Abstract
This study investigated the effect of high doses of monosodium glutamate (MSG), a known food additive on hepatic, memory and locomotor functions in mice, and the ameliorative potentials of Jobelyn® (JB), a unique dietary supplement. Twenty four male Swiss mice divided into 4 groups (n = 6) were given MSG (2, 4 and 8 g/kg) or normal saline (10 mL/kg) orally for 14 days. In the intervention study, another set of 30 male Swiss mice distributed into 5 groups (n = 6) received normal saline, MSG (8 g/kg) alone or in combination with JB (5, 10 and 20 mg/kg) orally, for 14 days. Memory and locomotor functions as well as brain oxido-nitrergic stress biomarkers were then assessed in both studies. The hepatic oxido-nitrergic stress biomarkers, liver enzymes functions and histomorphology of the liver were also assessed. MSG (2, 4 and 8 g/kg) produced memory dysfunction, hyperlocomotion, increased malondialdehyde and nitrite levels accompanied by decreased antioxidant status in the brain and hepatic tissues. MSG-treated mice had increased hepatic enzyme activities (alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase) and distorted cyto-architectural integrity of the liver. These findings further suggest that MSG compromised hepatic functioning, which might also contribute to its neurotoxicity. However, JB (5, 10 and 20 mg/kg, p.o) attenuated the memory deficit, hyperlocomotion, increased oxido-nitrergic stress responses in the brain and hepatic tissues induced by MSG (8 g/kg, p.o). JB also normalized hepatic enzymes activities and histomorphological changes in MSG-treated mice. Taken together, JB mitigated MSG-induced toxicity through mechanisms relating to enhancement of cellular antioxidant-machineries and normalization of hepatic enzymatic functions.
Keywords: Jobelyn®, Monosodium glutamate, Neurotoxicity, Hepatotoxicity, Oxido-nitrergic stress
Introduction
The unique flavo-savory-enhancing effect of monosodium glutamate (MSG) has earned it a worldwide recognition as one of the most extensively consumed food additives, common to restaurants and household menus [1, 2]. Monosodium glutamate [C5H8NO4NaH2O] is the non-essential sodium salt of L-glutamic amino acid occurring naturally in high abundance both in nature and in a variety of food /dairy products including milk, vegetables, meat, fish and cheese [2, 3]. Although, palatability, cost and availability may be the primary reasons for the widespread consumption of dietary MSG, the presumed ‘relative safety’ as adjudged by World Health Organization (WHO) and Food and Drug Administration (FDA) [4, 5] may perhaps be another reason for its wide spread consumption. Monosodium glutamate, generally regarded as safe (GRAS) by these regulatory bodies, has no fixed daily dose ratio [5], thus, its exact amount in various preparations flooding the markets lack manufacturer’s clarity leading to its disproportionate consumption [3]. In fact, the initial daily estimate (0.3–1 g) in terms of average daily intake of MSG per person in most industrialized and developing nations [3] has significantly increased in recent times to as high as 14 g in certain populations [5, 6].
The biosafety of MSG remain one of the most debated health-related issue in both scientific and public domains globally. In Nigeria, there are claims that MSG, widely sold as either Ajino-moto or Vedan, is a potent bleaching agent capable of erasing even tough stains from clothes [7]. This claim amongst others, further amplify the fears of the possible harmful effects of MSG. In fact, there are speculations that MSG facilitates the early formations of life threatening diseases especially in chronic consumers [1, 7]. Specifically, MSG has been reported to cause obesity [8], cellular hypertrophy, degeneration of male and female reproductive cells [9, 10], asthmatic-like airway irritations [11], thrombocytopenia [4], hyper-homocysteinemia and renal dysfunctions [12]. It is also known to cause MSG syndrome complex [4, 5], hepatic steatosis and cirrhosis [13, 14]. Besides, MSG has also been tagged ‘excitotoxin’ or ‘neurotoxin’ based on evidences of toxicity to several neuronal cells in distinct brain regions [5]. Studies have shown that MSG induces neurotoxicity, with several symptomatic manifestations such as depression, anxiety [1, 5], abnormal motor functions [15, 16] and cognitive deterioration [5].
In recent times, increased efforts to elucidate the exact molecular mechanisms underlying MSG-induced neurotoxicity are beginning to unravel the pathologic derangements including mechanisms relating to hypersensitization of specific glutamate taste receptors in gut-brain axis, impaired metabolism, abnormal generation of free radical moieties and inflammatory mediators [8, 17]. Meanwhile, food-containing MSG especially in high amounts have been reported to be highly palatable, thus enhancing craving in consumers [17]. Such increased cravings have been implicated in increased consumption of MSG resulting in abnormal spike (4.5–11 folds) in plasma glutamate concentration [5, 17] and consequently, its excessive bioaccumulation and damage to vital organs including the liver [1, 17]. It has been reported that impaired liver function plays a major role in the pathogenesis of several chronic diseases [1]. In fact, increased amount of circulating glutamate has been linked to cellular injury through calcium (Ca2+) and ammonium ions overload-induced formation of toxic reactive oxygen/nitrogen species (ROS/RNS) [1, 18]. Previous reports have indeed highlighted that most cellular/tissue injuries caused by MSG in experimental animals are consequences of severe activation of oxidative and inflammatory signaling pathways [5].
Harmoniously, as the scientific controversy about the mechanistic basis underlying MSG-induced neurotoxicity in humans increasingly become popular, agents that could mitigate its insults will be of public health significance. Accordingly, the uses of naturally occurring molecules with antioxidants, anti-inflammatory and neuroprotective activities against MSG-induced toxicities are the major focus of current preclinical investigations [19–21]. Jobelyn® (JB), commonly known as millet or guinea corn, is a dietary supplement derived from the leaf-sheaths of Sorghum bicolor [22, 23]. JB is one of the fastest selling dietary supplements in Nigeria owing to its wide spectrum of therapeutic usefulness and safety profiles [22]. JB first caught public attention following its clinical efficacy in boosting haemoglobin contents and immune functions [22, 23]. Pharmacological investigations have further shown that JB exhibits anti-amnesic, anti-depressant, antioxidant, anti-inflammatory and neuroprotective effects in rodents [22, 24–26]. These effects were attributed to its rich flavonoid-based polyphenolic phytochemicals such as luteolin, naringenin, apigenin, luteolinidins, apigeninidins and dimeric 3-deoxyanthocyanidin [22, 23]. Previous preclinical studies have also shown that JB mitigated the neurotoxic effects of scopolamine and attenuated neurodegeneration evoked by chronic alcohol treatment in rodents [24, 26]. However, this present study was designed to evaluate the effects of JB on high dose of MSG-induced neurotoxicity and hepatic dysfunctions in mice.
Materials and methods
Experimental animals
Adult male Swiss mice (28–34 g; 24 weeks old) were obtained from the Central Animal House, University of Ibadan, Ibadan, Nigeria, and housed five per plastic cage (42 × 30 × 27 cm) at a room temperature. They were allowed free access to pelletized rodent food and water ad libitum and acclimatized for 1 week prior to commencement of the experiments. The experimental procedures were approved by the University of Ibadan Animal Care and Use Research Ethics Committee (UI-ACUREC/App/16/0002). Also, efforts were made to minimize the suffering of the animals by careful handling during treatments and euthanization of the animals.
Drugs and chemicals procurements
Jobelyn®–JB (Health Forever Ltd, Lagos, Nigeria); Monosodium glutamate–MSG (Ajinomoto Co. Inc. Tokyo, Japan); Ellman Reagent [5′, 5′-Dithiosbis- (2-nitrobenzoate)–DTNB]; adrenaline (epinephrine), Tris-Potassium Chloride–KCl, Acetic acid- AA (Sigma-Aldrich, St. Loius, MO, USA); sodium bicarbonate, disodium phosphate, monosodium phosphate, dipotassium phosphate, potassium dichromate, hydrogen peroxide, (BDH Chemical Ltd, Poole, England); sodium Chloride–NaCl, sodium hydroxide (J.T Baker Chemicals Co., Phillipsburg, NJ, USA); trichloroacetic acid-TCA (Burgoyne Burbidges& Co., Mumbai, India); thiobabituric acid-TBA (Guanghua Chemical Factory Co. Ltd., China); and Agappe (Knonauerstrasse, 54- 6330, Cham, Switzerland GmbH) Diagnostic’s kits were used in this study.
