:
| 5 |
where L* and L0 are lightness of treated sample and untreated sample; a* and a0 are redness of the treated sample and untreated sample; b* and b0 are yellowness of the treated sample and untreated sample.
Total phenolic content
The total phenolic content was measured based on the Folin–Ciocalteu assay method (Islam et al. 2016) with some modifications. Gallic acid with concentrations between 10 and 100 µg/mL was used to built a standard curve. 0.1 mL of cloudy apple juice sample, 0.1 mL of Folin–Ciocalteu reagent (10%, v/v) and 0.7 mL of sodium carbonate( 20%, w/v) were mixed in 2 mL of water and incubated at 25 °C for 1 h in the dark. A spectrophotometer (Unico WFJ7200 Spectrophotometer, Shanghai, China) was used to measure the absorbance of the mixture at 740 nm. The result was expressed as mg of gallic acid equivalents (GAE) per 100 mL of sample (mg GAE/100 mL).
Ascorbic acid content
The ascorbic acid content was measured based on the 2, 6-dichloroindophenol titrimetric method of AOAC (1995). 20 mL of cloudy apple juice was added into 80 mL distilled water at 4 °C. The diluted apple juice was mixed with 25 mL of 20% glacial acetic acid and the mixture was titrated with standardized 2.6-dichloroindophenol solution. Ascorbic acid content was expressed as mg/100 mL of juice and calculated by the following equation:
| 6 |
where T is the Titre, indicating 1 mL standardized 2.6-dichloroindophenol solution is equivalent to the milligram amount of ascorbic acid, V is the volume of standardized 2.6-dichloroindophenol solution, and V0 is the volume of apple juice.
Antioxidant activity
Antioxidant activity was evaluated according to DPPH method. The DPPH scavenging activity was measured by spectrophotometric analysis as reported by Brand-Williams et al. (1995). The reaction mixture containing 0.1 mL of cloudy apple juice sample and 3.9 mL of 100 μM ethanolic DPPH solution was vortexed and incubated at 25 °C for 30 min in the dark. A spectrophotometer (UV-1801, Ruili Analytical Co., Ltd, Beijing, China) was used to measure the absorbance of the mixture at 517 nm. A mixture containing 0.1 mL of ethanol and 3.9 mL of 100 μM DPPH was served as control. The DPPH radical scavenging activity was calculated according to the following equation:
| 7 |
where A0 and A1 are the absorbance of untreated sample and treated sample, respectively.
Statistical analysis
All the experiments were performed in triplicate and statistical analysis were carried out by SPSS software (SPSS 20.0, IBM, Chicago, USA). The data were expressed as mean ± standard deviation and the significant difference at p < 0.05 level was determined using analysis of variance.
Results and discussions
POD, PPO and PME residual activity
PPO, POD and PME residual activities treated by PMF are shown in Fig. 1, Fig. 2 and Fig. 3. PMF decreased PPO activity in cloudy apple juice at the intensity of 5, 6 and 7 T with pulse number of 5, 10, 15, 20, 25 and 30. PMF intensity and pulse number affected the activity of PPO, but higher intensity and higher pulse number were not more effective in inhibiting PPO activity, which may be related to the “window effect” of PMF. The “window effect” indicates that targets inside biosystem only respond to the PMF within some time or intensity range, so the “window effect” includes of “time window” and “intensity window”. PPO reached minimum value at pulse number of 15 under all the pluses processed and 15 pulses was one of the “time window” in the inactivation of PPO by PMF. The “vale value” or “window effect” of PMF had been found during the inactivation of certain bacteria including E. coli and S. cerevisiae (Ma et al. 2008). However, “window effect” of PMF in enzyme inactivation has not been reported yet. The lowest residual activity of PPO was obtained at 6 T with 15 pulses, showing residual activity of 53.2%. Different inactivation values of PPO in cloudy apple juice treated by different non-thermal technologies have been reported in the literature. Residual activity of PPO was 13.4% in the cloudy apple juice after HPCD treatment at 20 MPa and 45 °C for 60 min (Illera et al. 2018a). However, US technology resulted in low inactivation efficiency on PPO, and even US combined with mild temperature of 52 °C for 20 min only decreased the residual activity of PPO to 71% (Illera et al. 2018b).
Fig. 1.

Effect of PMF on residual activity of PPO in cloudy apple juice
Fig. 2.
