Abstract
Resistance of Shiga toxin-producing Escherichia coli (STEC) O157:H7 and serogroups O103, O26 and O145 to synthetic gastric fluid (SGF, pH 1.5) were investigated during frankfurter storage. Pathogens were inoculated (5 ± 1 log10 cfu g−1) on frankfurters and frankfurters were stored at 4 °C for 75 days in vacuum packages. Population changes of the competitive flora and STEC, changes in the pH of the frankfurters and resistance of STEC to SGF were monitored on days 0, 15, 30, 45, 60 and 75 of frankfurter storage. Direct synthetic gastric fluid (DSGF) challenges were also conducted to assess pathogen resistance without being effected by frankfurters, by inoculating pathogen cultures directly into SGF. Results showed that acid resistance of O145 and O26 was stronger than that of O103 and O157 during frankfurter storage. Resistance of O103 to SGF was better than that of O157 during frankfurter storage but, was similar to that of O157 during DSGF challenges. Results indicate that acid resistance of some strains of STEC pathogens might differentiate during storage of frankfurters. Different resistance capabilities to SGF were observed in the STEC strains when inoculated and stored on frankfurters than directly inoculated in the SGF.
Keywords: Acid resistance, Competitive flora, STEC, Frankfurter, Synthetic gastric fluid
Introduction
Shiga toxin-producing E.coli (STEC) is a group of pathogenic E.coli which produce Shiga toxin and can cause illnesses with a wide range of symptoms, ranging from mild non-bloody diarrhea to hemolytic uremic syndrome (HUS) in humans (World Health Organization 2018). STEC serogroups other than O157:H7 are called non-O157 STECs and since the older laboratory practices did not identify non-O157 STECs, their role in STEC infections was unrecognized (CDC, 2014). The large O104:H4 outbreak in 2011 (originated from Germany) has gained more attention to non-O157 STECs (Burger 2012). The non-O157 STEC serogroups which frequently linked to diseases in the USA (O26, O45, O103, O111, O121 and O145) were named as the “big six” or “top six” (USDA 2010).
The main source of transmission is cattle but other ruminants can also cause STEC transmission to humans. The infectious dose of STEC is notably low and adequate cooking of ground meat is advised in order to avoid STEC infections (Hunt 2010). Infection can occur via undercooked ground beef, unpasteurized milk or juices, foods that are grown in manured gardens and consumed raw and even foods that have an acidic nature like fermented hard salami (Nataro and Kaper 1998; Purwar et al. 2016). STEC has multiple acid resistance systems that confer protection from acid stress. The acidic nature of the stomach is the first barrier in human body against food-borne pathogens. Therefore it is important to test the survival ability of acid resistant pathogens such as STEC in a simulated stomach environment. Previous studies evaluated the acid resistance ability of STEC strains usually in acidified media, but the resistance capabilities of these pathogens might differ in food matrices.
In many studies, E. coli O157:H7 and non-O157 STECs isolations have been made in many foods originating from beef and minced meat, and many food related epidemics have been reported (USDA 2010; CDC 2014; WHO, 2018). Outbreaks of E. coli O157 and non-O157 STECs have been reported for various types of sausages (Normanno et al. 2002, 2004; Ethelberg et al. 2009). Many reports show that frankfurters can be contaminated with various pathogens alongside with E.coli (Mahami et al. 2012; Bolghari 2016; Samaha et al. 2016) during post-heat process handling, ie. casing removal, slicing, packaging (Güngör and Gökoğlu 2010). Moreover frankfurters can be consumed without further cooking before consumption, ie. in cold salads, especially in Turkey and European countries. This consumption habit increases the risk of infection caused by food-borne pathogens, especially acid resistant pathogens like STEC due to their low infectious doses. The resistance of STEC to acidic environments, like the stomach, is known to be affected by some factors such as storage temperature or pH of the food product. Cold storage might provide acid resistance since the production of cold shock proteins might provide cross-protection (Smith and Fratamico 2012). Moreover, the pH of the frankfurter type sausage during storage, even though buffered by phosphates in the product formula, might gradually decrease due to the activity of lactic acid bacteria (Mantis et al. 2005). Acid adaptation in sub-lethal pH or gradually decreasing pH allows the organism to survive even in lethal acidic environments such as the stomach fluid (Yuk and Marshall 2004).
In the light of the information presented, we aimed to investigate the survival and resistance rates of STEC O157:H7, O103, O26, and O145 to synthetic gastric fluid (SGF) during frankfurter storage, according to the scenario of contamination after heat treatment. Since acid stress responses of STEC might be affected by the acidulant type and pH, direct SGF (DSGF) trials (direct inoculation of strains to SGF) were also conducted to assess the acid resistance of the strains, without being affected by the frankfurter matrix. The evaluation of the resistance of STEC strains to SGF before inoculation onto the frankfurters by direct synthetic gastric fluid (DSGF) trials would help to draw an objective conclusion on how the frankfurter matrix and storage conditions affected the acid resistance capabilities of the strains. To our knowledge this is the first study in the literature in regards of STEC survival and resistance to gastric fluid during storage of emulsified meat products like frankfurters.
