Abstract
Six types of cooking pots with five different food stuffs were used to investigate the influence of cooking pots on macro and micronutrients of cooked foods. A general trend observed was that cooking pot forged from titanium offered best protection (retention) of micronutrients while pitted aluminum pot offered the lowest irrespective of the food sample cooked. Titanium and enamel coated cooking pots required less quantity of water to get food done resulting into a low (68.67%) moisture content for food cooked in such pots in contrast to values as high as 77.89% when other pots were used. Our research evidenced that cooking pot may have impact on people’s morbidity since steady consumption of food cooked in some pots may aggravate, micronutrient malnutrition. Our findings suggest a contrary view to the previous idea of using pressure pot to cook food. Pots that offered low-pressure cooking (82 °C/0.53 bar) was found to preserve the most heat liable nutrients. Our recommendation, therefore, is the use of titanium and enamel coated cooking pots which offered better retention of food nutrients. Cooking may cause changes to food nutrient depending on foodstuff, materials used in forging the pot as well as the fitness of the pot lid.
Keywords: Cooking pot, Food, Nutrient, Morbidity
Introduction
There is abundant evidence that cooking reduces the level of nutrients in foods, but the reductions are exacerbated by such factors as the type of cooking pot used (Greger et al. 1985; Mougin et al. 2015; Weidenhamer et al. 2014; Stahl et al. 2017a, b, c). A foodstuff that is naturally nutrient-dense can be turned into poison-dense before it gets into the mouth depending on its handling and processing operations. Several factors, including materials (composition) and design of the cooking pot (Mougin et al. 2015; Weidenhamer et al. 2014) influence the losses and changes associated with food nutrients during cooking. In addition to cooking duration or time, temperature/pressure as well as the interaction between pot surface and food materials are other major factors that affect the nutrient components of food during cooking. From thermodynamics point of view, the temperature at which solution boils in a cooking pot affects the nutrient status of the food being cooked. The boiling temperature depends on the prevailing atmospheric pressure of the system and changes with elevation. Liquid boils at higher temperature under increased atmospheric pressure. So boiling occurs when the vapor pressure (the degree to which the liquid molecules are escaping into the vapor phase) reaches or exceeds the surrounding pressure from the atmosphere or whatever else is in contact with the liquid. In addition to temperature and pressure of cooking there could be a chemical reaction between the food and the surface of the cooking pot (Stahl et al. 2017a; https://www.canada.ca › services › household-products › safe. Accessed 12th May, 2018) depending on the materials used to forge the cooking pot.
The regular kitchen pots in the developing world seldom have tight fitting lids, so they mostly operate at atmospheric pressure and hence boil water at 100 °C which is the boiling point of water at 1 atmosphere. These regular pots are therefore unable to operate below 100 °C. On the other hand, pressure pots operate at higher temperatures because of the peculiarity of their construction (Mougin et al. 2015). The relationship between the vapour pressure in a pot and the atmospheric pressure greatly influences the optimal or boiling temperature achievable in the pot while cooking. According to standard chemical laws high atmospheric pressures causes water to boil at high temperature. And high temperature always causes loss of nutrients in food. This relationship is freely applied in the vacuum drying of thermo sensitive food materials where high temperatures can lead to loss of food value. In situations like this, low operating temperatures are achieved by the use of vacuum pump (that maintains the vapour pressure inside the drying bin below atmospheric pressure) and so keep the process temperature at a value below 100 °C. The vacuum pump has a one-way valve that allows the removal or passage of vapour in just one direction and consequently and effectively breaks the links between the inside of the bin and the atmosphere. For this reason, process temperatures with a vacuum are usually below 100 °C.
It is also to be noted that the cooking mixture is a solution with the food as the solute. According to Felder et al. (2016), the presence of nutrient/food in the pure solvent water used for cooking lowers the vapour pressure of the solvent water from its pure solvent value (Raoult’s law, ). Here, is the mole fraction of nutrient/food in the solution, is the vapour pressure of pure solvent (water) and is the effective vapour pressure of water when food/nutrient is in the cooking pot. The consequence of the above is the lowering of the solvent vapour pressure which leads to the solution to boil at a higher temperature at any given pressure. According to standard chemical laws, this boiling point elevation can be related to the mole fraction (concentration) of the solute (nutrient/food) (), pure solvent boiling point temperature () and heat of vaporization of the pure solvent water at its boiling point () as:
| 1 |
That is at atmospheric pressure, presence of food/nutrient causes water/solution to boil at higher temperature. High temperature always causes loss of nutrients in food.