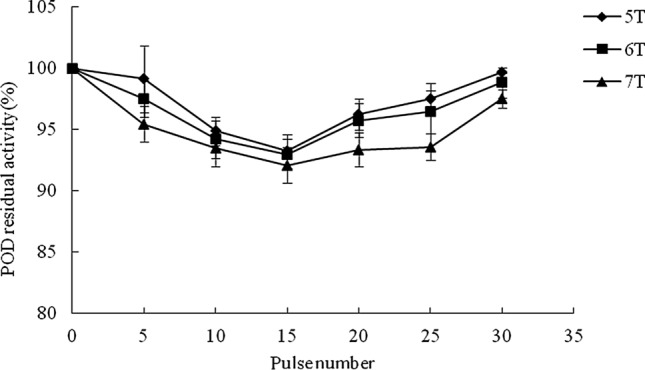
Effect of PMF on residual activity of POD in cloudy apple juice
Fig. 3.
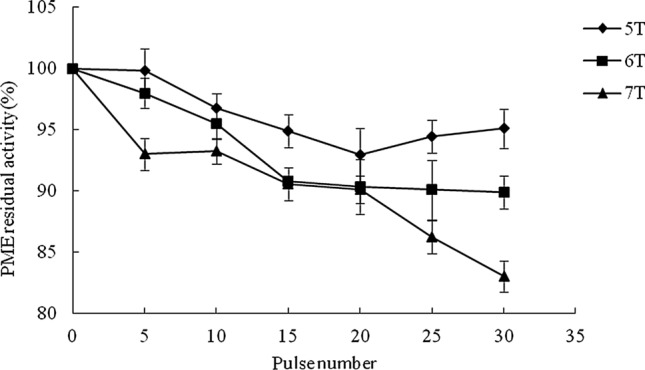
Effect of PMF on residual activity of PME in cloudy apple juice
POD and PME were more resistant to PMF than PPO. It has been reported that POD exhibited lower inactivation than PPO in PEF-treated cloudy apple juice (Wibowo et al. 2019) and PME in cloudy apple juice showed more stability to HPCD compared to PPO (Illera et al. 2018a). Although POD reached to minimum value also at pulse number of 15 during all the treated pluses, the POD activity was not reduced dramatically as PPO in samples. The residual activity of POD were 93.23, 92.96 and 92.09% respectively when cloudy apple juice was treated by PMF at 5, 6 and 7 T with the pulse number of 15. Wibowo et al (2019) found PEF treatment (12.5 kV/cm, 76.4 kJ/L, inlet and outlet temperatures of 37.6 °C and 59.5 °C) achieved 49 and 36% reduction in POD and PPO activity and suggested that temperature played an important role in the inactivation of POD and PPO due to the high outlet temperature after PEF treatment. The high residual activity of POD treated by PMF may be partly attributed to relative low temperature (28 °C) in PMF treatment chamber. The “time window” appeared again when PMF was applied to inactivate POD in cloudy apple juice. The “window effect” of PPO and POD inactivation in cloudy apple juice by PMF is pioneering and further research will be required to evaluate the effect of PMF on enzymes in other fruit juices.
The residual activity of PME in cloudy apple juice ranged from 83.01 to 99.82% after PMF treatment. Increased PMF intensity and pulse number led to lower PME activity and the lowest residual activity of PME was obtained at the intensity of 7 T with pulse number of 30. Residual activity of PME in cloudy apple juice treated by HPCD was around 60% at 20 MPa and 45 °C for 60 min (Illera et al. 2018a) and 18% at 20 MPa and 65 °C for 20 min (Niu et al. 2010). It seems that the temperature used by non-thermal technologies to process apple juice affected the enzyme inactivation. Furthermore, enzyme inactivation may be more effective when PMF treatment combined with mild temperature.
The different inactivation degree of enzymes is related to the type of enzyme and the processing conditions. The activity of enzyme depends on its active site and conformational structure. The change of PPO activity can be attributed to the exposure or enclosure of active site, resulting in the increase or decrease of PPO activity, respectively. Conformational changes of apoenzyme and modification of prosthetic group can lead to POD inactivation (Cruz et al. 2008). The Lorentz force induced by PMF can affect charged particles such as enzymes, which may lead to the changes in the active sites and structures. PMF may close or denature the active site of PPO, resulting in the decrease of the residual activity. The decrease of POD activity may be due to the conformational change of apolipolase or the degradation of prosthesis induced by PMF. On the other hand, the secondary structure and tertiary structure of the enzymes may also be disrupted by PMF. The conformations of POD and PME are more resistant to the PMF treatment compared with PPO, so the relative activities did not change dramatically as PPO.