Materials and methods
This research is divided into two parts; the first part of the study consisted of direct synthetic gastric fluid (DSGF) trials, the second part consisted of the evaluation of the STEC survival during storage and SGF trials of inoculated frankfurters. Microbiological analyses, SGF experiments and pH measurements were performed at days 0, 15, 30, 45, 60 and 75 of storage. All analyses were performed in duplicates and inoculations, SGF and DSGF trials were repeated 3 times.
Bacterial culture preparation
Bacteria cultures were prepared from agar slants. Slant cultures were transferred to Tryptic Soy Broth (TSB, Merck, Germany) and incubated at 35 °C for 6 h then streaked on Sorbitol MacConkey (SMAC, Merck, Germany) agar plates and incubated for 24 h. A single colony was transferred to TSB for each strain individually and incubated statically for 18 h at 35 °C. A cocktail of ATCC 35,150 and ATCC 43,895 strains were used for E.coli O157:H7 inoculation. The non-O157 strains; O103, O26, and O145 were provided from Instituto Superiore di Sanita (ISS; National Institute of Health, Italy).
Direct SGF trials of strains
Synthetic gastric fluid (SGF) was prepared according to Beumer et al. (1992). Briefly, protease peptone (8.3 g L−1; Difco), d-glucose (3.5 g L−1) NaCl (2.05 g L−1), KH2PO4 (0.6 g L−1), CaCl2 (0.11 g L−1) and KCl (0.37 g L−1) were mixed in deionized water and autoclaved. Ox bile (0.05 g/liter), lysozyme (0.10 g L−1), and pepsin (0.0133 g L−1) were filter sterilized and aseptically mixed. The pH of SGF was adjusted to 1.5 with 1 N HCl. For direct SGF trials, bacterial strains were prepared as mentioned above. 1 ml from 18-h TSB cultures were transferred into 99 ml of pre-warmed SGF (37 °C) and the homogenate was kept in an incubator at 37 °C (Yuk and Marshall 2004). Enumeration of STEC strains was performed at 30, 60 and 90 min of exposure to SGF. The number of STEC at minute “0” was enumerated directly from the culture by serially diluting the 18-h TSB cultures.
Inoculation and storage of frankfurters
Frankfurters used in this study were produced at a local meat processor, based on the manufacturer formulation and production process to mimic the product that is available to consumers. The producer did not allow us to share the specific formula but the frankfurter batter was prepared by mixing the ground meat (beef), ice, starch, sodium nitrite, sodium polyphosphate, ascorbic acid, salt, seasonings and liquid smoke in a cutter. The results of proximate analyses of the frankfurters show that the material had 57.61 ± 1.62 moisture content (%), 14.84 ± 0.58 fat content (%), 14.96 ± 0.24 protein content (%) and 1.85 ± 0.04 salt content. The batter was filled in the casings and cooked to an internal temperature of 72 °C. The cooked frankfurters were cooled and kept overnight at 4 °C.
The 18-h TSB cultures were used for inoculation. The cells in the 50 ml of TSB were centrifuged at 1792 g-force at 4 °C for 15 min; the supernatant was discarded and the pellet was washed twice with sterile saline solution (0.85 NaCl). The cell pellet of each strain studied was re-suspended in 300 ml of sterile saline solution and frankfurters were dipped in this suspension. The suspension was stirred for 2 min to allow the bacteria to attach on the surface of frankfurters. A total of 42–45 frankfurters were inoculated per serogroup by using the suspensions 2–3 times. For E.coli O157:H7 inoculation two strains were prepared individually and the washed cell pellets were re-suspended in 1 ml of saline solution. A volume of 0.7 ml from this suspension from each strain culture was mixed and added to 300 ml of saline solution. The initial populations of STEC strains in the suspensions were around 107–108 cfu ml−1. A total of 3.3–3.4 kg of frankfurter/per trial were used in the study (one frankfurter is about 20 g). The inoculated frankfurters were vacuum packed (7 frankfurter/per package, 20 g/per frankfurter) and stored at 4 °C, for 75 days. On each of the sampling day, a separate package was opened aseptically and the analyses were conducted.
Microbiological analysis
STEC, total plate count (TPC), psychrophilic bacteria (PB) and lactic acid bacteria (LAB) were enumerated during storage at 0, 15, 30, 45, 60, 75 days of cold storage at 4 °C. TPC and PB were enumerated on pour plated Plate Count Agar (Merck, Germany) plates after incubation at 30 °C for 72 h (BAM, 2001) and at 7 °C for 7 days, respectively (Delaquis et al. 1992). For LAB enumeration double layered De Man Rogosa-Sharpe Agar (MRS Agar, Merck, Germany) plates were incubated at 35 °C for 72 h. STEC serogroups were enumerated from surface plated Sorbitol MacConkey Agar (SMAC, Merck, Germany) plates after incubation at 35 °C for 36 h (Dikici et al. 2015).