Drug preparation and treatments
Monosodium glutamate (MSG) and Jobelyn® (JB) were freshly prepared by dissolution in normal saline as vehicle and were administered orally. The doses of MSG (2, 4 and 8 g/kg; p.o.) used in the study were selected based on previous scientific documentations [4, 12, 27]. Also, the doses of JB (5, 10 and 20 mg/kg; p.o) used in this study were selected based on previous investigations [24].
Treatment groupings
Male Swiss mice were randomly distributed into four (4) treatment groups (n = 6): group I (control) were treated with normal saline (10 mL/kg) while groups 2–4 received MSG (2, 4 or 8 g/kg) orally for 14 consecutive days. In the intervention studies, mice were also randomly distributed into 5 treatment groups (n = 6): group I (control) received normal saline (10 mL/kg), group 2 MSG (8 g/kg) while groups 3–5 had MSG (8 g/kg) + JB (5, 10 and 20 mg/kg), respectively. Neurobehavioral changes (memory, motor functions and rearing behavior) were evaluated on day 15 post-treatment between the hours of 8:00 am–12:00 pm by trained observers blinded to the treatments.
Memory function
Memory function was evaluated using the Y-maze test (YMT) as previously described [28]. The Y-maze apparatus is made up of a triple-arm chamber labeled A, B and C symmetrically separated at 120°. Each mouse was placed into arm A of the Y-maze apparatus and allowed to explore the three arms for 5 min. The parameters assessed in this test were frequency of arm entries and alternations (consecutive navigations across all three arms i.e. ABC, CAB or BCA but not BAB or ABA). Thereafter, the percentage (%) of correct alternation (an index of memory performance) was calculated as: (Total alternation number/Total number of arm entries–2) × 100 [29]. The maze was cleaned with 10% ethanol after each test to eliminate residual odor.
Locomotor activity and rearing behavior
Immediately after the test for memory function on day 15, the locomotor activity and rearing behavior were evaluated using the automated activity cage [30]. The animals were placed individually into the central arena of the activity cage. The horizontal movements (locomotor activity) and vertical movements (rearing behaviors) were recorded for 5 min. The activity cage was also cleaned with 10% ethanol after each test to eliminate residual odor.
Preparation of brain, liver and blood tissues for biochemical assays
Immediately after the behavioral testing, blood was collected via ocular puncture from three mice per group using capillary tubes and then centrifuged (3000 rpm; 10 min). The serum was stored at – 40 ºC. Thereafter, each animal was euthanized using diethyl ether, and the whole brain and liver organs, were removed, weighed, rinsed with 10% w/v sodium phosphate buffer; 0.1 M, 5 mL; pH 7.4), and homogenized using cold centrifuge (10,000 rpm, 4 ºC) for 15 min. The supernatants of each brain and liver tissue homogenates were stored for the different biochemical assays.
Biochemical assays
Oxidative and nitrergic status in brain and liver tissues of mice
The oxidative and nitrergic status in the brain and liver tissues of mice exposed to MSG alone or in combination with JB were assayed using spectrophotometric techniques. The supernatants of the brain or liver tissue were used to estimate the content (µmol/g protein) of reduced GSH [31], and activities of catalase and SOD [32, 33]. The activity of SOD and catalase were expressed in µmoles/min/mg protein and unit/mg protein, respectively. The concentrations of MDA (nmole/mg protein) and NO (µmol/mg protein) were determined in the supernatants of the brain or liver tissue using the thiobarturic method [34] and griess reagent [35], respectively. The protein content in each tissue was estimated using the Biuret reagent [36].
Liver enzymes assays
In the independent studies, immediately after defrosting frozen clear supernatants of sampled sera, indices of the serum enzyme [aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP)] activities were assayed spectrophotometrically using Randox Diagnostic’s kits (for AST and ALT) and Teco Diagnostic’s kits (for ALP), respectively, in accordance to the manufacturers’ instructions. The activities of the liver enzymes were expressed as UI/L units.
Liver serum proteins
The deleterious effects of MSG on the liver and the protective effect of JB were further assessed in mice by estimating the concentrations of liver serum proteins such as albumin, bilirubin and total protein. For albumin, the manufacturer’s (Agappe Diagnostic kits) assay protocol was employed as previously described [37]. Randox Diagnostic kits were used for the assay of bilirubin and total protein contents according to the manufacturer’s instructions. The contents of serum albumin and total proteins were expressed as g/dL and mg/dL for bilirubin.
Liver histomorphologic studies
After the behavioral tests, three mice in each group (n = 3) were euthanized using light ether, and perfused intracardially with formalin before the liver tissues were harvested. Standard routine procedures for embedded tissue paraffinization/deparaffinization in block media were utilized. Thereafter, representative portions of the harvested liver (centrilobular) tissue of the animals were sectioned (5–6 µm thick) transversally, using a microtome (Leica, Germany) and stained using Hematoxylin & Eosin (H & E) medium [38]. Stained tissue sections were each fixed-on glass slides for light microscopic evaluations of liver cyto-architectural alterations (density and size /shape). Images were then captured utilizing an Optronics Digital Camera connected to a computer interface (MagnaFire) and an Olympus BX-51 binocularmicroscope. An Inter-reader variability device was employed for characterizations of the general morphology of the liver (lobular, trabecular and portal regions) tissue. Also, the density of viable hepatocytes was quantified using Image J (X400 magnification) based on typical characteristics: absence of nuclear condensation distortion, intact cytoplasmic membrane and round/oval-shaped cells. The ratio of cellular viability to circularly viewed square area/section was calculated and used as determinant of hepatocytes densities.
Statistical analysis
The data obtained from the independent investigations were expressed as Mean ± SEM (standard error of mean) for each treatment group. Results were analyzed utilizing one-way analysis of variance (ANOVA); and Bonferroni post-hoc test was done to determine the source of significant mean effect using GraphPad Prism software (Version 5.0, USA). The level of significance for all tests was set at p < 0.05.
Results
MSG impairs spatial working memory in Y-maze test and effect of JB intervention
The effect of MSG on spatial working memory is depicted in Fig. 1a. As shown in Fig. 1a, mice treated with oral doses of MSG (2–8 g/kg) once daily for 14 days exhibited memory deficits as evidenced by a significant (p < 0.05) [F (3,16) = 15.50, p < 0.0001] decrease in % correct alternations in a dose-related manner. JB (5, 10 and 20 mg/kg, p.o.) treatment significantly (p < 0.05) [F (4,20) = 32.84, p < 0.0001] attenuated MSG (8 g/kg, p.o.)–induced memory decline as shown by increased % correct alternation (Fig. 1b).
Fig. 1.
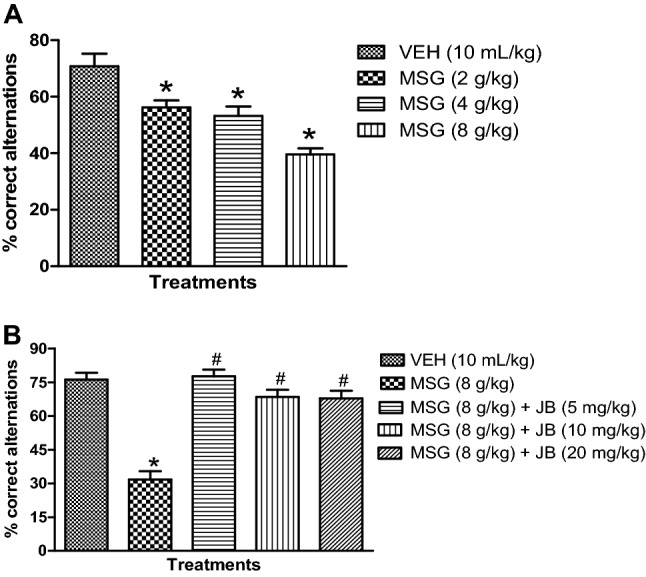
Monosodium impairs spatial working memory (a) and ameliorating effect of Jobelyn® intervention (b) in mice. Each column represents the mean ± SEM for 3 animals per group. *p < 0.05 as compared to vehicle group; #p < 0.05 as compared to MSG (8 g/kg) (one-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
MSG elicits hyperlocomotion and its attenuation by JB
As shown in Fig. 2b, daily oral administration of MSG (2–8 g/kg, p.o.) induced hyperlocomotion as depicted by a significant (p < 0.05) [F (3,16) = 33.21, p < 0.0001] increase in horizontal activity counts/5 min in the automated activity cage relative to control. However, daily doses of JB (5, 10 and 20 mg/kg, p.o.) for 14 days significantly (p < 0.05) [F (4,20) = 27.92, p < 0.0001] suppressed MSG (8 g/kg, p.o.)-induced hyperlocomotion as indexed by decreased horizontal activity counts/5 min (Fig. 2b).