In addition to processing condition involved in PMF intensity and pulse number, the inactivation rate may also be affected by the source of enzyme. It has already been reported that PEF induced a greater decrease of PPO activity in apple than in pear (Giner et al. 2001). Similar results were observed on inactivation of enzymes in different juices by high pressure (HPP). Yi et al. (2017) reported HPP reduced PPO and POD activity by 45 and 34% in apple juice at 600 MPa for 3 min, while Chang et al. (2017) reported PPO and POD activity in grape juice decreased by 48.8 and 47.9% under HPP treatment at 600 MPa for 3 min. Therefore, inactivation of the same enzyme in different juices treated by PMF should be further investigated.
PH, soluble solid and titratable acidity
Table 1 presented the results of pH, soluble solids content and titratable acidity in cloudy apple juice treated by PMF. The pH decreased from 4.10 to 4.03 after PMF treatment. The results were consistent with the observation of Liao et al. (2018) who reported the pH in apple juice was reduced from 3.71 to 3.62 after exposure to cold plasma at 50 W for 30 s. No significant differences in soluble solids (p > 0.05) were observed in PMF-treated cloudy apple juice, and PMF did not change titratable acidity dramatically. Juarez-Enriquez et al. (2015) reported HPP processing at 430 MPa for 7 min had no significant differences in soluble solids content even after apple juice was stored at 20 °C for 34 days. Apple juice treated by combination of US and HHP also showed no significant changes in soluble solids and titratable acidity (Abid et al. 2014a, b). Apple juices can be regarded as a water electrolyte system composed of multiple ions. Under PMF treatment, some dissociation reactions can take place and release H+ ions, which will reduce the pH value. On the other hand, free radicals such as OH· caused by PMF can oxidize some aldehydes into acidic compounds, resulting in the increase of the acidity in apple juice. However, the decrease of pH did not completely correspond to the increase of titratable acidity. The pH value continued to decrease slowly in cloudy apple juice treated by PMF, while titratable acidity decreased firstly, then increased and decreased again. This phenomenon may be attributed to treatment conditions. Under the low pulses, PMF destroyed the acidic compounds in cloudy apple juice. With the increase of pulses, the synthesis of acidic compounds by free radicals was accelerated and titratable acidity increased. When the pulses continued to increase, the synthesized acidic compounds were decomposed and titratable acidity decreased.
Table 1.
Effects of PMF on pH, titratable acid and soluble solid in cloudy apple juice
| Treatment conditions | pH | Titratable acid | Soluble solid | |
|---|---|---|---|---|
| Intensity (T) | Pulse number | |||
| 0 | 0 | 4.10 ± 0.01a | 0.107 ± 0.02ab | 7.04 ± 0.02a |
| 5 | 5 | 4.09 ± 0.01ab | 0.093 ± 0.003bc | 7.04 ± 0.01a |
| 10 | 4.07 ± 0.02bc | 0.121 ± 0.004a | 7.04 ± 0.01a | |
| 15 | 4.07 ± 0.02bc | 0.121 ± 0.004a | 7.04 ± 0.02a | |
| 20 | 4.06 ± 0.01cd | 0.114 ± 0.003a | 7.04 ± 0.01a | |
| 25 | 4.06 ± 0.01cd | 0.107 ± 0.004ab | 7.04 ± 0.02a | |
| 30 | 4.06 ± 0.01cd | 0.093 ± 0.002bc | 7.04 ± 0.02a | |
| 6 | 5 | 4.05 ± 0.00cde | 0.093 ± 0.003bc | 7.03 ± 0.01a |
| 10 | 4.05 ± 0.01cde | 0.107 ± 0.002ab | 7.04 ± 0.02a | |
| 15 | 4.05 ± 0.00cde | 0.119 ± 0.003a | 7.04 ± 0.01a | |
| 20 | 4.04 ± 0.01de | 0.121 ± 0.003a | 7.03 ± 0.02a | |
| 25 | 4.04 ± 0.01de | 0.121 ± 0.004a | 7.04 ± 0.01a | |
| 30 | 4.04 ± 0.01de | 0.114 ± 0.002a | 7.04 ± 0.02a | |
| 7 | 5 | 4.05 ± 0.01cde | 0.121 ± 0.002a | 7.04 ± 0.01a |
| 10 | 4.05 ± 0.02cde | 0.121 ± 0.003a | 7.04 ± 0.02a | |
| 15 | 4.04 ± 0.01de | 0.114 ± 0.002a | 7.03 ± 0.01a | |
| 20 | 4.04 ± 0.02de | 0.107 ± 0.004ab | 7.04 ± 0.00a | |
| 25 | 4.04 ± 0.01de | 0.086 ± 0.003c | 7.04 ± 0.01a | |
| 30 | 4.03 ± 0.01e | 0.086 ± 0.002c | 7.04 ± 0.02a | |
Values are expressed as the mean ± standard deviation of triplicate data. A different letter denotes significant difference at p < 0.05
Color
Color plays an important role in consumers choice of beverages. As shown in Table 2, the L* (lightness), a* (redness) and b*(yellowness) values in untreated cloudy apple juice were 79.47 ± 1.24, 6.48 ± 0.05 and 51.92 ± 0.96, respectively. PMF treatment resulted in no significant changes in L* and b* values (p > 0.05), but changed a* value significantly. Xiang et al. (2018) classified the total colour difference (∆E) as not noticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), well visible (3.0–6.0) and great (6.0–12.0). The values of ΔE were lower than 0.5 in apple juice treated by PMF at 5 T with 15 pulses, 6 T with 5 and 15 pulses, and 7 T with 20, 25 and 30 pulses, indicating these treated samples showed not noticeable color difference compared with the control sample. The color change was noticeable after being treated at 5 T with 10 and 25 pulses. The total color differences were slight noticeable under other treatment conditions. No significant color changes were observed in apple juice treated by PEF at 0–25 kV/cm, but the increases in L*, b* and ΔE values were obtained at 30 and 35 kV/cm, which suggested the color was mainly affected by electric field strength (Bi et al. 2013).