Nutrient loss orchestrated by cooking pot is of public concern because such loss affects the entire family over a life time. Such salient nutrient loss can lead to “hidden hunger.” It has been scientifically and medically proven, that “hidden hunger” which is caused by deficiency of micronutrients is one of the major causes of morbidity. Hidden hunger is a subtle enemy that drains away health and vitality unnoticed until it is too late to reverse (Harvestplus 2015; Gbogbo et al. 2018). Since hidden hunger is mainly caused by deficiency of micronutrients, and is a public concern, it is imperative that the investigation of the retention of food micronutrient as influenced by cooking pots be examined. This is especially important in developing economies where Food and Health administration laws may be weak and unenforceable for material-type used for fabricating cooking utensils.
Evaluation of the impact of cooking pot on heat sensitive micronutrients such as vitamin C, A, B1, Ca, etc. is an indirect way of assessing the impact of the use of cooking pots on public health. Vitamin C is an essential nutrient in human diet, but is easily reduced or destroyed by exposure to heat and oxygen while vitamin B1 is most unstable under heat (Lee et al. 2018). The vitamins selected for this assessment belong to the two main groups of vitamins: fat-soluble and water-soluble vitamins. Hence the true retention of these heat sensitive nutrients can be used as yardstick for food nutrient retention in any cooking condition. The above mentioned essential micronutrients are powerful antioxidants that are expected to be present in human daily diets (Gerald et al. 2016; Tian et al. 2016). True retention is an important component defining the ultimate importance of vitamins in the consumed food, (https://www.ars.usda.gov accessed on 10th January, 2020). The objective of this study was to determine the magnitude of loss of nutrients as influenced by type of cooking pot.
Materials and methods
The cooking pots used included new (non-pitted) aluminum (NPA), old (pitted) aluminum (OPA), stainless steel (SSP), titanium (clad) (TCP), enamel coated (ECP), and iron-cast (ICP). The choice of the pots was based on their structural material (thermal diffusivity), design of their lid and availability. The titanium (clad) pots cook under vacuum while the rest of the pots cook under varying pressures depending on the fitness of their lids. Food stuff used for the experiments were chosen from the major classes of food as to include tubers (yam), cereals (rice), fleshy (beef), vegetables (tomato) and legumes (cowpea). The heating regime given to each cooking pot was the same for each food, and enough to cook each food to doneness.
Cooking of the food items
All the food items were divided into seven (7) equal parts. One portion was kept for raw analysis while the remaining portions were cooked differently with the six experimental cooking pots. Except for tomatoes which was cooked with lowest setting (number 1) of gas stove, the other food stuffs were cooked with the gas stove set at number 2. Cooking time was 5 min for tomatoes, 40 min for yam, 45 for rice and beef, and 70 min for beans (Oloyin). Each food item was washed and rinsed with distilled water before cooking with the different pots. For tomatoes, 1 kg was sliced (10 mm thickness) into each pot and boiled with 20 ml distilled water. Five hundred grams each of rice and beans were cooked differently in the different cooking pots using 800 ml of distilled water. Seven slices (2 cm thickness) of yam tuber was cooked with the different pots while 500 g of beef was manually cut into smaller pieces (10 mm) using a sterile sharp stainless steel knife for each pot. Generally, after cooking all the samples were sieved to remove water, cooled in glass beakers at room temperature and coded for laboratory analysis.
Analysis
Proximate analyses were done using the standard methods of analysis of AOAC (2010). The vitamin profile of the samples was analysed following AOAC methods 992.03, 992.04 and 992.26 (2006). The analysis was done under standard conditions in a HP (Hewleth Packard) Gas chromatography (Model Rev, A09.01 equipped with 1206 Software) calibrated with selected standards.
Analysis of vitamins A
This was done using AOAC (2010) spectrophotometric method involving extraction of pigment with 1:1 v/v acetone-n-hexane solution, saponification and isolation of unsaponified extract using methanolic potassium hydroxide. The saponified extract was dried over anhydrous sodium hydroxide, evaporated to dryness and then made up to 10 ml with acetone-n-hexane. The mixture was chromatograph in a column of manganese oxide-hydro super-gel using 3.5% acetone-n-hexane. This separates carotene from other pigments that were not removed by saponification. The concentration of β-carotene was thus calculated by the following expression:
| 2 |
where T = absorbance, V = volume of eluate-100, L = depth of cuvette − 1 cm, W = original weight of sample, = 43,336 nm.