Fig. 2.
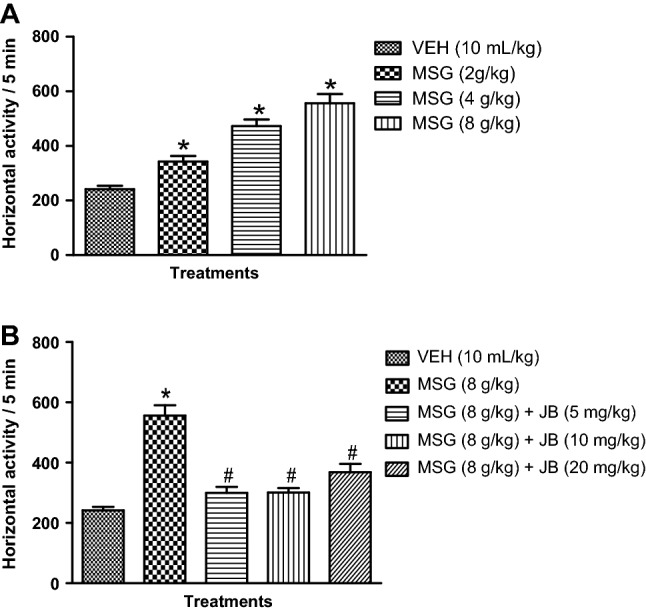
Monosodium glutamate produces hyperlocomotion (a) in mice and the inhibitory effect of Jobelyn® (b). Each bar represents the mean ± SEM of 3 animals / group. *p < 0.05 as compared to vehicle group; #p < 0.05 as compared to MSG (8 g/kg) (one-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
MSG increases rearing activity and its suppression by JB intervention
Daily oral administration of MSG (2–8 g/kg, p.o.) produced a significant (p < 0.05) [F (3,16) = 52.37, p < 0.0001] dose-dependent spike in the frequency of rearing behavior shown by increased vertical activity/5 min in the automated activity when compared with control (Fig. 3a). As presented in Fig. 3b, daily oral doses of JB (5, 10 and 20 mg/kg) for 14 days significantly (p < 0.05) [F (4,20) = 47.05, p < 0.0001] reduced the frequency of rearing behavior induced by MSG (8 g/kg, p.o.).
Fig. 3.
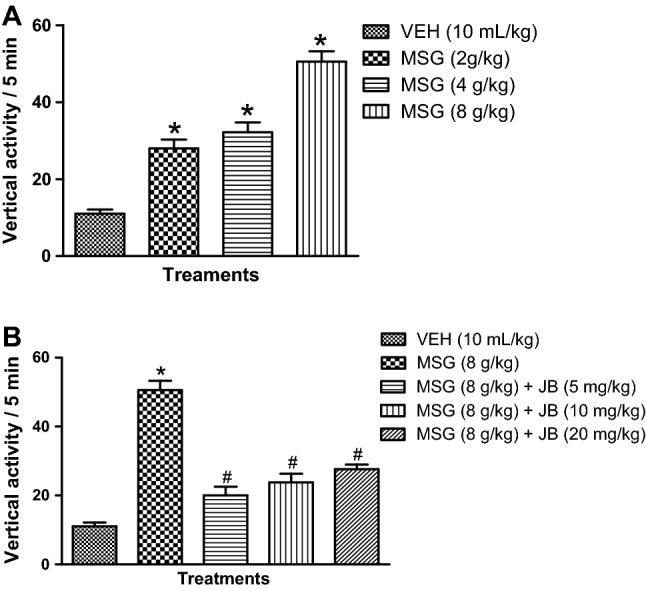
Monosodium glutamate increases rearing behavior (a) in mice and inhibitory effect of Jobelyn® (b). Each bar represents the mean ± SEM of three animals/group. *Denotes p < 0.05 as compared to vehicle group; #Denotes p < 0.05 as compared to MSG (8 g/kg) (one-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
MSG induces oxido-nitrergic stress in mouse brain and effects of JB intervention
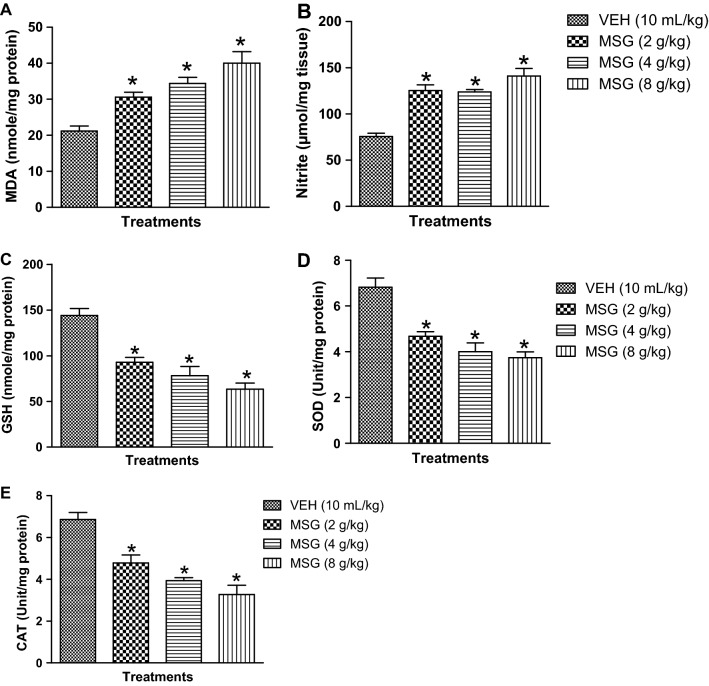
The effects of MSG on biomarkers of oxidative and nitrergic stresses in mouse brain are shown in Fig. 4a–d. As presented in Fig. 4a–d, administration of oral doses of MSG (2–8 mg/kg) for 14 days caused a dose-dependent elevation of MDA [F (3,16) = 14.74, p < 0.0001] (Fig. 4a) and nitrite [F (3,16) = 55.21, p < 0.0001] (Fig. 4b) contents accompanied by depletion of endogenous antioxidant status [reduced GSH [F (3,16) = 21.08, p < 0.0001] (Fig. 4c), SOD [F (3,16) = 18.67, p < 0.0001] (Fig. 4d) and catalase, CAT [F (3,16) = 20.70, p < 0.0001] (Fig. 4e) in mouse brain when compared with control (p < 0.05). As shown in Table 1, daily administration of JB (5, 10 and 20 mg/kg, p.o) for 14 days reduced MDA [F (4,20) = 47.05, p < 0.0001] and nitrite [F (4,20) = 47.05, p < 0.0001] contents, and boosted the antioxidant defense armory of the brain tissues of mice exposed to MSG (8 g/kg, p.o.).
Fig. 4.
Effect of monosodium glutamate on oxido-nitrergic stress biomarkers: MDA (a), nitrite (b), GSH (c), SOD (d) and CAT (e) in mouse brain. Each column represents the mean ± SEM for 3 animals per group. *p < 0.05 compared to control group (one-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, MSG monosodium glutamate
Table 1.