Table 2.
Effect of PMF on color in cloudy apple juice
| Treatment conditions | Color parameters | ||||
|---|---|---|---|---|---|
| Intensity (T) | Pulse number | L* | a* | b* | △E |
| 0 | 0 | 79.47 ± 1.24a | 6.48 ± 0.05ef | 51.92 ± 0.96a | |
| 5 | 5 | 78.79 ± 0.83a | 6.10 ± 0.03h | 51.19 ± 0.67a | 1.07 |
| 10 | 78.11 ± 0.89a | 6.76 ± 0.08cd | 52.70 ± 1.14a | 1.59 | |
| 15 | 79.42 ± 1.58a | 6.54 ± 0.07e | 52.23 ± 0.78a | 0.32 | |
| 20 | 78.76 ± 0.77a | 6.24 ± 0.08g | 51.09 ± 0.82a | 1.12 | |
| 25 | 78.63 ± 0.69a | 7.10 ± 0.07a | 53.04 ± 1.55a | 1.54 | |
| 30 | 78.91 ± 2.21a | 6.84 ± 0.05c | 52.58 ± 2.10a | 0.93 | |
| 6 | 5 | 79.38 ± 1.68a | 6.42 ± 0.05f | 51.91 ± 1.49a | 0.11 |
| 10 | 78.71 ± 0.70a | 6.95 ± 0.04b | 53.01 ± 1.66a | 1.41 | |
| 15 | 79.63 ± 0.74a | 6.22 ± 0.09g | 51.68 ± 2.23a | 0.38 | |
| 20 | 79.06 ± 0.93a | 6.67 ± 0.06d | 52.32 ± 1.88a | 0.61 | |
| 25 | 79.38 ± 1.19a | 6.67 ± 0.06d | 52.30 ± 1.67a | 0.43 | |
| 30 | 78.65 ± 0.92a | 6.98 ± 0.08b | 53.06 ± 1.99a | 1.49 | |
| 7 | 5 | 78.67 ± 1.34a | 7.03 ± 0.05ab | 53.02 ± 1.61a | 1.47 |
| 10 | 78.66 ± 0.77a | 6.95 ± 0.08b | 52.92 ± 2.23a | 1.37 | |
| 15 | 79.72 ± 0.85a | 6.23 ± 0.04g | 51.12 ± 1.67a | 0.88 | |
| 20 | 79.17 ± 1.69a | 6.54 ± 0.03e | 51.95 ± 2.18a | 0.30 | |
| 25 | 79.26 ± 1.57a | 6.46 ± 0.06ef | 52.01 ± 1.89a | 0.23 | |
| 30 | 79.55 ± 1.46a | 6.26 ± 0.07g | 51.75 ± 1.33a | 0.29 | |
Values are expressed as the mean ± standard deviation of triplicate data. A different letter denotes significant difference at p < 0.05
Color change is correlated to enzymatic activities such as PPO and POD, which are responsible for phenolic oxidation and enzymatic browning. When cloudy apple juice was treated by PMF at 5 T with 15 pluses and 6 T with 15 pulses, the residual activities of PPO and POD were low, resulting in not noticeable color difference. PME also affected the color of apple juice. PME residual activity was low in cloudy apple juice treated by PMF at 7 T with 20, 25 and 30 pluses, and there was not noticeable color difference in apple juice. The inactivation of PPO, POD and PME by PMF contributed to the stability of color in cloudy apple juice. Since inactivation of enzyme was affected by the operating conditions of PMF, color change was also related to the operating conditions of PMF.