Analysis of vitamin B1
The method adopted was as described by Anderson and Becky (2002) with little modifications. The vitamin was first extracted before being determined using a fluorimetric/colorimetric approach. Fifty grams of the sample were homogenized with ethanoic sodium hydroxide (50 ml) and filtered into a 100 ml flask. Then, 10 ml of the filtrate was pipetted and the color developed by addition of 10 ml potassium dichromate. Both the filtrate and the blank samples were read with the colorimeter at 360 nm wavelength.
Analysis of vitamins C
The vitamin C content was determined by spectrophotometry method as described by AOAC (2006) and Rahman et al. (2007) using 2,4-dinitrophenylhydrazine. Both cooked and uncooked samples (10 g) were homogenized with 50 ml of distilled water and 2 ml of each sample homogenate was pipetted into test tubes; 38 ml of 0.5% oxalic acid was added, mixed and incubated at 50 °C for 1 h. The tubes were transferred to an ice-water bath for 5 min, 2.5 ml of 85% H2SO4 was added to the tubes in the ice-water bath drop-wise with mixing after each drop. The test tubes were removed from the ice-bath and left to cool at room temperature for 30 min. The absorbance was measured at 520 nm against the blank (2 ml of 4% TCA with 1 ml of 2,4-dinitrophenylhydrazine).
| 3 |
Analysis of mineral content
The mineral content of the samples was analyzed using Atomic Absorption Spectrophotometer (Chem. Tech. AAS Model CTA2000) while Flame Photometer (Jenway PFP7, Sheffield, UK) was used to determine sodium and potassium contents.
Determination of boiling point
The boiling point of pure solvent (water) inside the cooking pot was estimated using Antoine Eq. (2).
| 4 |
where p* = vapour pressure of pure solvent in mmHg, T = Temperature in °C, A, B, C are constants. For water, the values for A, B and C are respectively 8.10765, 1750.286 and 235 for temperatures from 0 to 60 °C For temperatures between 60 and 150 °C, the respective values are 7.96681, 1668.210 and 228.0 (Felder et al. 2016).
The vapor pressure used in equation (4) is estimated to be equal to the ambient pressure of the cooking pot. With the mole fraction of the solute (food being cooked) being relatively low () in comparison to that of solvent (water), we can use Raoult’s law and estimate the effective vapour pressure of water during the cooking solution. The estimate of the boiling temperature elevation of our samples at any given vapour (cooking pot pressure) pressure can be estimated using Eq. (1) above.
Determination of true retention of nutrients during cooking
True retention (TR) values for all vitamins were calculated using the formula given by the United States Department of Agriculture, USDA in 2007 (https://www.ars.usda.gov/ accessed on 10th January, 2020) as shown in Eq. 5.
| 5 |
Data obtained were subjected to ANOVA on windows using SASS 9.2 and the means separated by LSD, with a significance level of p < 0.05.
Results and discussion
The comparison of proximate composition of food cooked with different pots is shown in Table 1 With respect to the moisture content all the pots show increased moisture content of food relative to the raw food. This is expected. Interesting observation is that while the old aluminum pot has the highest moisture content, both the enamel ware and titanium pots have the least amount of moisture in the cooked food. A further comparison of other food contents shows a decrease in protein, ash, fiber and fat contents of cooked food relative to the raw food. With respect to the order of importance, Table 1 shows that the Titanium pot cooked food consistently retained proximate food composition closer to the raw food. This suggests that based on the afore-mentioned (protein, fiber, fat, ash and moisture) content of the cooked food, titanium is most preferable to use for cooking. The reason adduced for the desirable performance of Titanium and enamel ware is their low thermal diffusivity. While Aluminum has a thermal diffusivity of , pure titanium has a diffusivity of and this low thermal diffusivity could account for high values observed in Table 1 for Titanium pot. An important point from Table 1 is the suggestion to do away with an old aluminum cooking pot since the results obtained with the old aluminum pot are consistently the worst of all the examined pots. Advisedly, it is suggested to get rid of an aluminum pot after two or three years of use.
Table 1.