SGF trials of pathogens inoculated on frankfurters
120 ml of pre-warmed (37 °C) SGF were mixed with 20 g of frankfurter (1:6) with a bag mixer (Interscience, Bag Mixer 400, France) for 2 min. The homogenate was kept in an incubator at 37 °C and 0.1 ml was sampled at 30, 60 and 90 min of exposure to SGF. Pathogen counts of the samples before SGF exposure (Time 0) were performed by mixing 225 ml of sterile peptone water with 25 g of sample. In this study, a static SGF challenge was performed. Since in an actual digestion, stomach is gradually acidified, in static digestion challenges like the procedure used in this study, a pH limit is usually determined in order to avoid false results caused by the buffering effect of food. The pH limit we set for our trials was 2.5 and in none of the SGF experiment results included in this study, pH rose above this limit (Dikici et al. 2015).
pH measurement
The pH changes of frankfurters were also monitored at 0, 15, 30, 45, 60 and 75 days of storage. Briefly, 10 g of frankfurter was mixed with 90 ml of deionized water with a bag mixer (Interscience, Bag Mixer 400, France) and the pH was measured by a pH-meter.
Statistical analysis
Colony counts obtained from the microbiological analysis were converted to log10 cfu/g. The data were subjected to analysis of variance (ANOVA) according to serogroup × rep × time (storage day/SGF exposure minutes) design; mean values of counts were separated by GLM procedure using Fisher’s Least Square Differences method. Analyses were performed by the Statistical Analysis System (SAS) program (SAS 1999).
Results and discussion
Direct SGF trials
Average counts of direct SGF trials are shown in Table 1. The lowest viability loss was detected in O145 (1.11 log10 cfu g−1) whereas the maximum decrease in viable counts was observed in O157 (2.31 log10 cfu g−1). Statistical analysis showed that the most resistant serogroup to SGF was O145, followed by O26 and O103/O157 at the end of 90 min of exposure. Resistance of STEC O103 and O157 to SGF was statistically similar. These trials showed the acid resistance of strains to SGF without taking into account the effects of frankfurter matrix.
Table 1.
Average Counts of STEC Serogroups During Direct SGF Challenges (log10 cfu/ml ± SD, results of triplicate trials)
| Serogroup | Minutes of Exposure to SGF | |||
|---|---|---|---|---|
| 0 | 30 | 60 | 90 | |
| O103 | 9.08 ± 0.77Ax | 7.21 ± 0.79Bz | 7.22 ± 0.94By | 6.91 ± 0.84Bz |
| O26 | 9.25 ± 0.94Ax | 8.19 ± 1.08Bxy | 8.17 ± 1.44Bx | 7.65 ± 1.21By |
| O145 | 9.40 ± 0.76Ax | 8.80 ± 1.11ABx | 8.50 ± 1.17Bx | 8.29 ± 1.44Bx |
| O157 | 9.31 ± 0.89Ax | 7.94 ± 1.28By | 7.37 ± 1.14BCy | 7.00 ± 1.09Cz |
A,B,CDifferent letters in the same row indicate statistically significant differences (p < 0.05)
x,y,zDifferent letters in the same column indicate statistically significant differences (p < 0.05)
Studies usually show that acid resistance of STEC O157 is stronger than that of non-O157 strains or similar (Bergholz and Whittam, 2007; Molina et al. 2003; Rivera-Reyes et al. 2019). But comparing the results of the studies is challenging, since strain-to-strain differences, methodological differences, the type and pH of the acidulant may affect bacterial responses to acid challenges. Acid resistance of O157 and non-O157 STECs differ based on the medium (food or broth medium), and testing conditions. Elhadidy and Mohammed (2013) observed O26:H11 survived better in acidified dairy products than O157:H7. Whereas Molina et al. (2003) reported contrary results in LB-broth medium, but they reported some strains of non-O157 belonging to other serogroups had greater stress resistance than O157. Different test conditions and mediums affect acid resistance, however; strain- to-strain differences might also play a role in these varying results. Miszcycha et al. (2014) also reported that O26:H11 had higher average counts than that of O157:H7 during in vitro digestion challenges of contaminated cheese and they hypothesized that the O157:H7 strain that they tested had a defective gene. Therefore in this study, direct SGF trials were performed using the same procedure to prepare the bacterial culture suspensions and the same acidulant (SGF) to assess the resistance of STEC strains before inoculation onto frankfurters by performing the direct SGF trials, we aimed to assess the resistance of STEC strains used in this study, without being affected by the frankfurter matrix or storage conditions.