Total phenol, ascorbic acid and DPPH radical scavenging activity
After exposure of cloudy apple juice to PMF, a decrease in total phenols was observed (Table 3). Total phenolic content in control was 9.78 mg GAE 100 mL−1, while ranged from 9.77 to 9.46 mg GAE 100 mL−1 after PMF treatment. The highest decrease of total phenol was obtained by 3.27% in apple juice treated by PMF at 7 T with 30 pulses. Total phenol was well retained when apple juice was subjected to UV–C (Islam et al. 2016). However, total phenolic contents decreased with an increase of temperature in apple juice treated by thermosonication, reaching the decrease by 3.32 and 20.90% at 25 kHz, 750 W and 40 °C and at 25 kHz, 750 W and 60 °C for 5 min, respectively (Abid et al. 2014a). The decrease of total phenolic contents may be due to the physical and oxidative degradation of phenolic components. The oxidative degradation of phenolic compounds in fruit and vegetable depends on PPO (Amiot et al. 1997). The inactivation of PPO by PMF can reduce the oxidative degradation and compensate for the loss of phenolic compounds caused by the physical degradation, resulting in the the maintenance of total phenols in apple juice.
Table 3.
Effects of PMF on total phenol, ascorbic acid and DPPH in cloudy apple juice
| Treatment conditions | Total phenol (mg GAE/100 mL) | Ascorbic acid (mg/100 mL) | DPPH (%) | |
|---|---|---|---|---|
| Intensity (T) | Pulse number | |||
| 0 | 0 | 9.78 ± 0.06a | 8.51 ± 0.04e | 93.6 ± 0.71cd |
| 5 | 5 | 9.77 ± 0.08a | 8.34 ± 0.06f | 94.03 ± 0.54bc |
| 10 | 9.75 ± 0.08ab | 8.33 ± 0.05f | 95.47 ± 0.74a | |
| 15 | 9.69 ± 0.07abc | 8.39 ± 0.07ef | 95.50 ± 0.72a | |
| 20 | 9.68 ± 0.08abcd | 9.02 ± 0.08d | 95.53 ± 0.41a | |
| 25 | 9.62 ± 0.10bcdef | 11.0 ± 0.08a | 94.90 ± 0.29ab | |
| 30 | 9.54 ± 0.06defg | 10.5 ± 0.15b | 93.87 ± 0.68c | |
| 6 | 5 | 9.74 ± 0.08ab | 8.04 ± 0.11g | 93.70 ± 0.41c |
| 10 | 9.70 ± 0.08abc | 7.89 ± 0.07h | 93.90 ± 0.67c | |
| 15 | 9.64 ± 0.13abcde | 7.17 ± 0.04i | 94.17 ± 0.34bc | |
| 20 | 9.67 ± 0.08abcd | 8.10 ± 0.12g | 93.63 ± 0.75cd | |
| 25 | 9.58 ± 0.10cdefg | 9.62 ± 0.13c | 93.20 ± 0.36cde | |
| 30 | 9.51 ± 0.05efg | 9.51 ± 0.06c | 92.63 ± 0.62def | |
| 7 | 5 | 9.71 ± 0.09abc | 6.88 ± 0.05j | 93.13 ± 0.41cde |
| 10 | 9.62 ± 0.08bcdef | 6.83 ± 0.03jk | 93.10 ± 0.43cde | |
| 15 | 9.57 ± 0.03cdefg | 6.54 ± 0.09m | 93.23 ± 0.21cde | |
| 20 | 9.59 ± 0.07cdefg | 6.72 ± 0.06kl | 92.50 ± 0.42ef | |
| 25 | 9.49 ± 0.08fg | 6.61 ± 0.03lm | 92.47 ± 0.41ef | |
| 30 | 9.46 ± 0.03g | 6.56 ± 0.04m | 91.90 ± 0.45f | |
Values are expressed as the mean ± standard deviation of triplicate data. A different letter denotes significant difference at p<0.05
Ascorbic acid content in control sample was 8.51 mg/100 mL (Table 3). PMF increased ascorbic acid content at 5 T with 20, 25 and 30 pulses and at 6 T with 25 and 30 pulses, but decreased ascorbic acid content under other processing parameters. Different results on ascorbic acid after different non-thermal treatments have been reported previously. Ascorbic acid content in apple juice treated by US at 25 kHz, 70% amplitude and 20 °C for 60 min increased from 5.46 to 6.28 mg/100 mL, while treated by HHP at 250, 350 and 450 MPa for 10 min ranged from 5.19 to 5.47 mg/100 mL (Abid et al. 2014b). Solomon, et al. (1995) has reported that occluded oxygen can influence the stability of ascorbic acid. PMF treatment may remove the occluded oxygen in apple juice, resulting in an increase in ascorbic acid. The decrease of ascorbic acid can be attributed to chemical decomposition. Ascorbic acid is not a stable compound, and can be decomposed under severe conditions. The significant decrease of ascorbic acid was observed when apple juice was treated by PMF at the intensity of 7 T with 5–30 pluses.