Effect of Jobelyn® on monosodium glutamate-induced oxidative and nitrergic changes in mouse brain
| Treatments groups | GSH (nmole/mg protein) | SOD (Unit/mg protein) | CAT (Unit/ mg protein) | MDA (nmole/mg protein) | Nitrite (µmol/mg protein) |
|---|---|---|---|---|---|
| VEH (10 mL/kg) | 144.0 ± 7.79 | 6.82 ± 0.40 | 3.740 ± 0.26 | 21.20 ± 1.56 | 75.60 ± 3.56 |
| MSG (8 g/kg) | 63.40 ± 6.64* | 3.74 ± 0.26* | 3.74 ± 0.26* | 40.00 ± 3.24* | 140.0 ± 5.12* |
| MSG (8 g/kg) + JB (5 mg/kg) | 104.6 ± 3.54# | 7.36 ± 0.22# | 7.36 ± 0.22# | 18.00 ± 1.14# | 73.60 ± 7.19# |
| MSG (8 g/kg) + JB (10 mg/kg) | 100.4 ± 4.94# | 5.54 ± 0.35# | 5.54 ± 0.35# | 21.80 ± 1.72# | 86.20 ± 5.78# |
| MSG (8 g/kg) + JB (20 mg/kg) | 92.20 ± 8.13# | 5.20 ± 0.20# | 5.20 ± 0.20# | 21.60 ± 0.68# | 97.80 ± 6.67# |
Values represents the mean ± SEM of grouped mice (n = 3). *p < 0.05 compared to vehicle group. #p < 0.05 compared to MSG (8 g/kg) (one-way ANOVA followed by Bonferroni post-hoc test)
VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
MSG increases oxido-nitrergic stress in liver tissues and the defensive effect confers by JB
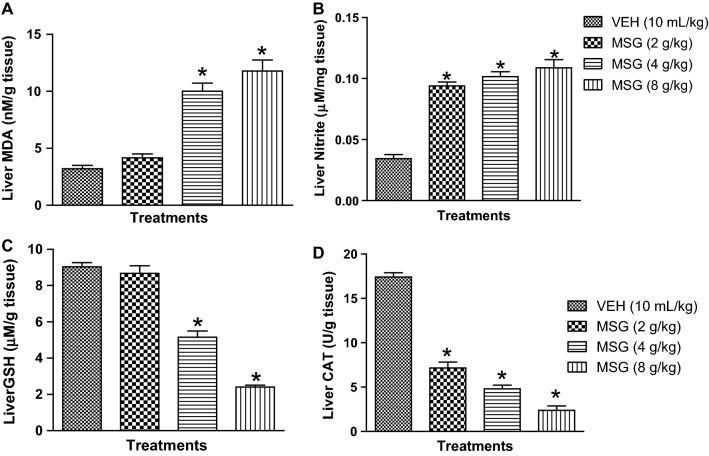
Figure 5a–d showed that oral administration of MSG (4–8 g/kg) significantly (p < 0.05) elevated the liver contents of MDA [F (3,16) = 43.23, p < 0.0001] and nitrite [F (3,16) = 56.57, p < 0.0001] followed by depletion of GSH [F (3,16) = 106.1, p < 0.0001] and CAT [F (3,16) = 162.6, p < 0.0001] relative to control. Meanwhile, JB (5, 10 and 20 mg/kg, p.o) significantly (p < 0.05) attenuated MSG (8 g/kg, p.o.)-induced increase in MDA [F (4,20) = 32.40, p < 0.0001] and nitrite [F (4,20) = 31.02, p < 0.0001] contents and boosted the antioxidant machineries, GSH [F (4,20) = 48.68, p < 0.0001] and CAT [F (4,20) = 85.29, p < 0.0001] of the liver tissues (Table 2).
Fig. 5.
Effects of monosodium glutamate on oxido-nitrergic stress biomarkers: MDA (a), nitrite (b), GSH (c) and CAT (d) in mouse liver tissue. Each column represents the mean ± SEM for three animals per group. *p < 0.05 compared to control group (one-way ANOVA followed by Bonferroni post-hoc test). VEH: Vehicle, MSG: Monosodium glutamate
Table 2.
Effects of Jobelyn® on monosodium glutamate-induced oxidative stress and nitrergic changes in mouse liver tissue
| Treatments groups | GSH (nmole/mg protein) | CAT (Unit/ g protein) | MDA (nmole/g protein) | Nitrite (µmol/g protein) |
|---|---|---|---|---|
| VEH (10 mL/kg) | 9.03 ± 0.23 | 17.42 ± 0.48 | 3.20 ± 0.30 | 0.03 ± 0.003 |
| MSG (8 g/kg) | 2.404 ± 0.11* | 2.40 ± 0.48* | 11.78 ± 0.90* | 0.11 ± 0.001* |
| MSG (8 g/kg) + JB (5 mg/kg) | 7.63 ± 0.63# | 12.96 ± 0.43# | 5.40 ± 0.69# | 0.09 ± 0.003# |
| MSG (8 g/kg) + JB (10 mg/kg) | 7.87 ± 0.34# | 12.74 ± 0.96# | 3.97 ± 0.44# | 0.10 ± 0.007# |
| MSG (8 g/kg) + JB (20 mg/kg) | 8.11 ± 0.35# | 13.69 ± 0.40# | 6.51 ± 0.27# | 0.06 ± 0.005# |
Values represents the mean ± SEM of grouped mice (n = 3). *Denotes p < 0.05 compared to vehicle group. #Denotes p < 0.05 compared to MSG (8 g/kg) (ANOVA followed by Bonferroni test)
VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
MSG elevates liver enzymes of mice and inhibitory effect of JB
As presented in Fig. 6a–c, MSG (2–8 g/kg, p.o.) produced a significant (p < 0.05) perturbations in liver enzymes functioning as shown by elevated ALT [F (3,20) = 67.97, p < 0.0001], AST [F (3,20) = 84.17, p < 0.0001]and ALP [F (3,20) = 40.04, p < 0.0001] activities in comparison with controls. However, oral treatment with JB (5, 10 and 20 mg/kg, p.o) significantly (p < 0.05) restored ALT [F (4,25) = 124.3, p < 0.0001], AST [F (4,25) = 112.7, p < 0.0001] and ALP [F (4,25) = 41.60, p < 0.0001] activities in the liver of MSG (8 g/kg)-treated mice (Table 3).
Fig. 6.
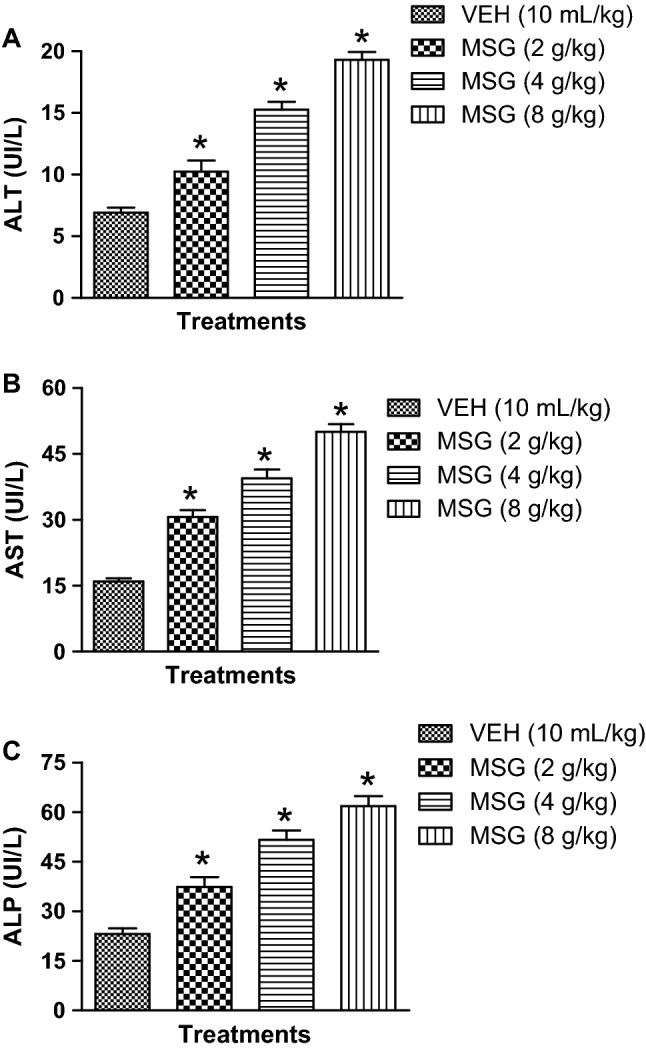
Monosodium glutamate elevates liver enzymes activities: ALT (a), AST (b) and ALP (c) in mice. Each bar represents the mean ± SEM for three animals per group. *p < 0.05 compared to control group (one-way ANOVA followed by Bonferroni post-hoc test).VEH: Vehicle, MSG: Monosodium glutamate
Table 3.