Proximate composition of food as affected by cooking pot
| Pot type | Proximate content of cooked food (g/100 g) | ||||
|---|---|---|---|---|---|
| Moisture | Protein | Ash | Fiber | Fat | |
| Raw food | 47.67a ± 2.61 | 11.51a | 1.5a | 2.36a ± 1.69 | 1.40a ± 0.86 |
| Titanium | 68.67b ± 3.09 | 8.63b | 0.95b | 1.78b ± 1.18 | 0.95b ± 0.83 |
| Enamelware | 68.83b ± 1.29 | 7.95b | 0.91b | 1.68b ± 1.15 | 0.85bc ± 0.51 |
| Stainless | 73.91b ± 0.69 | 6.75b | 0.77bc | 1.46bc ± 1.12 | 0.81bc ± 0.87 |
| Cast iron | 74.26b ± 1.62 | 6.57b | 0.74bc | 1.24c ± 0.15 | 0.65c ± 0.79 |
| New aluminum | 76.53b ± 4.19 | 6.57b | 0.65c | 1.19c ± 0.95 | 0.68c ± 0.79 |
| Old aluminum | 77.89b ± 3.39 | 6.51b | 0.59c | 1.14c ± 0.91 | 0.63c ± 0.68 |
| LSD | 14.01 | 0.021 | 0.20 | 0.022 | 0.18 |
Average of five different food stuffs
Mean values in the same column having the same superscript are not significantly different (p < 0.05)
The micronutrients of the cooked foods were more negatively affected compared to macronutrients (Table 2). Significant (p < 0.05) portions of some micronutrients namely vitamin C, vitamin B1 and selenium were mostly affected compared with others. Among the micronutrients, the vitamins were more negatively affected than the minerals (Tables 2 and 3). Within the vitamins, thiamine was mostly lost followed by vitamin C and then A while selenium was mostly lost among the minerals. Cooking pot forged from titanium offered best protection (retention) of micronutrients while OPA offered the least retention irrespective of the food sample cooked. There was no significant difference (p < 0.05) in vitamin A (retinol) content of all the samples analyzed, though uncooked samples had highest content of 206.57 µg/kg, followed by samples cooked in TCP with value of 198.94 µg/kg. This observation may be attributed to the fact that vitamin A is rarely lost during cooking due to its thermo-stable and water-insoluble nature. This result is in agreement with previous reports which stated that cooking had little or no significant deleterious effect on retinol content of foods (FAO 2002; Kabiru and Sal 2004), rather cooking increases the bioavailability of the vitamin A for easy digestion (Kabiru and Sal 2004). Although the uncooked sample showed no significant difference (p < 0.05) from cooked ones, the vitamin A content in the cooked foods maintained a decreasing trend. According to Kreauler et al. (2006) there are many other factors other than heat and water responsible for the destruction of vitamins in foods such as exposure to air (oxidation), exposure to light, pH of the solution, and storage time.
Table 2.
Effect of cooking pot on quantity of vitamins and minerals preserved in cooked food
| Nutrient | Types of cooking pot | ||||||
|---|---|---|---|---|---|---|---|
| Raw food | Titanium | Stainless steel | Enamel ware | Cast iron | Old aluminum | New aluminum | |
| Vitamins (µg/kg) | |||||||
| Vitamin A | 206.57a ± 16.89 | 198.94a ± 9.35 | 157.91a ± 10.37 | 188.94a ± 5.35 | 147.94a ± 3.65 | 141.39a ± 28.04 | 148.88a ± 29.21 |
| Vitamin B1 | 2.27a ± 2.88 | 1.93ab ± 2.52 | 1.66bc ± 2.26 | 1.76bc ± 1.29 | 1.46c ± 2.12 | 1.29c ± 2.10 | 1.47c ± 2.09 |
| Vitamin C | 9.88a ± 8.11 | 9.28ab ± 7.92 | 8.43b ± 7.23 | 7.13b ± 5.34 | 6.42c ± 2.23 | 6.04c ± 6.27 | 6.24c ± 5.40 |
| Minerals (mg/kg) | |||||||
| Sodium | 111.29a ± 2.38 | 107.48a ± 0.23 | 97.15a ± 5.62 | 95.55a ± 6.66 | 93.91a ± 5.44 | 93.18a ± 7.30 | 93.77a ± 3.80 |
| Potassium | 410.67a ± 18.20 | 351.81b ± 2.70 | 323.11bc ± 56.19 | 343.21c ± 1.16 | 315.01 ± 8.26 | 293.74c ± 4.17 | 302.36c ± 9.00 |
| Selenium | 9.19a ± 1.46 | 7.85b ± 0.81 | 7.04c ± 0.87 | 7.79c ± 06.19 | 6.26c ± 0.83 | 6.43d ± 0.55 | 6.69d ± 1.71 |
| Magnesium | 136.53a ± 19.36 | 122.59ab ± 12.90 | 113.69bc ± 10.97 | 161.7 9 ± 2.19 | 143.62e ± 0.67 | 136.53a ± 29.36 | 109.10c ± 9.86 |
| Calcium | 30.83a ± 21.83 | 26.75b ± 19.47 | 25.94b ± 18.69 | 26.25b ± 18.69 | 24.69b ± 17.42 | 24.59b ± 7.61 | 24.79b ± 17.42 |
| Zinc | 5.08a ± 0.23 | 4.68b ± 0.10 | 4.25c ± 0.61 | 5.04 ± 0.46 | 4.27 ± 0.21 | 4.05d ± 0.66 | 4.25c ± 0.51 |
Mean values in the same row having the same superscript are not significantly different (p < 0.05). Average of five different food stuffs
Table 3.