The percent inhibition of DPPH was measured to evaluate the antioxidant capacity in cloudy apple juice treated by PMF. As presented in Table 3, DPPH radical scavenging activity in control was 93.60%, while ranged from 91.90 to 95.53% in PMF treated cloudy apple juice. DPPH radical scavenging activity increased at 5 T with 5–30 pluses, and deceased at 7 T with 5–30 pluses compared to control. There were no significant changes in DPPH radical scavenging activity in the juice samples treated by PMF at 6 T with 5–30 pluses (p > 0.05). The intensity of PMF seems to have an important effect on the inhibition rate of DPPH. Furthermore, the results of DPPH radical scavenging activity in apple juice are different under different non-thermal treatments. No significant changes in DPPH radical scavenging activity were observed in apple juice treated by PEF at 1, 3 and 5 kV/cm with 30 pulses (Schilling et al. 2007), However, UV–C irradiation at the dose of 40 mJ cm−1 and higher than 40 mJ cm−1 caused significant decrease in DPPH radical scavenging activity (Islam et al. 2016), while cold plasma at 10.5 kV for 3, 4 and 5 min increased DPPH radical scavenging activity from 79.4 to approximately 93% in cloudy apple juice (Illera et al. 2019a). Antioxidant activity in apple is related to the contents of polyphenols and ascorbic acid (Eberhardt et al. 2000). PMF caused changes in contents of polyphenols and ascorbic acid in cloudy apple juice, resulting in the change in antioxidant capacity.
Conclusion
PMF treatment inhibited PPO, POD and PME activities in cloudy apple juice, but POD and PME showed more resistance to PMF than PPO. PMF had no significant changes in soluble solid content, induced small changes in pH, titratable acidity, color, and DPPH radical scavenging activity, and impaired the stability of total phenolic and ascorbic acid in cloudy apple juice slightly. The findings from this study demonstrated that PMF would be a promising non-thermal technology for enzyme inactivation in fruit juice with minor quality changes.
Acknowledgements
This project is funded under Jiangsu Provincial Natural Science Foundation (No. BK20150498); National Key Research and Development Program of China (No. 2016YFD0400705-04, 2017YFD0400903-01); Jiangsu University Senior Professionals Scientific Research Foundation (No.15JDG082) and Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC (1995) (16 ed) Official methods of analysis. AOAC International, Arlington VA.
- Abid M, Jabbar S, Hu B, Hashim MM, Wu T, Lei SC, Khan MA, Zeng XX. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason Sonochem. 2014;21:984–990. doi: 10.1016/j.ultsonch.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Abid M, Jabbar S, Hu B, Hashim MM, Wu T, Wu ZW, Khan MA, Zeng XX. Synergistic impact of sonication and high hydrostatic pressure on microbial and enzymatic inactivation of apple juice. LWT- Food Sci Technol. 2014;59(1):70–76. doi: 10.1016/j.lwt.2014.04.039. [DOI] [Google Scholar]
- Amiot MJ, Fleuriet A, Cheynier V, Nicolas J. Phenolic compounds and oxidativemechanisms in fruits and vegetables. In: Tomás-Barberán FA, Robins RJ, editors. Phytochemistry of fruit and vegetables. Oxford: Science Publications; 1997. pp. 51–85. [Google Scholar]
- Augustin MA, Ghazali HM, Hashim H. Polyphenoloxidase from guava (Psidium guajava L.) J Sci Food Agr. 1985;36:1259–1265. doi: 10.1002/jsfa.2740361209. [DOI] [Google Scholar]
- Bi XF, Liu FX, Rao L, Li J, Liu BJ, Liao XJ, Wu JH. Effects of electric field strength and pulse rise time on physicochemical and sensoryproperties of apple juice by pulsed electric field. Inno Food Sci Emerg. 2013;17:85–92. doi: 10.1016/j.ifset.2012.10.008. [DOI] [Google Scholar]