In the present study, the O157 and non-O157 strains had variable but strong SGF resistance during direct SGF trials. But contrary to the most of the study results in the literature, non-O157 strains of O145 and O26 had stronger resistance to SGF than O157.
Bergholz and Whittam (2007) reported that survival of acid-adapted O157:H7, O26:H11 and O111:H8 at 4 °C was higher than that of strains adapted at 22 °C and non-O157 STECs had lower average counts than O157 in subsequent gastric acidity challenge. On the other hand, Kim et al. (2016), studied the SGF resistance of O26, O103, O104, O111, and O157 after acid adaptation in pineapple juice, they reported non-O157 strains of O103:H2 and O111:H8 showed high acid resistance similar to O157. In the present study acid resistance of O103 and O157 was also similar but lower than that of the other strains of non-O157 serogroups. Mand et al. (2013), reported some non-O157 strains (some strains of serogroups O103, O26 and O111) survived significantly better in acidified Luria–Bertani broth (pH 3.0) than O157 (ATCC 43,895), but the survival of other tested strains of non-O157 serogroups were lower than that of O157:H7. Rivera-Reyes et al. (2019), investigated cross-protection in E. coli O26, O45, O103, O111, O121, O145, and O157:H7 to different production related stresses after acid adaptation in TSB (pH 5.0). They reported that O157:H7 survived the subsequent acid treatment at pH 4.5 better than non-O157 strains but overall non-O157 strains behaved similarly to O157:H7. No difference was observed in the acid resistance of non-O157 strains. As the authors stated, this pH level might not be low enough to see any differences in the acid resistance of non-O157 strains.
Changes in the populations of STEC serogroups and natural flora during frankfurter storage
Average counts of STEC serogroups during 75 days of frankfurter storage is shown in Table 2. Average counts of STEC O145 and O26 during storage decreased non-significantly whereas average counts of O103, and O157 decreased 1.43 log10 cfu g−1, and 1.63 log10 cfu g−1 respectively.
Table 2.
Average Counts of STEC Serogroups during Cold Storage of Frankfurters for 75 Days At 4 °C (log10 cfu/g ± SD, results of triplicate trials)
| Serogroup | Storage Days | |||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | |
| O103 | 5.44 ± 0.41Ax | 5.62 ± 0.21Ax | 5.43 ± 0.36Ax | 4.78 ± 0.21ABx | 4.12 ± 0.38By | 4.01 ± 0.14By |
| O26 | 5.89 ± 0.20Ax | 5.16 ± 0.87ABx | 5.25 ± 1.02ABx | 5.09 ± 0.99ABx | 4.61 ± 0.26Bxy | 5.17 ± 1.21ABx |
| O145 | 5.80 ± 0.16Ax | 5.64 ± 0.41Ax | 5.56 ± 0.35Ax | 5.10 ± 0.63Ax | 5.36 ± 0.29Ax | 5.32 ± 0.60Ax |
| O157 | 5.71 ± 0.14Ax | 4.80 ± 1.54ABx | 4.74 ± 1.00Bx | 3.97 ± 0.94Cy | 4.11 ± 1.64BCy | 4.08 ± 1.61BCy |
A,B,CDifferent letters in the same row indicate statistically significant differences (p < 0.05)
x,y,zDifferent letters in the same column indicate statistically significant differences (p < 0.05)
LAB and PB counts of frankfurters were below the detection limit at the 1st day of storage (Day 0) but increased significantly during storage to around 6–7 log10 cfu g−1. The number of TPC also increased significantly and TPC was enumerated around 7 log10 cfu/g at the end of the storage (Fig. 1).
Fig. 1.
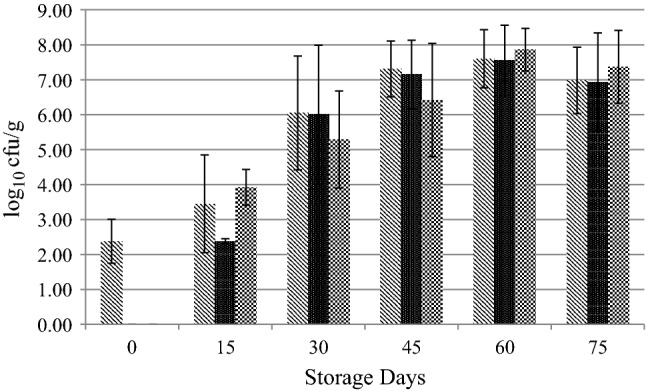
Changes in the Average Counts of Competitive Flora of Frankfurters Stored at 4ºC for 75 Days, TPC ( ), LAB (
), LAB ( ), PB (
), PB ( )
)
In this study survival of STEC was not affected significantly by the competitive flora of frankfurters (Table 2). There are studies show that high levels of background flora especially LAB decreased STEC survival (Vold et al. 2000; Hwang and Huang 2018; Gao et al. 2019). Our previous study on commercially available frankfurters also indicated that STEC survival was affected by competitive flora (unpublished data). The results obtained in the present study might be a consequence of the frankfurter pH. The pH of the frankfurters did not change significantly during storage. The pH was 5.18 ± 0.26 at the beginning of the storage and was measured as 5.56 ± 0.1 at the end of the storage. Sub-lethal pH values around 4.5–5.5 are known to cause a stress adaptation response in E.coli, depending on the adaptation period (Öztürk and Halkman 2015). This kind of adaptation also explains the high average counts of pathogens during exposure to SGF throughout the storage. Acid resistance might also confer resistance to other stressors, which is known as cross-protection (Capozzi et al. 2009). Possible cross-protection to competitive flora stress might have occurred in this study. In the study conducted by Öztürk and Halkman (2015), acid-adapted E.coli O157:H7 (pH 5.5) cells were not inhibited by the high LAB levels that increased during fermentation.