Jobelyn® attenuates monosodium glutamate-induced increase in activity of liver enzymes in mice
| Treatments groups | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|
| VEH (10 mL/kg) | 6.92 ± 0.40 | 15.98 ± 0.69 | 23.17 ± 1.69 |
| MSG (8 g/kg) | 19.33 ± 0.61* | 50.04 ± 1.75* | 61.82 ± 2.95* |
| MSG (8 g/kg) + JB (5 mg/kg) | 9.19 ± 0.39# | 23.47 ± 0.81# | 31.36 ± 2.42# |
| MSG (8 g/kg) + JB (10 mg/kg) | 9.34 ± 0.25# | 20.58 ± 1.17# | 33.46 ± 2.30# |
| MSG (8 g/kg) + JB (20 mg/kg) | 10.85 ± 0.41# | 22.78 ± 1.57# | 29.08 ± 2.13# |
Each value represents the mean ± SEM of grouped mice (n = 3). *p < 0.05 compared to vehicle group, #p < 0.05 compared to MSG (8 g/kg) (one-way ANOVA followed by Bonferroni post-hoc test)
VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
Monosodium glutamate alters liver protein contents and the protective effects of JB
The effect of MSG on liver proteins (albumin, bilirubin and total protein) contents is depicted in Fig. 7a–c. As shown in Fig. 7a–c, MSG (2–8 g/kg, p.o.) given daily for 14 days produced a significant (p < 0.05) depletion of liver albumin [F (3,20) = 12.14, p < 0.0001], total protein [F (3,20) = 14.92, p < 0.0001] concentrations but elevated bilirubin [F (3,20) = 31.14, p < 0.0001] levels in the serum of mice relative to controls (Fig. 7a-c). However, Table 4 showed that oral treatments with JB (5, 10 and 20 mg/kg, p.o.) ameliorated the MSG (8 g/kg, p.o)-induced abnormal levels of liver bilirubin [F (4,25) = 79.03, p < 0.0001], albumin [F (4,25) = 10.07, p < 0.0001] and total proteins [F (4,25) = 10.75, p < 0.0001] contents (Table 4).
Fig. 7.
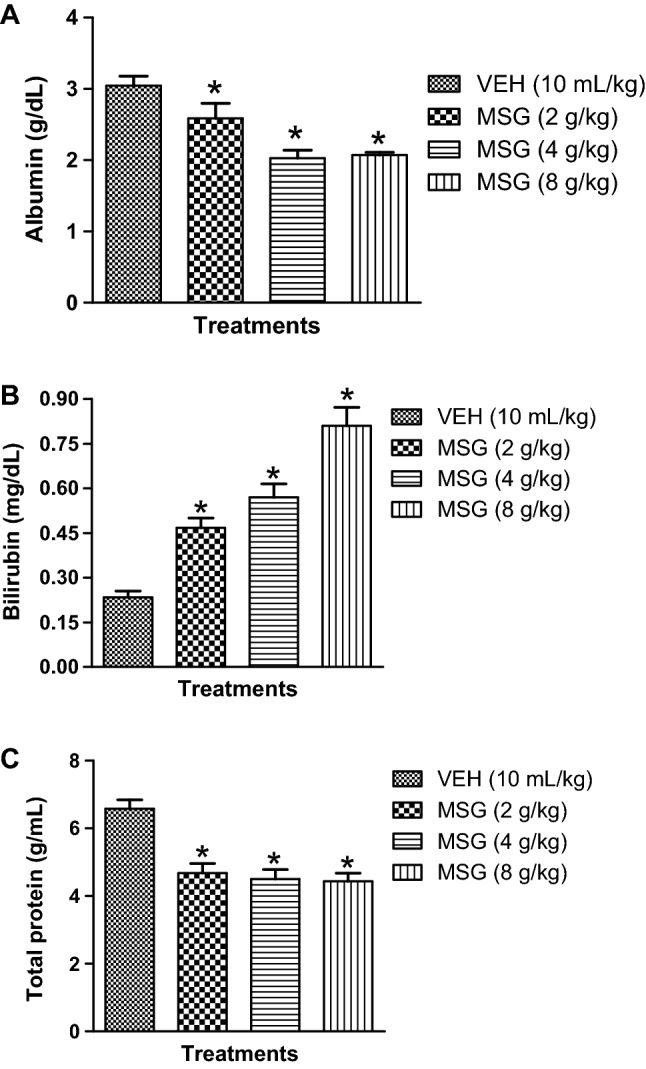
Monosodium glutamate alters liver protein contents: albumin (a), bilirubin (b) and total protein (c) in serum of mice. Each bars represent the mean ± SEM for three animals per group. *p < 0.05 compared to vehicle (control) group (one-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, MSG monosodium glutamate
Table 4.
Effect of Jobelyn® on monosodium glutamate-induced alterations of liver proteins in mice serum
| Treatments groups | Bilurubin (mg/dL) | Albumin (g/dL) | Total protein (g/mL) |
|---|---|---|---|
| VEH (10 mL/kg) | 0.23 ± 0.02 | 3.05 ± 0.13 | 6.59 ± 0.26 |
| MSG (8 g/kg) | 0.97 ± 0.02* | 2.07 ± 0.04* | 3.44 ± 0.24* |
| MSG (8 g/kg) + JB (5 mg/kg) | 0.53 ± 0.02# | 2.83 ± 0.10# | 6.42 ± 0.27# |
| MSG (8 g/kg) + JB (10 mg/kg) | 0.57 ± 0.02# | 2.77 ± 0.19# | 6.00 ± 0.38# |
| MSG (8 g/kg) + JB (20 mg/kg) | 0.58 ± 0.30# | 2.95 ± 0.15# | 6.00 ± 0.04# |
Values represents the mean ± SEM of grouped mice (n = 3). *p < 0.05 compared to vehicle group. #p < 0.05 compared to MSG (8 g/kg) (one-way ANOVA proceeded by Bonferroni post-hoc test)
VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
Jobelyn®confers cyto-protection on mice liver tissue treated with monosodium glutamate
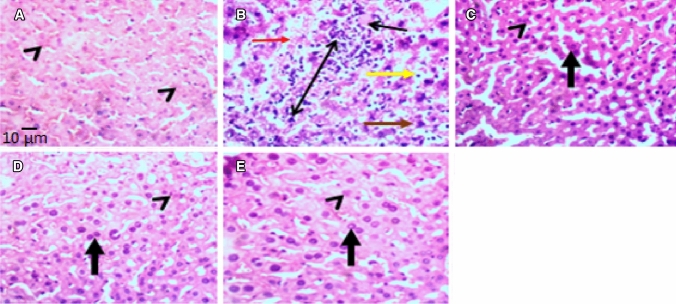
The photomicrographs of the effects of JB on MSG-induced histomorphologic (density of viable cells) changes in mice liver tissues are shown in Fig. 8. Hematoxylin and Eosin (H&E) staining revealed that daily administration of MSG (8 g/kg, p.o.) for 14 days produced cyto-architectural perturbations in the centrilobular (zone 3) regions of the liver as evidenced by the increased diffused zone 3 hepatocellular degeneration (kupffer cell hyperplasia, vacuolar degeneration, necrotic coagulation and neutrophilic cellular infiltrates) (Fig. 8). As shown in Fig. 9a–c, MSG also reduced the population of viable hepatocyte counts [F (4,10) = 19.74, p < 0.0001] and caused atrophy (decreased diameter) of hepatic cord [F (4,10) = 8.165, p < 0.0001] and central vein [F (4,10) = 7.591, p < 0.0001] relative to control. However, JB (5, 10 and 20 mg/kg, p.o) treatment attenuated the MSG (8 g/kg, p.o.)-induced cytoarchitectural distortions of the liver tissues of mice (Fig. 8). Besides, JB also significantly (p < 0.05) increased the population of viable cells, diameters of hepatic cord, and central vein in the centrilobular (zone 3) region of the liver of the MSG-treated mice (Fig. 9a–c).
Fig. 8.
Representative photomicrographs (H & E staining) of the effect of Jobelyn® on monosodium glutamate-induced hepatotoxicity in the centrilobular (zone 3) region of mice liver. a = Vehicle (10 mL/kg), b = MSG (8 g/kg), c = MSG + JB (5 mg/kg), d = MSG + JB (10 mg/kg), and e = MSG + JB (20 mg/kg). a revealed normal histomorphometric orientations of viable hepatocytes with intact hepatic cord and vein (arrow head). b exhibited decreased population of viable hepatocytes, atrophy of hepatic cord and central vein (red arrow), kupffer cell hyperplasia (double-head arrow), cytoplasmic vacuolar degeneration (yellow arrow), necrotic coagulation (brown arrow) and neutrophilic cellular infiltrates (black single-head arrow). c, d, e showed population of viable hepatocytes with normal hepatic cord and central vein (arrow head) and binucleation (regeneration) of hepatocytes (thick vertical arrows). Mag = × 400; Scale bar for all figures = 0.01 mm (10 µm)
Fig. 9.