Percentage (%) retention of food nutrients by the various cooking pots
| Nutrient | Types of cooking pot | |||||
|---|---|---|---|---|---|---|
| Titanium | Stainless steel | Enamel ware | Cast Iron | New aluminum | Old aluminum | |
| Vitamins | ||||||
| Vitamin A | 96.31 | 76.44 | 91.47 | 71.62 | 72.07 | 68.45 |
| Vitamin B1 | 85.02 | 73.13 | 77.53 | 64.32 | 64.76 | 61.03 |
| Vitamin C | 93.93 | 85.32 | 72.17 | 62.98 | 63.18 | 61.23 |
| Minerals | ||||||
| Sodium | 96.58 | 87.29 | 85.86 | 84.38 | 84.26 | 83.73 |
| Potassium | 85.67 | 78.68 | 83.57 | 76.71 | 73.63 | 72.53 |
| Selenium | 85.42 | 76.61 | 84.77 | 68.12 | 72.8 | 72.14 |
| Magnesium | 89.79 | 83.27 | 118.5 | 105.19 | 79.91 | 99.93 |
| Calcium | 86.77 | 84.14 | 85.14 | 80.08 | 80.41 | 79.76 |
| Zinc | 92.13 | 83.66 | 99.21 | 84.06 | 83.66 | 79.72 |
The vitamin B1 content (2.27 µg/kg) of uncooked and those cooked with TCP (1.93 µg/kg) did not vary significantly (p < 0.05). But there were significant differences in thiamine contents between the raw and samples cooked in the other tested pots. Apart from TCP enamel coated pot retained vitamin B1 more than the other pots. The lowest (61.03%) vitamin B1 retention occurred in foods cooked with OPA pot (Table 3). This may be attributed to high temperature and high thermal diffusivity and high pressure of the boiling water in the pot (Table 4). Our result tallies with the report of Lee et al. (2018)who reported that vitamin B1 is lost during cooking due to heat and leaching. The percentage loss of vitamin B1 from our study is in accordance with reports by Netdoctors (2015) where 20-35% of vitamin B1 was lost in cooking water. The proof that heat intensity and leaching are involved in the loss of thiamine offers explanation why TCP, the pot that cooks with little water, had higher vitamin B1 retention (85.02%) among other pots tested. In the same vein, food cooked with TCP had the highest (93.93%) value of vitamin C retention (Table 3) compared with the other pots. This may be attributed to effects of heat and leaching which are all dependent on structural material of the cooking pots. Hence foods cooked with aluminum and cast-iron pots recorded higher loss of vitamin C.
Table 4.
Boiling temperature and pressure of solvent and solute inside the various cooking pots
| Cooking pot | With water (solvent) | With Food (solute) | ||
|---|---|---|---|---|
| Temperature (°C) | Pressure (kPa) | Temperature (°C) | Pressure (kPa) | |
| Stainless steel | 105.28 | 123.60 | 116.48 | 177.90 |
| New aluminum | 121.30 | 207.40 | 129.92 | 269.70 |
| Old aluminum | 122.08 | 212.60 | 127.68 | 252.60 |
| Enamelware | 108.08 | 134.80 | 110.88 | 149.30 |
| Cast Iron | 118.17 | 187.90 | 125.44 | 237.60 |
| Titanium | 82.88 | 53.40 | 87.36 | 63.70 |
It has been reported that vitamin C is easily destroyed during processing and storage through the action of metals such as copper and iron, exposure to oxygen and prolonged heating (Lee et al. 2018). Afful, (2003) and Gernah (2007) reported that the rate of thermal diffusivity of a cooking pot affects the rate it conducts heat by convection and hence the rate it cooks and retains nutrients in food. Further explanation could be derived from low thermal diffusivity rate of titanium, from which TCP was forged from (Cookwares 2010). In addition to the above, Garba (2011) stated that heating (especially cooking in open pots) can cause huge losses of vitamin C because of its thermo liable nature. Our findings agree with Garba (2011) because we observed that food cooked in OPA, with loose lid fitting, recorded the lowest retention (61.13%) of vitamins C.