- Brand-William W, Cuvelier ME, Berset C. Use of a free radical method toevaluate antioxidant activity. LWT - Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chang YH, Wu SJ, Chen BY, Huang HW, Wang CY. Effect of high-pressure processing and thermal pasteurization on overall quality parameters of white grape juice. J Sci Food Agr. 2017;97(10):3166–3172. doi: 10.1002/jsfa.8160. [DOI] [PubMed] [Google Scholar]
- Chisari M, Barbagola RN, Spaga G. Characterization on polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J Agr Food Chem. 2007;55:3469–3476. doi: 10.1021/jf063402k. [DOI] [PubMed] [Google Scholar]
- Cruz RMS, Vieira MC, Silva CLM. Effect of heat and thermosonication treatments on watercress (Nasturtium officinale) vitamin C degradation kinetics. Innov Food Sci Emerg. 2008;9:483–488. doi: 10.1016/j.ifset.2007.10.005. [DOI] [Google Scholar]
- Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apple. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- Giner J, Gimeno V, Barbosa-Canovas GV, Martin O. Effects of pulsed electric field processing on apple and pear polyphenoloxidase. Food Sci Technol Int. 2001;7(4):339–345. doi: 10.1106/MJ46-8J9U-1H11-T0ML. [DOI] [Google Scholar]
- Gong Z, Li D, Liu C, Cheng A, Wang W. Partial purification and characterization of polyphenol oxidase and peroxidase from chestnut kernel. LWT - Food Sci Technol. 2015;60:1095–1099. doi: 10.1016/j.lwt.2014.10.012. [DOI] [Google Scholar]
- Illera AE, Sanz MT, Beltrán S, Melgosa R, Solaesa AG, Ruiz MO. Evaluation of HPCD batch treatments on enzyme inactivation kinetics and selected quality characteristics of cloudy juice from golden delicious apples. J Food Eng. 2018;221:141–150. doi: 10.1016/j.jfoodeng.2017.10.017. [DOI] [Google Scholar]
- Illera AE, Sanz MT, Benito-Román O, Varona S, Beltrán S, Melgosa R, Solaesa AG. Effect of thermosonication batch treatment on enzyme inactiva tion kinetics and other quality parameters of cloudy apple juice. Innov Food Sci Emerg. 2018;47:71–80. doi: 10.1016/j.ifset.2018.02.001. [DOI] [Google Scholar]
- Illera AE, Chaple S, Sanz MT, Ng S, Lu P, Jones J, Carey E, Bourke P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem: X. 2019;3:10049. doi: 10.1016/j.fochx.2019.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illera AE, Sanz MT, Beltrán S. Structural changes of a protein extract from apple with polyphenoloxidase activity obtained by cationic reversed micellar extraction induced by high-pressure carbon dioxide and thermosonication. Sci Rep. 2019;9:13749. doi: 10.1038/s41598-019-50209-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Patras A, Pokharel B, Wu Y, Vergne MJ, Shade L, Xiao H, Sasges M. UV-C irradiation as an alternative disinfection technique: study of its effect on polyphenols and antioxidant activity of apple juice. Innov Food Sci Emerg. 2016;34:344–351. doi: 10.1016/j.ifset.2016.02.009. [DOI] [Google Scholar]
- Juarez-Enriquez E, Salmeron-Ochoa I, Gutierrez-Mendez N, Ramaswamy HS, Ortega-Rivas E. Shelf life studies on apple juice pasteurised by ultrahigh hydrostatic pressure. LWT-Food Sci Technol. 2015;62(1):915–919. doi: 10.1016/j.lwt.2014.07.041. [DOI] [Google Scholar]
- Kwak SS, Kim SK, Lee MS, Jung KH, Park IH, Liu JR. Acidic peroxidases from suspension-cultures of sweet potato. Phytochemistry. 1995;39:981–984. doi: 10.1016/0031-9422(95)00098-R. [DOI] [Google Scholar]
- Liao XY, Li J, Muhammad AI, Suo YJ, Chen SG, Ye XQ, Liu DH, Ding T. Application of a dielectric barrier discharge atmospheric cold plasma (dbd-acp) for E. coli inactivation in apple juice. J Food Sci. 2018;83(2):401–408. doi: 10.1111/1750-3841.14045. [DOI] [PubMed] [Google Scholar]
- Ma HL, Pan ZL, Gao MX, Luo L. Efficacy in microbial sterilization of pulsed magnetic field treatment. Int J Food Eng. 2008;4(4):15. [Google Scholar]