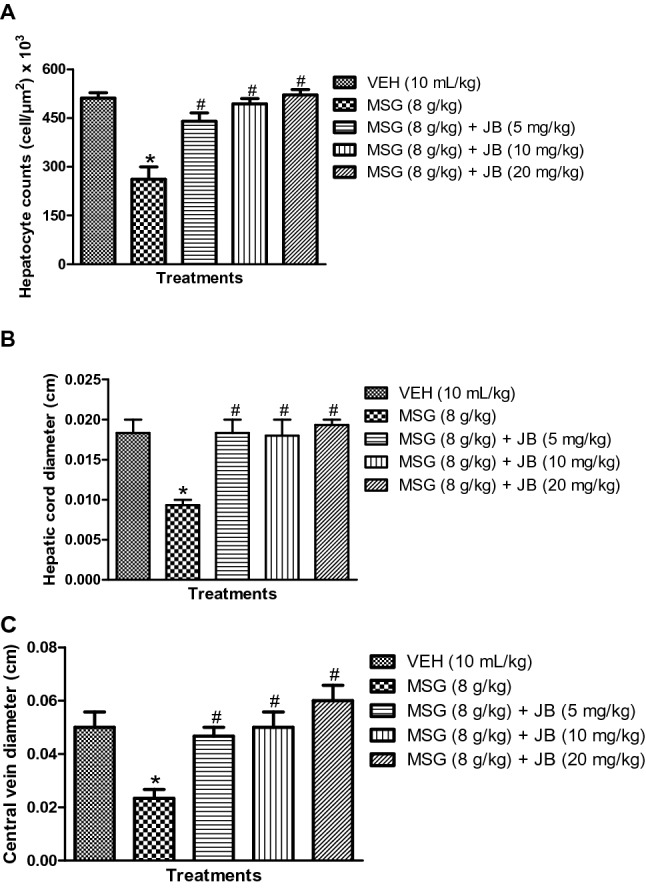
Jobelyn® attenuates monosodium glutamate-induced loss of liver cell viability: hepatocyte counts (a), hepatic cord diameter (b) and central vein diameter (c) of mice. Bars represent the mean ± SEM of grouped mice (n = 3). *p < 0.05 compared with vehicle group; #p < 0.05 compared to MSG (8 g/kg) (one-way ANOVA proceeded by Bonferroni post-hoc test). VEH vehicle, MSG monosodium glutamate, JB Jobelyn®
Discussion
The results of this study showed that repeated administration of high doses of MSG impaired spatial working memory, enhanced locomotion and rearing behavior in mice. However, pretreatment with JB improves memory function and attenuated hyperlocomotion, and abnormal rearing behavior in mice exposed to oral dose of 8 mg/kg of MSG. In the biochemical studies, MSG caused a dose-dependent elevation in the concentrations of MDA and nitrite accompanied by profound depletion of endogenous antioxidant-protective substances in the brain and liver tissues of mice indicating the involvement of oxidative/nitrergic pathways in its toxic effects. Meanwhile, JB attenuated the oxidative and nitrergic insults as evidenced by the profound suppression of MDA and nitrite levels followed by increased antioxidant defense armory of the brain and liver cells of mice treated with MSG (8 g/kg, p.o.). Studies have shown that compromised hepatic function promotes the neurotoxic effect of MSG by increasing its brain concentrations [38, 39]. Hence, further investigations focused on specific biomarkers of liver damage such as enzymes functioning, serum proteins (albumin, bilirubin and total proteins) profiles and hepatic histomorphologic features were evaluated. Our data showed that MSG caused pathologic perturbations in liver enzymes as shown by elevated ALT, AST and ALP activities. MSG further altered the liver protein (albumin, bilirubin and total protein) contents and distorted the cyto-architectural integrity of the liver tissues of mice, which further suggest hepatotoxicity. Interestingly, JB pretreatment restores the deregulated hepatic enzymes functioning, serum protein contents and cyto-architectural changes in MSG-treated mice suggesting hepatoprotective activity.
The YMT is an animal paradigm employed routinely for screening of novel compounds with memory boosting effect in rodents [28]. This test relies wholly on the innate capability of rodents to recognize the sequence of arms entries (also known as spontaneous alternations) [28]. Voluntary visitations by mice into a choice-arm at any given time is a decisive functional activity generally believed to be held in specific brain neural circuits relevant to learning and memory processes [40]. Thus, the avoidance of the last arm recently visited, is an indication that the animal has this information in its working memory, making this behavioral task, a model for short-term memory [28, 29]. In the dose finding study, administration of high doses of MSG impaired memory performance in mice as shown by reduced correct alternations in the YMT. Interestingly, we have earlier reported that low doses (0.1–0.5 g/kg) of orally administered MSG did not exert any significant alterations in alternation behaviors of mice [1], which was also in agreement with literature [41]. However, studies that employed 2 g/kg [42] or 3 g/kg [43] of oral dose of MSG reported impaired memory function. In line with the later, our findings further revealed that MSG-induced memory deteriorations, and this may in part, have a direct proportionality to its dose and duration of administration. However, in the intervention studies, JB ameliorated MSG-induced memory deficits as shown by increased correct alternation behavior in mice. This finding further confirmed earlier preclinical studies, which showed that JB improved memory deficits induced by scopolamine and attenuated neurodegeneration evoked by chronic alcohol treatment in rodents [24].
The automated activity cage was used in assessing the effect of JB on MSG-induced motor dysfunctions in mice. Our results showed that high doses of MSG caused hyperlocomotion and increased rearing activity. It is worthy of note that various studies have shown that MSG produced divergent effects on motor function, namely hyperactivity, hypo-activity or no significant effect [16, 41, 42]. The reports of Seo et al. [16] showed that MSG produced a time-dependent bi-phasic modulation of locomotor function; an initial nonspecific spike of exploratory motor activity, preceded by retarded voluntary motor movements, with some stereotypic manifestations. However, the results of Quines et al. [15] are in agreement with our findings, which showed that MSG caused hyperactivity in rats as revealed by increase in the frequency of rearing, velocity and total distance travelled. These behavioral changes have been attributed to MSG-mediated imbalance between glutamate and GABA transmitters in the brain [44]. Specifically, MSG-induced hyperactive behavior has been linked to presynaptic glutamate-triggered Ca2+ release and increased sympathetic discharge due to GABAergic disinhibition [45]. However, in the intervention studies, JB attenuated the hyperlocomotion and increased rearing activity induced by MSG in mice, which further suggest its potential benefit in ameliorating the deleterious effects of this widely consumed food additive. Nevertheless, further studies are needed to pinpoint the mechanistic role of glutamate transmitter in the ability of JB to mitigate MSG-induced neurotoxicity.
Previous studies have shown that MSG induced tissue injury via increased cellular oxidative and nitrergic perturbations [1, 20], which might be connected with glutamate excitotoxicity [18]. In agreement with previous findings [16, 20], our data showed that high doses of MSG produced abnormal increases in MDA and NO contents followed by depletion of antioxidant-protective machineries (GSH, SOD and CAT) in the brain and liver tissues of mice suggesting increase in oxido-nitrergic stress. Several studies have reported copious hepatocellular injuries including steatosis, fibrosis and chronic hepatitis, attributable to MSG-mediated formation of oxido-nitrergic moieties [46, 47]. Therefore, the increased lipid pro-oxidant (MDA and NO) activities coupled with depletion of antioxidant status of the liver of MSG-treated mice indicate hepatic tissue injury. However, JB intervention resulted in sharp fall in MDA and NO with corresponding increased antioxidant status of the brain and liver tissues of MSG-treated mice, which suggest neuroprotection and hepatoprotection. Meanwhile, previous studies have also shown that JB attenuated scopolamine-induced memory deficit via suppression of oxidative stress in mice brains [24]. Thus, the ability of JB to mitigate MSG-induced memory dysfunction may be related to the inhibition of oxidative and nitrergic pathways and augmentation of antioxidant systems.