The order of the stresses that bacteria encounter might also play a part in the resistance of bacteria to subsequent stresses (Rivera-Reyes et.al. 2019). In our previous study on commercially available frankfurters (unpublished data), we observed in one brand of samples as LAB grew (around 7–8 log at the end of the storage) they impaired the survival and acid resistance of STEC. But in the present study even though LAB levels increased during storage (Fig. 1), STEC survival and acid resistance was not affected. These contradictory results might be a consequence of the order of the stresses that the strains encounter. In the previous study LAB levels were already high, therefore their possibly inhibitory metabolites were already present at the beginning of the storage (at the time of inoculation of the strains), but in this study, LAB level was under the detection limit at the beginning of the storage and the product pH was favorable for adaptation of STEC strains. Resistance of STEC strains might have been triggered at this pH. Even though LAB counts increased during storage, the resistance of STEC strains was not affected.
Resistance of STEC serogroups inoculated onto frankfurters against synthetic gastric fluid during refrigerated storage
Gastric fluid resistance of non-O157 strains was better than that of O157 during frankfurter storage (Table 3). Viability of STEC O26 was not significantly affected by SGF between 15–60 days of storage whereas viable counts of STEC O145 did not decrease significantly between 30–60 days of storage. Survival of STEC O103 during SGF challenges was weakened by day 75 of storage but, the viable counts decreased by only around 2 log10 cfu g−1, between 30–60 days of storage. SGF resistance of STEC O157 was the weakest. The resistance of pathogen weakened by day 30 of the storage and the viable counts of the pathogen were undetectable on the 75th day of storage.
Table 3.
Average Counts of STEC Serogroups during Synthetic Gastric Fluid Exposure throughout Cold Storage of Experimentally Produced Frankfurters (log10 cfu g-1 ± SD, results of triplicate trials)
| Organism | Days | Minutes of Exposure to SGF | |||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | ||
| STEC O103 | 0 | 5.44 ± 0.41Ax | 4.18 ± 1.10Bx | 4.02 ± 1.27Bx | 4.39 ± 0.07ABx |
| 15 | 5.62 ± 0.21Ax | 3.74 ± 1.5Bx | 4.37 ± 0.46ABx | 4.05 ± 0.39Bxy | |
| 30 | 5.43 ± 0.36Ax | 3.89 ± 1.06Bx | 3.36 ± 0.74Bx | 3.22 ± 0.75Bxyz | |
| 45 | 4.78 ± 0.21Axy | 3.10 ± 1.08Bxy | 3.46 ± 0.07Bx | 2.73 ± 0.15Byz | |
| 60 | 4.12 ± 0.38Ay | 4.27 ± 0.33Ax | 3.70 ± 0.09Ax | 3.38 ± 0.09Axyz | |
| 75 | 4.01 ± 0.14Axy | 2.32 ± 0.39By | ND | 1.89 ± 0.12Bz | |
| STEC O26 | 0 | 5.89 ± 0.2Ax | 4.88 ± 0.53ABx | 4.73 ± 0.64ABxy | 4.39 ± 0.34Bxy |
| 15 | 5.16 ± 0.87Axy | 5.19 ± 0.31Ax | 4.97 ± 0.48Axy | 4.98 ± 0.16Ax | |
| 30 | 5.25 ± 1.01Axy | 5.02 ± 0.18Ax | 4.76 ± 0.29Axy | 4.99 ± 0.19Ax | |
| 45 | 5.09 ± 0.99Axy | 5.04 ± 0.54Ax | 5.07 ± 0.74Ax | 4.89 ± 0.61Ax | |
| 60 | 4.61 ± 0.26Ay | 5.04 ± 0.14Ax | 4.87 ± 0.22Axy | 4.87 ± 0.25Ax | |
| 75 | 5.17 ± 1.21Axy | 4.08 ± 0.25ABx | 3.58 ± 0.28By | 3.33 ± 0.26By | |
| STEC O145 | 0 | 5.80 ± 0.15Ax | 4.62 ± 0.73Bx | 4.32 ± 0.90Bx | 3.93 ± 1.29Bx |
| 15 | 5.64 ± 0.41Ax | 4.74 ± 1.08ABx | 4.50 ± 1.28ABx | 4.11 ± 1.53Bx | |
| 30 | 5.56 ± 0.35Ax | 4.37 ± 1.18Bx | 4.30 ± 1.37Bx | 4.88 ± 0.27ABx | |
| 45 | 5.10 ± 0.63Ax | 5.12 ± 0.34Ax | 4.75 ± 0.28Ax | 4.69 ± 0.40Ax | |
| 60 | 5.36 ± 0.29Ax | 4.56 ± 0.5Ax | 4.24 ± 0.37Ax | 4.30 ± 0.2Ax | |
| 75 | 5.32 ± 0.60Ax | 4.36 ± 0.19ABx | 4.12 ± 0.3ABx | 3.67 ± 0.25Bx | |
| STEC O157 | 0 | 5.71 ± 0.14Ax | 4.62 ± 0.85ABx | 3.77 ± 0.79Bx | 3.94 ± 0.05Bx |
| 15 | 4.80 ± 1.54Axy | 4.17 ± 0.11ABx | 3.50 ± 0.09Bx | 3.22 ± 0.14Bx | |
| 30 | 4.74 ± 1.00Axy | 3.38 ± 0.09Bx | 2.77 ± 0.18Bx | 2.85 ± 0.48Bx | |