- Marszalek K, Kruszewski B, Woźniak Ł, Skąpska S. The application of supercritical carbon dioxide for the stabilization of native and commercial polyphenol oxidases and peroxidases in cloudy apple juice (cv. Golden Delicious) Innov Food Sci Emerg. 2017;39:42–48. doi: 10.1016/j.ifset.2016.11.006. [DOI] [Google Scholar]
- Marszalek K, Szczepanska J, Starzonek S, Woźniak Ł, Trych U, Skąpska S, Saraiva JA, Rzoska S, Lorenzo JM, Barba FJ. Enzyme inactivation and evaluation of physicochemical properties, sugar and phenolic profile changes in cloudy apple juices after high pressure processing, and subsequent refrigerated storage. J Food Process Eng. 2019;42(4):e13034. doi: 10.1111/jfpe.13034. [DOI] [Google Scholar]
- Niu S, Xu Z, Fang Y, Zhang L, Yang Y, Liao X, Hu X. Comparative study on cloudy apple juice qualities from apple slices treated by high pressure carbon dioxide and mild heat. Innov Food Sci Emerg. 2010;11:91–97. doi: 10.1016/j.ifset.2009.09.002. [DOI] [Google Scholar]
- Ortuño C, Duong T, Balaban M, Benedito J. Combined high hydrostatic pressure and carbon dioxide inactivation of pectin methylesterase, polyphenol oxidase and peroxidase in feijoa puree. J Supercrit Fluids. 2013;82:56–62. doi: 10.1016/j.supflu.2013.06.005. [DOI] [Google Scholar]
- Qian JY, Zhou CS, Ma HL, Li SJ, Yagoub AEA, Abdualrahman MAY. Proteomics analyses and morphological structure of Bacillus subtilis inactivated by pulsed magnetic field. Food Biophys. 2016;11(4):436–445. doi: 10.1007/s11483-016-9444-5. [DOI] [Google Scholar]
- Qian JY, Zhou CS, Ma HL, Li SJ, Yagoub AEA, Abdualrahman MAY. Biological effect and inactivation mechanism of Bacillus subtilis exposed to pulsed magnetic field: morphology, membrane permeability and intracellular Contents. Food Biophys. 2016;11(4):429–435. doi: 10.1007/s11483-016-9442-7. [DOI] [Google Scholar]
- Qian JY, Zhang M, Dai CH, Huo SH, Ma HL. Transcriptomic analysis of Listeria monocytogenes under pulsed magnetic field treatment. Food Res Int. 2020;133:109195. doi: 10.1016/j.foodres.2020.109195. [DOI] [PubMed] [Google Scholar]
- Rawson A, Hossain MB, Patras A, Tuohy M, Brunton N. Effect of boiling and roasting on the polyacetylene and polyphenol content of fennel (Foeniculum vulgare) bulb. Food Res Int. 2013;50(2):513–518. doi: 10.1016/j.foodres.2011.01.009. [DOI] [Google Scholar]
- Schilling S, Alber T, Toepfl S, Neidhart S, Knorr D, Schieber A, Carle R. Effects of pulsed electric field treatment of apple mash on juice yield and quality attributes of apple juices. Innov Food Sci Emerg. 2007;8:127–134. doi: 10.1016/j.ifset.2006.08.005. [DOI] [Google Scholar]
- Solomon O, Vanberg US, Sahlstrom A. Food Chem. 1995;53:363–368. doi: 10.1016/0308-8146(95)99828-N. [DOI] [Google Scholar]
- Sun YJ, Zhong LZ, Cao LF, Lin WW, Ye XQ. Sonication inhibited browning but decreased polyphenols contents and antioxidant activity of fresh apple (malus pumila mill, cv. Red Fuji) juice. J Food Sci Technol. 2015;52(12):8336–8342. doi: 10.1007/s13197-015-1896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo S, Essel EA, Man SD, Bernaert N, Droogenbroeck BV, Grauwet T, Loey AV, Hendrickx M. comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov Food Sci Emerg. 2019;54:64–77. doi: 10.1016/j.ifset.2019.03.004. [DOI] [Google Scholar]
- Xiang QS, Liu XF, Li JG, Liu SN, Zhang H, Bai YH. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018;254:201–207. doi: 10.1016/j.foodchem.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Yi J, Kebede BT, Dang DNH, Buvé C, Grauwet T, Loey AV, Hu X, Hendrickx M. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT-Food Sci Technol. 2017;75:85–92. doi: 10.1016/j.lwt.2016.08.041. [DOI] [Google Scholar]