There are several evidences suggesting that compromised liver functions contribute substantially to neurotoxicity of chemical toxicants [48, 49]. Thus, the quantification of liver enzymes (ALT, AST and ALP) functioning and proteins (bilirubin, albumin and total proteins) contents as biomarkers of liver injuries [39] was carried out in this study. Indeed, several investigations have shown that MSG-induced organ toxicities in rodents were closely associated with abnormal serum ALT, AST and ALP activities [1, 19, 46]. Monosodium glutamate was also shown to increased bilirubin concentrations and depleted albumin and total proteins [50] contents, which further indicate hepatotoxicity. Consistent with these findings, our data also revealed that high doses of MSG induced liver damage as shown by increased hepatic transaminases (ALT, AST) and phosphatase (ALP) enzymes. Besides, MSG also increased hepatic bilirubin and reduced albumin and total protein contents, which further suggest hepatotoxicity in mice. The histomorphological studies further confirmed the hepatotoxicity of high doses of MSG, as shown by the distortions of the cyto-architecture of the liver. Moreover, previous studies have also established reduced viable hepatocytes, atrophy of hepatic cord, hepatic parenchyma edema, multiple pyknosis, vacuolar degeneration, necrotic coagulation, neutrophilic cellular infiltrates, massive lymphocytic/macrophage exudation around portal regions and hyper-proliferated bile ducts in MSG-treated animals [14, 51]. It is generally believed that MSG-induced hepatic dysfunction, which may in turn aid its neurotoxicity in experimental animals [52]. Moreover, micromolar concentrations of plasma bilirubin and reduced albumin/total protein contents in circulation have been found to promote neurotoxicity [52, 53]. Meanwhile, in the intervention study, JB attenuated MSG-induced deregulations of serum liver enzymes (ALT, AST and ALT) and proteins (bilirubin, albumin and total protein) contents suggesting hepatoprotection. The findings that JB abrogated the MSG-induced pathologic cyto-architectural changes of mice liver tissue as shown by increased population of viable hepatocyte (density count), hepatic cord/central vein (diameter size), and increased hepatocytic binucleation, suggests a remediated regeneration. This hepatoprotective activity of JB may be related in part to its acclaimed anti-oxidant flavonoid-based polyphenolic constituents [23, 54], although more in-depth molecular investigations are strongly recommended to justify this assumption.
It may be concluded from the results obtained from this study that high doses of monosodium glutamate impairs memory, motor activity and hepatic functions in mice via induction of oxido-nitrergic stress and impaired hepatic enzyme functioning and structural integrity. This study also identified the beneficial effects of JB administration in mitigating the toxic effects of MSG by augmenting the cellular antioxidant defense machineries.
Acknowledgements
The authors thank the technical staff of the Department of Pharmacology and Therapeutics, University of Ibadan for their assistance.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ALP
Alkaline phosphatase
- CAT
Catalase
- GSH
Glutathione
- JB
Jobelyn®
- MSG
Monosodium glutamate
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
Compliance with ethical standards
Conflict of interest
Authors have no potential conflict of interest to declare.
References
- 1.Umukoro S, Oluwole OG, Olamijowon HE, Omogbiya AI, Eduviere AT. Effect of monosodium glutamate on behavioral phenotypes, biomarkers of oxidative stress in brain tissues and liver enzymes in mice. W J Neurosci. 2015;5:1–11. doi: 10.4236/wjns.2015.55033. [DOI] [Google Scholar]
- 2.Shredah MT. Molecular study to the effect of monosodium glutamate on rat gingiva. Tanta Dent J. 2017;14:155–163. doi: 10.4103/tdj.tdj_21_17. [DOI] [Google Scholar]
- 3.Wijayasekara K, Wansapala J. Uses, effects and properties of monosodium glutamate (MSG) on food & nutrition. Int J Food Sci Nutr. 2017;2:132–143. [Google Scholar]
- 4.Husarova V, Ostatnikova D. Monosodium glutamate toxic effects and their implications for human intake: a review. J Med Res. 2013;2013:608765. doi: 10.5171/2013.608765. [DOI] [Google Scholar]
- 5.Moneim WMA, Yassa HA, Makboul RA, Mohamed NA. Monosodium glutamate affects cognitive functions in male albino rats. Egypt J Forensic Sci. 2018;8:1–10. doi: 10.1186/s41935-018-0038-x. [DOI] [Google Scholar]
- 6.Onaolapo OJ, Onaolapo AY, Akanmu MA, Gbola O. Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology. 2016;23:147–156. doi: 10.1016/j.pathophys.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Gbore FA, Olumomi OR, Aworetan IM, Gabriel-Ajobiewe RAO. Oral administration of monosodium glutamate alters growth and blood parameters in female rabbits. Eur J Biol Res. 2016;6:218–225. doi: 10.5281/zenodo.150297. [DOI] [Google Scholar]
- 8.Khalaf HA, Arafat EA. Effect of different doses of monosodium glutamate on the thyroid follicular cells of adult male albino rats: a histological study. Int J Clin Exp Pathol. 2015;8:15498–15510. [PMC free article] [PubMed] [Google Scholar]
- 9.Hamza RZ, AL-Harbi MS. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol Rep. 2014;1:1037–1045. doi: 10.1016/j.toxrep.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondal M, Sarkar K, Nath PP, Khatun A, Pal S, Paul G. Monosodium glutamate impairs the contraction of uterine visceral smooth muscle ex vivo of rat through augmentation of acetylcholine and nitric oxide signaling pathways. Reprod Biol. 2018;18:83–93. doi: 10.1016/j.repbio.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda J, Chin K, Torii K, Sakai R. Effects of oral monosodium glutamate in mouse models of asthma. Food Chem Toxicol. 2011;49:299–304. doi: 10.1016/j.fct.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Sharma J, Singh K, Ahluwalia P, Kaur AP. Alteration in serum homocysteine levels upon ingestion of monosodium glutamate (MSG) to adult male rats. J Life Sci. 2009;1:103–105. doi: 10.1080/09751270.2009.11885140. [DOI] [Google Scholar]
- 13.de Oliveira MC, Torrezan R, da Costa CEM, Ambiel CR, Constantin RP, Ishii-Iwamoto EL, Salgueiro-Pagadigorri CL. Changes in calcium fluxes in mitochondria, microsomes, and plasma membrane vesicles of livers from monosodium L-glutamate–obese rats. Metab Clin Exp. 2011;60:1433–1441. doi: 10.1016/j.metabol.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 14.AL-Mosaibih MA. Effects of monosodium glutamate and acrylamide on the liver tissue of adult Wistar rats. Life Sci J. 2013;10:35–42. [Google Scholar]
- 15.Quines CB, Rosa SG, Velasquez D, Da Rocha JT, Neto JSS, Nogueira CW. Diphenyl diselenide elicits antidepressant-like activity in rats exposed to monosodium glutamate: a contribution of serotonin uptake and Na+, K+-ATPase activity. Behav Brain Res. 2016;301:161–167. doi: 10.1016/j.bbr.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Seo HJ, Ham H, Jin HY, Lee WH, Hwang HS, Park S, Kim YS, Choi SC, Lee S, Oh KJ, Kim BS, Park BR, Lee MY. Chronic administration of monosodium glutamate under chronic variable stress impaired hypothalamic- pituitary-adrenal axis function in rats. Korean J Physiol Pharmacol. 2010;14:213–221. doi: 10.4196/kjpp.2010.14.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torii K. Brain activation by the umami taste substance monosodium L-glutamate via gustatory and visceral signaling pathways, and its physiological significance due to homeostasis after a meal. J Oral Biosci. 2012;54:144–150. doi: 10.1016/j.job.2012.03.005. [DOI] [Google Scholar]
- 18.Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashry MA, Abd-Ellah HF, Gheth EMM (2012) The possible ameliorative effect of propolis in rat’s liver treated with monosodium glutamate (MSG). Nat Sci 10:209–219. http://research.asu.edu.eg/handle/123456789/1566