Samples cooked in TCP had a better potassium retention (85.67%) followed by those cooked with ECP (Table 3). Samples from NPA and OPA showed no significant variation (p < 0.05) among themselves and with that from SSP. The statistical similarity observed between NPA, OPA and SSP cooking pots in terms of potassium retention is contrary to expectations since the thermal diffusivity of aluminum material is much higher than that of stainless steel. Limited literature exist on how cooking conditions affects the selenium concentration in stable foods. Lestienne et al. (2005) analyzed the content of Se in cowpea with and without previous soaking and noted a reduction in the Se content after the cooking treatment. However, these results were evaluated in the soaked form, whereas in our studies, the Se content was observed after heat treatment, because most food stuffs are typically consumed as cooked product. Again, like in all the nutrients tested the samples cooked in TCP had a higher value of Se while the least was observed in the OPA pot.
We observed that titanium and enamel coated cooking pots required less quantity of water to get food done. The use of less water to cook resulted to low (68.67%) moisture content of food cooked in such pots compared to values as high as 77.89% when other pots were used (Table 1). Food cooked with less water may have better taste (Mohammad et al. 2011) since they are more nutrient-dense; having considerable lower moisture content. According to Miller (1996), loss of minerals during cooking is not caused by destruction but by leaching (extraction of minerals by water) into the cooking water which is later discarded. So pots that cook with little water are likely to preserve the minerals and volatiles in the food being cooked. Previous study by Zheng et al. (2011) reported high moisture cooked food to have low taste, low storage quality and lesser nutritional value due to the loss of vitamins, minerals and volatiles (Lee et al. 2018).
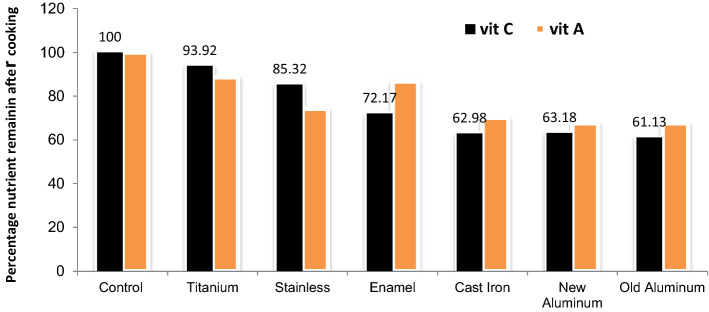
We observed that Titanium pot cooks food under vacuum (low pressure), making it possible for water therein to boil below 100 °C precisely around 87.36 °C (Table 4). Our observation concurs with the report of Schurle (2006). This may be as a result of the pot lid design. Unlike the regular cooking pots in Nigeria, the titanium (salad master) pot have inbuilt sensitive one-way valves that operates like vacuum pump. These valves at the pot lid opens up at the vapour pressure of the cooking mixture () which is well below the vapour pressure of pure solvent at that condition and shuts off immediately. The implication is that food materials in the pots do not reach the boiling point of pure water which is 100 °C at 1 atm and so retains their nutrients as our analysis showed in Tables 2, 3 and Fig. 1. It is crucial to state here that our findings, complemented and is in agreement with those of Mougin et al. (2015), who provided an alternative to the previous idea of using pressure pot to cook food. Pressure pot mounts “pressure” on the nutrients of food, elevating boiling temperature (see Eq. (1)) which is not desirable and in the best interest of public health. The regular cooking pots having loose lids were unable to cook food at temperatures below 100 °C (Table 3). The net effect is that regular cooking pots are unable to operate below 100 °C or at best are controlled subjectively. Low nutrient content of foods cooked with the aluminum pots could be as a result of the obvious interaction between food material and aluminum, and again the design of the pot lids being loose could not permit vacuum cooking conditions. Aluminum is not an inert material hence is prone to react with food materials at extreme pH conditions.