| 45 | 3.97 ± 0.93Ay | 1.84 ± 0.002By | ND | ND | |
| 60 | 4.06 ± 1.72Ay | 1.99 ± 0.37By | ND | ND | |
| 75 | 4.08 ± 1.61y | ND | ND | ND | |
A,B,CDifferent letters in the same row indicate statistically significant differences (p < 0.05)
x,y,zDifferent letters in the same column indicate statistically significant differences (p < 0.05)
ND non-detected
SGF trials show that the pathogens had strong resistance to acid on the frankfurters (Table 3). But these trials and direct SGF trials were not completely in correlation. The direct SGF challenges showed that O157 and O103 had similar acid resistance (Table 1) but during storage of frankfurters STEC O157 had the lowest average counts in SGF trials (Table 3). Moreover, the results of the direct SGF trials indicated that acid resistance of STEC O145 was stronger than that of STEC O26. But the average counts of STEC O26 inoculated on frankfurters did not decrease significantly during SGF challenges between 15–60 days of storage whereas the average counts of O145 during SGF trials was statistically lower. The stress responses of STEC pathogens, as the results indicated in the present study, might differ when food is introduced in the acid challenges. Rode et al. (2017), investigated the survival of STEC strains in a digestion challenge after sausage fermentation and they reported non-O157 isolates had survival patterns similar to that of O157 strains. Miszczycha et al. (2014), reported the fate of STEC O157 and O26 during a digestion challenge of contaminated cheeses was dependent on the strain and O26 survived significantly better than O157. In the present study, the ability of non-O157 strains of the serogroups of O145 and O26 to endure gastric acid stress was better than the strains of O157:H7 and O103 when inoculated directly in the SGF. But even though average counts of STEC O103 were similar to that of O157 when directly inoculated in SGF, it was higher than that of O157:H7 throughout frankfurter storage.
Conclusion
In this study, the ability of STEC to survive and withstand synthetic gastric fluid during frankfurter storage was demonstrated. STEC pathogens had remarkable SGF resistance during frankfurter storage. Since the low infectious dose of these pathogens is mostly related to their acid resistance capabilities, the results obtained from this study are raising concerns. Non-O157 strains included in this study had better survival and acid endurance ability than O157. Even though the majority of the researches in this area show that O157:H7 has superior/similar acid resistance capabilities to non-O157 strains, there are also studies show that they can behave differently especially in a food matrix. Non-O157 STEC is a diverse group of pathogens and relatively less information is available on their behavior, especially in foods. Even though the results of the studies conducted with laboratory media provides valuable information about these pathogens, the exclusion of variables specific to food matrix in the studies might lead to underestimated risk profiling. Therefore, more studies should be undertaken on related foodstuffs regarding STEC behavior and stress resistance. The O157 strains used in this study might have had different acid resistance systems that provide more protection in different environments but not during frankfurter storage. The resistance mechanisms can be further studied under different stress environments.
Acknowledgments
This research was supported by Scientific Research Projects Coordination Unit of Uşak University, [Project Number: MF011]; Uşak, TURKEY. This study is a part of a detailed study (PhD thesis) on profiling the STEC infection risk in frankfurters available to consumers in Turkey. The results included in that manuscript have not been published in any journal as an article.