- 20.Hussein UK, Hassan NEY, Elhalwagy MEA, Zaki AR, Abubakr HO, Venkata KCN, Jang KY, Bishayee A. Ginger and propolis exert neuroprotective effects against monosodium glutamate-induced neurotoxicity in rats. Molecules. 2017;22:1928. doi: 10.3390/molecules22111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramalho JB, Izaguirry AP, Soares MB, Spiazzi ZZ, Pavin NF, Affeldt RF, Ludtke DS, Pinto S, Santos FW, Prigol M. Selenofuranoside improves long-term memory deficits in rats after exposure to monosodium glutamate: Involvement of Na+, K+-ATPase activity. Physiol Behav. 2018;184:27–33. doi: 10.1016/j.physbeh.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Umukoro S, Oghwere EE, Ben-Azu B, Owoeye O, Ajayi AM, Omorogbe O, Okubena O. Jobelyn® ameliorates neurological deficits in rats with ischemic stroke through inhibition of release of pro-inflammatory cytokines and NF-ĸB signaling pathway. Pathophysiology. 2018;26:77–88. doi: 10.1016/j.pathophys.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Okubena O, Makanjuola S, Ajonuma LC, Dosunmu A, Umukoro S, Erah PO. The West African Sorghum bicolor leaf sheath extract Jobelyn® and its diverse therapeutic potentials. MOJ Drug Des Dev Therapy. 2018;2:20–28. doi: 10.15406/mojddt.2018.02.00025. [DOI] [Google Scholar]
- 24.Umukoro S, Ugbomah A, Aderibigbe A, Omogbiya A. Antioxidant property of Jobelyn® as the possible mechanism underlying its anti-amnesic effect in rodents. Basic Clin Neurosci. 2013;4:42–49. [PMC free article] [PubMed] [Google Scholar]
- 25.Omorogbe O, Ajayi AM, Ben-Azu B, Oghwere EE, Adebesin A, Aderibigbe AO, Okubena O, Umukoro S. Jobelyn® attenuates inflammatory responses and neurobehavioral deficits associated with complete Freund-adjuvant-induced arthritis in mice. Biomed Pharmacother. 2018;98:585–593. doi: 10.1016/j.biopha.2017.12.098. [DOI] [PubMed] [Google Scholar]
- 26.Oyinbo CA, Igbigbi PS, Avwioro OG. Jobelyn® supplement lowered neuronal degeneration: significance of altered p53 and ɤ-Enolase protein expressions in prefrontal cortex of rat exposed to ethanol. Ann Neurosci. 2016;23:139–148. doi: 10.1159/000449179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh K, Ahluwalia P. The effect of monosodium glutamate (MSG) administration on the activity of xanthine oxidase, superoxide dismutase and catalase in hepatic tissue of adult male mice. Indian J Clin Biochem. 2002;17:29–33. doi: 10.1007/BF02867938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Eduviere AT, Umukoro S, Aderibigbe AO, Ajayi AM, Adewole FA. Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice. Life Sci. 2015;132:20–26. doi: 10.1016/j.lfs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Taweel GM. Effect of monosodium glutamate and aspartame on behavioral and biochemical parameters of male albino mice. Afr J Biotech. 2016;15:601–612. doi: 10.5897/AJB2015.15199. [DOI] [Google Scholar]
- 31.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 32.Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. doi: 10.1172/JCI10805510.1172/JCI108055. [DOI] [PubMed] [Google Scholar]
- 33.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 34.Adam-Vizi A, Seregi V. Receptor independent stimulatory effect of noradrenaline on Na+/K+-ATPase in rat brain homogenate: role of lipid peroxidation. Biochem Pharmacol. 1982;34(2):2231–2236. doi: 10.1016/0006-2952(82)90106-X. [DOI] [PubMed] [Google Scholar]
- 35.Green LC, Wagner DA, Glowgowski J, et al. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 36.Gornall AG, Bardawill CJ, David MM. Determination of serum protein by means of biuret reaction. J Biol Chem. 1949;177:751–753. doi: 10.1016/S0021-9258(18)57021-6. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsson JEC. Improved specificity of serum albumin determination and estimation of acute phase reactant by the use of bromocresol green reaction. Clin Chem. 1976;22:616–622. doi: 10.1093/clinchem/22.5.616. [DOI] [PubMed] [Google Scholar]
- 38.Baratta JL, Lopez NA, Kasabwalla BN, Longmuir KJ, Robertson RT. Cellular organization of normal mouse liver: a histological, quantitative immunocytochemical, and fine structural analysis. Histochem Cell Biol. 2009;131:713–726. doi: 10.1007/s00418-009-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akanya HO, Peter S, Ossamulu IF, Oibiokpa FI, Adeyemi HY. Evaluation of the changes in some liver function and hematological parameters in MSG fed rats. Int J Biochem Res Rev. 2015;6:113–120. doi: 10.9734/IJBCRR/2015/15433. [DOI] [Google Scholar]
- 40.Lee M, Yun B, Zhang D, Liu L, Wang Z, Wang C, et al. Effect of aqueous antler extract on scopolamine induced memory impairment in mice and antioxidant activities. Food Sci Biotechnol. 2010;19:655–661. doi: 10.1007/s10068-010-0092-0. [DOI] [Google Scholar]
- 41.Onaolapo OJ, Onaolapo AY, Mosaku TJ, Akanji OO, Abiodun OR. Elevated plus maze and Y-maze behavioral effects of subchronic, oral low dose monosodium glutamate in Swiss Albino Mice. IOSR J Pharm Biol Sci. 2012;3:21–27. doi: 10.9790/3008-0342127. [DOI] [Google Scholar]
- 42.Dief AE, Kamha ES, Baraka AM, Elshorbagy AK. Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: a potential role for cyclic AMP protein kinase. Neurotoxicology. 2014;42:76–82. doi: 10.1016/j.neuro.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Hashem HE, Safwat E, Algaidi S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of Vitamin C (Histological and Immunohistochemical Study) J Mol Histol. 2012;43:179–186. doi: 10.1007/s10735-011-9380-0. [DOI] [PubMed] [Google Scholar]
- 44.Rosa SG, Quines CB, Stangherlin EC, Nogueira CW. Diphenyl diselenide ameliorates monosodium glutamate-induced anxiety-like behavior in rats by modulating hippocampal BDNF-Akt pathway and uptake of GABA and serotonin neurotransmitters. Physiol Behav. 2016;155:1–8. doi: 10.1016/j.physbeh.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Sun Y, Wang L, Gao Z. Glutamate transporters / Na2+, K+-ATPase involving in the neuroprotective effect as a potential regulatory target of glutamate uptake. Mol Neurobiol. 2015;53:1124–1131. doi: 10.1007/s12035-014-9071-4. [DOI] [PubMed] [Google Scholar]
- 46.Soliman AM. Extract of Coelatura aegyptiaca, a freshwater clam, ameliorates hepatic oxidative stress induced by monosodium glutamate in rats. Afr J Pharm Pharmacol. 2011;5:398–408. doi: 10.5897/AJPP11.085. [DOI] [Google Scholar]
- 47.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Song Y, Chen Z, Leng SX. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/1972714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duvert B, Senejani AG. Role of systemic inflammation in neurodegenerative diseases. J Biotechnol Biores. 2019;1:1–7. [Google Scholar]
- 50.Tawfik MS, Al-Badr N. Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamins C and E. Food Nutr Sci. 2012;3:651–659. doi: 10.4236/fns.2012.35089. [DOI] [Google Scholar]
- 51.Yaqub H, Abdel Baky NA, Attia HA, Faddah LM. Hepatoprotective effect of N-acetyl cysteine and/or β-carotene on monosodium glutamate-induced toxicity in rats. Res J Med Med Sci. 2008;3:206–215. [Google Scholar]
- 52.Mendez NV, Wharton JA, Leclerc JL, Blackburn SL, Douglas-Escobar MV, Weiss MD, Seubert CN, Dore S. Clinical implications of bilirubin-associated neuroprotection and neurotoxicity. Int J Clin Anesthesiol. 2013;1:1310. [PMC free article] [PubMed] [Google Scholar]
- 53.Hassel B, Iverson EG, Fonnum F. Neurotoxicity of albumin in vivo. Neurosci Lett. 1994;167:29–32. doi: 10.1016/0304-3940(94)91020-0. [DOI] [PubMed] [Google Scholar]
- 54.Benson KF, Beaman JL, Ou B, Okubena A, Okubena O, Jensen GS. West African Sorghum bicolor leaf sheaths have anti-inflammatory and immune-modulating properties in vitro. J Med Foods. 2013;16:230–238. doi: 10.1089/jmf.2012.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]