Fig. 1.
Influence of cooking pot on loss of vitamins A and C of cooked food
The difference in nutritional values of food cooked in the different pots may be as a result of the structural differences in the materials used in forging each cooking pot. These structural materials determine the thermal diffusivity of particular cooking pot hence the rate of heat transfer and quantity of water to be used for cooking (Cookwares 2010; Zheng et al. 2011). Materials with high thermal diffusivity cook faster due to their fast conduction and convection rate but also leads to quicker evaporations and loss of food nutrients due to destruction of heat unstable nutrients, leaching as well as increased demand for replacement of water. One could infer from our data that aluminum cooking pots have high thermal diffusivity compared to TCP and SSP. From Table 4 it can be observed that solvent inside OPA boils at 122.08 °C and had steam pressure of 212.6 kpa meaning that the solvent boiling occurred at above 1 atm. This implies that OPA cooks food with pressures above 1 atm hence the resultant negative impact on food nutrients. OPA also had the most unfitted lid (probably because of the long usage of the pot) giving rise to higher temperature of boiling (Felder et al. 2016).
Conclusion
The material compositions, as well as the pot lid design are vital parameters that influence the nutrient retention of food during cooking. Our research has evidence that cooking pot may have a strong impact on people’s health and morbidity. The basis for this claim is that cooking pot which reduces the micronutrients of cooked food will indirectly affect the vitality of the food consumers. The nutrient content of cooked food can be enhanced by using cooking pots that operates under low-pressure conditions. Food cooked with low temperature and low pressure would definitely be healthier because nutritive and biological values of such foods remain intact. Our findings stress the use of titanium and enamel cooking pots which offer better retention (protection) of food nutrients than the other pots tested. The result discourages use of an old aluminum pot for cooking. We believe that the information generated by this work is vital for food consumers, manufacturers of cook wares, operators and key players in the food processing industry.
Author contributions
Prof U.E.O., conceived the concept, supervised the work and edited the manuscript while Mr. O.N.I. carried out the experiments and wrote part of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afful RK (2003) Leaching from cooking surfaces in school of champions. http://www.school-for-champions.com/health/cooking-surfaces.htm#vsn-mv2t.09. Accessed 22 Jan 2014
- Anderson UV, Becky CD. Trace of heavy metal level of same bouillon cubes and food condiments readily consumed in Nigeria. Pak J Nutr. 2002;6(2):46. [Google Scholar]
- AOAC (2006) Official methods of analysis. Association of Official Analysis Chemists. Methods 922.06, 920.87, 925.09 925.10, and 967.21
- AOAC . Method 992.23, in “Official methods of analysis”. 17. Gaithersburg: AOAC International; 2010. [Google Scholar]
- Cookwares (2010) Types of pots and pans. In: Cookware materials. http://www.m.recipetips.com/kitchen-tips/t—586/types-of-cookware-asp. Accessed 11 July 2018
- FAO . Nutrient composition and science of food. Rome: Food and Agriculture Organization of the United Nations; 2002. [Google Scholar]
- Felder RM, Rousseau RW, Bullard LG. Elementary principles of chemical processes. 4. Hoboken NJ: Wiley; 2016. [Google Scholar]
- Garba CU. Vitamin C as antioxidant: evaluation of its role in disease prevention. J Am Chem Nutr. 2011;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Gbogbo F, Arthur-Yartel A, Bondzie JA, Dorleku WP, Dadzie S, Kwansa-Bentum B, et al. Risk of heavy metal ingestion from the consumption of two commercially valuable species of fish from the fresh and coastal waters of Ghana. PLoS ONE. 2018;13(3):e0194682. doi: 10.1371/journal.pone.0194682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald F, Combs JR, McClung JP. The vitamins: fundamental aspects in nutrition and health. 5. Cambridge: Academic Press; 2016. pp. 3–6. [Google Scholar]