Compliance with ethical standard
Conflict Of interest
The authors declare that they have no Conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- BAM, Bacteriological analytical manual. 2001. Aerobic Plate Count. Retrieved from: https://www.fda.gov/food/laboratory-methods-food/bam-aerobic-plate-count (Accessed 10 Mar 2019)
- Bergholz TM, Wittam TS. Variation in acid resistance among enterohaemorrhagic Escherichia coli in a simulated gastric environment. J Appl Microbiol. 2007;102:352–362. doi: 10.1111/j.1365-2672.2006.03099.x. [DOI] [PubMed] [Google Scholar]
- Beumer RR, de Vries J, Rombouts FM. Campylobacter jejuni non-culturable coccoid cells. Int J Food Microbiol. 1992;15:153–163. doi: 10.1016/0168-1605(92)90144-r. [DOI] [PubMed] [Google Scholar]
- Bolghari N. Analysis of the microbial load and the amount of acryl amide in heated sausages using microbiological methods and HPLC chromatography in Gilan Province of Iran. Res J Appl Sci. 2016;11:523–529. doi: 10.36478/rjasci.2016.523.529. [DOI] [Google Scholar]
- Burger R (2012) EHEC O104:H4 in Germany (2011): large outbreak of bloody diarrhea and haemolytic uraemic syndrome by Shiga toxin-producing E. coli via contaminated food. Improving food safety through a one health approach: workshop. Institute of medicine (US). Washington (DC): National Academies Press (US). En: https://www.ncbi.nlm.nih.gov/books/NBK114499/
- CDC, Centers for disease control and prevention (2014) E.coli–General information. Retrieved from: https://www.cdc.gov/ecoli/general/index.html (Accessed 10 Mar 2019)
- Capozzi V, Fiocco D, Amodio ML, Gallone A, Spano G. Bacterial stressors in minimally processed food. Int J Mol Sci. 2009;10:3076–3105. doi: 10.3390/ijms10073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaquis PJ, Gariepy C, Dussault F. Bacteriology of hot and cold boned pork preblends. J Food Prot. 1992;55:910–912. doi: 10.4315/0362-028X-55.11.910. [DOI] [PubMed] [Google Scholar]
- Dikici A, Koluman A, Çalıcıoğlu M. Comparison of effects of mild heat combined with lactic acid on Shiga toxin producing Escherichia coli O157: H7, O103, O111, O145 and O26 inoculated to spinach and soybean sprout. Food Control. 2015;50:184–189. doi: 10.1016/j.foodcont.2014.08.038. [DOI] [Google Scholar]
- Elhadidy M, Mohammed MA. Shiga toxin–producing Escherichia coli from raw milk cheese in Egypt: prevalence, molecular characterization and survival to stress conditions. Lett Appl Microbiol. 2013;56:120–127. doi: 10.1111/lam.12023. [DOI] [PubMed] [Google Scholar]
- Ethelberg S, Smith B, Torpdahl M, Lisby M, Boel J, Jensen T, Nielsen EM, Mølbak K. Outbreak of non-O157 Shiga toxin-producing Escherichia coli Infection from consumption of beef sausage. Arch Clin Infect Dis. 2009;48(8):78–81. doi: 10.1086/597502. [DOI] [PubMed] [Google Scholar]
- Gao Z, Daliri EBM, Wang J, Liu D, Chen S, Ye X, Ding T. Inhibitory effect of lactic acid bacteria on foodborne pathogens: a review. J Food Prot. 2019;82:441–453. doi: 10.4315/0362-028X.JFP-18-303. [DOI] [PubMed] [Google Scholar]
- Güngör E, Gökoglu N. Determination of microbial contamination sources at a frankfurter sausage processing line. Turk J Vet Anim Sci. 2010;34:53–59. doi: 10.3906/vet-0805-28. [DOI] [Google Scholar]
- Hunt JM. Shiga toxin–producing Escherichia coli (STEC) Clin Lab Med. 2010;30:21–45. doi: 10.1016/j.cll.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C-A, Huang L. Dynamic analysis of competitive growth of Escherichia coli O157:H7 in raw ground beef. Food Control. 2018;93:251–259. doi: 10.1016/j.foodcont.2018.06.017. [DOI] [Google Scholar]
- Kim GH, Fratamico P, Breidt F, Oh D-H. Survival and expression of acid resistance genes in Shiga toxin-producing Escherichia coli acid adapted in pineapple juice and exposed to synthetic gastric fluid. J Appl Microbiol. 2016;121:1416–1426. doi: 10.1111/jam.13223. [DOI] [PubMed] [Google Scholar]
- Mahami T, Amafu-dey H, Odonkor ST. Microbial food safety risk: cooked and smoked sausages as a potential Source. IJBPAS. 2012;1:99–107. doi: 10.4315/0362-028X-60.6.724. [DOI] [Google Scholar]