- Gernah HH (2007) Early pottery. Science and Information News. 5 June, 2007, p 44
- Greger JL, Goetz W, Sullivan D. Aluminum levels in foods cooked and stored in aluminum pans, trays and foil. J Food Protect. 1985;48(9):772–777. doi: 10.4315/0362-028X-48.9.772. [DOI] [PubMed] [Google Scholar]
- Harvestplus (2015) What is hidden hunger. http://www.Harvestplus.org. Accessed 14 Jan 2014
- Kabiru KI, Sal CF. Proximate composition and vitamin levels of different blanching treatments on vitamin retention in green leafy vegetables. J Food Agric. 2004;12(2):5112–5211. [Google Scholar]
- Kreauler SS, Owen FO, Berdanier LY, George W. Advance nutrient micronutrient. London: CRC Press; 2006. pp. 22–39. [Google Scholar]
- Lee S, Choi Y, Jeong HS, Lee J, Sung J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci Biotechnol. 2018;27(2):333–342. doi: 10.1007/s10068-017-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestienne I, Icard-Vernière C, Mouquet C, Picq C, Trèche S. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 2005;89(3):421–425. doi: 10.1016/j.foodchem.2004.03.040. [DOI] [Google Scholar]
- Miller D. Minerals. In: Fennema OR, editor. Food chemistry. 3. New York: Marcel Dekker books; 1996. pp. 639–640. [Google Scholar]
- Mohammad FS, AlZubaidy EAH, Bassioni G. Effect of Aluminum leaching processes of cooking wares on food. Int J Electrochem Sci. 2011;6:222–230. [Google Scholar]
- Mougin A, Mauroux O, Matthey-Doret W, Barcos EM, Beaud F, Bousbaine A, Viton F, Smarrito-Menozzi C. Impact of boiling conditions on the molecular and sensory profile of a vegetable broth. J Agric Food Chem. 2015;63(5):1393–1400. doi: 10.1021/jf506173m. [DOI] [PubMed] [Google Scholar]
- Netdoctors (2015) Vitamin B1 (thamine). http://M.netdoctor.co.uk/diet-and-nutrition/vitamin–B1.htm. Accessed 12 Jan 2014
- Rahman MM, Hosain MM, Khan MM. Analysis of vitamin C (ascorbic acid) content in various fruits and vegetable by UV-Spectrophotometry. Bangladesh J Sci Industr Res. 2007;42(4):417–424. doi: 10.3329/bjsir.v42i4.749. [DOI] [Google Scholar]
- Schurle UK (2006) Salad master 316 TI stainless steel titanium cookware. http://www.happycooking.co.com/316-ti-titanium-stainless-steel-cookware.cfm. Accessed 4 Aug 2014
- Stahl T, Falk S, Rohrbeck A, Georgii S, Herzog C, Wiegand A, Hotz S, Boschek B, Zorn H, Brunn H. Migration of aluminum from food contact materials to food—a health risk for consumers? Part I of III: exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environ Sci Eur. 2017;29(1):19. doi: 10.1186/s12302-017-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl T, Falk S, Rohrbeck A, Georgii S, Herzog C, Wiegand A, Hotz S, Boschek B, Zorn H, Brunn H. Migration of aluminum from food contact materials to food—a health risk for consumers? Part II of III: migration of aluminum from drinking bottles and moka pots made of aluminum to beverages. Environ Sci Eur. 2017;29(1):18. doi: 10.1186/s12302-017-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl T, Falk S, Rohrbeck A, Georgii S, Herzog C, Wiegand A, Hotz S, Boschek B, Zorn H, Brunn H. Migration of aluminum from food contact materials to food—a health risk for consumers? Part III of III: migration of aluminum to food from camping dishes and utensils made of aluminum. Environ Sci Eur. 2017;29(1):17. doi: 10.1186/s12302-017-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Safe Use of Cookware-Canada.ca. https://www.canada.ca/en/health-canada/services/household-products.html. Accessed 12 May 2018
- Tian J, Chen J, Lv F, Chen S, Chen J, Liu D, Ye X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016;197:1264–1270. doi: 10.1016/j.foodchem.2015.11.049. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, USDA Table of Nutrient Retention Factors, Release 6 (2007) https://www.ars.usda.gov. Accessed 10 Jan 2019
- Weidenhamer JD, Kobunski PA, Kuepouo G, Corbin RW, Gottesfeld P. Lead exposure from aluminum cookware in Cameroon. Science of the Total Environment. 2014;496:339–347. doi: 10.1016/j.scitotenv.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Zheng X-Z, Liu C-H, Chen Z-Y, Ding N-Y, Jin C-J. Effect of drying conditions on the texture and taste characteristics of rough rice. Dry Technol. 2011;29:2011. doi: 10.1080/07373937.2011.592032. [DOI] [Google Scholar]