- Mand TD, Döpfer D, Ingham B, Ané C, Kaspar CW. Growth and survival parameter estimates and relation to RpoS levels in serotype O157: H7 and non-O157 Shiga toxin-producing Escherichia coli. J App Microbiol. 2013;114:242–255. doi: 10.1111/jam.12021. [DOI] [PubMed] [Google Scholar]
- Mantis FN, Tsachev I, Sabatakou O, Burriel AR, Vacalopoulos A, Ramantanis SB. Safety and shelf-life of widely distributed vacuum packed, heat treated sausages. BJVM. 2005;8:245–254. [Google Scholar]
- Miszczycha SD, Thévenot J, Denis S, Callon C, Livrelli V, Alric M, Thevenot-Sergentet D. Survival of Escherichia coli O26: H11 exceeds that of Escherichia coli O157: H7 as assessed by simulated human digestion of contaminated raw milk cheeses. Int J Food Microbiol. 2014;172:40–48. doi: 10.1016/j.ijfoodmicro.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Molina PM, Parma AE, Sanz ME. Survival in acidic and alcoholic medium of Shiga toxin-producing Escherichia coli O157:H7 and non-O157:H7 isolated in Argentina. BMC Microbiol. 2003;3:17. doi: 10.1186/1471-2180-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno G, Dambrosio A, Lassandro L, Quaglia N, Sebastio C, Parisi A, Germinario L, Celano GV. Ricerca di E. coli O157:H7 in insaccati freschi. Ind Aliment. 2002;41:406. [Google Scholar]
- Normanno G, Parisi A, Dambrosio A, Quaglia NC, Montagna D, Chiocco D, Celano GV. Typing of E. coli O157:H7 strains from fresh sausage. Food Microbiol. 2004;21:79–82. doi: 10.1016/S0740-0020(03)00019-4. [DOI] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/CMR.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk F, Halkman A. Determination of the survival levels of acid-adapted Escherichia coli O157:H7 in sucuk (Turkish-typefermented sausage) Turk J Vet Anim Sci. 2015;39:485–492. doi: 10.3906/vet-1401-96. [DOI] [Google Scholar]
- Purwar S, Roy S, Metgud S. Non-O157:H7 Shiga Toxin Producing Diarrhoeagenic Escherichia coli (STEC) in Southern India: a Tinderbox for Starting Epidemic. J clin diagn res:JCDR. 2016;10:DC11. doi: 10.7860/JCDR/2016/21462.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Reyes M, Campbell JA, Cutter CN. Survival of acid-adapted and non-adapted Shiga toxin-producing Escherichia coli using an in vitro model. Food Control. 2019;104:28–33. doi: 10.1016/j.foodcont.2019.04.009. [DOI] [Google Scholar]
- Rode TM, McLeod A, Måge I, Heir E, Axelsson L, Holck AL. Survival of five strains of Shiga Toxigenic Escherichia coli in a sausage fermentation model and subsequent sensitivity to stress from gastric acid and intestinal fluid. Hindawi Int J Microbiol. 2017 doi: 10.1155/2017/5176384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha IA, Nossair MA, Kassem AA. Microbial Evaluation of Heat Treated Meat Products. Alex J Vet Sci. 2016;49:153–159. doi: 10.5455/ajvs.206988. [DOI] [Google Scholar]
- SAS (1999) Statistical analysis system Version 6.1. Cary, Nort Caroline, USA: S.A.S. Institute
- Smith JL, Fratamico PM. Effect of stress on non-O157 Shiga toxin–producing Escherichia coli. J Food Prot. 2012;75:2241–2250. doi: 10.4315/0362-028X.JFP-12-255. [DOI] [PubMed] [Google Scholar]
- USDA (2010) Food Safety and inspection service (FSIS). Detection and isolation of non-O157 Shiga-toxin producing Escherichia coli strains (STEC) from meat products. Microbiological Laboratory Guidebook, version 5B.00. USDA, Food Safety Inspection Service, Washington, DC
- Vold L, Holck A, Wasteson Y, Nissen H. High levels of background flora inhibits growth of Escherichia coli O157:H7 in ground beef. Int J Food Microbiol. 2000;56:219–225. doi: 10.1016/s0168-1605(00)00215-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2018) Shiga toxin-producing Escherichia coli (STEC) and food: attribution, characterization, and monitoring: report. (Vol. 19). World Health Organization
- Yuk HG, Marshall DL. Adaptation of Escherichia coli O157: H7 to pH alters membrane lipid composition, verotoxin secretion, and resistance to simulated gastric fluid acid. Appl Environ Microbiol. 2004;70:3500–3505. doi: 10.1128/AEM.70.6.3500-3505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


