Abstract
To access the nutritional quality of the Ruditapes philippinarum, a comprehensive quality evaluation procedure is always important to be established. In this study, fifteen nutritional quality evaluation indicators of R. philippinarum from 7 months were analyzed, and the most important indicators were determined using a combination of multiple chemometric methods such as correlation analysis (CA), principal component analysis (PCA), and system cluster analysis (SCA). Significant differences in nutritional quality were observed across the 7 months, as per the ANOVA results (P < 0.05). The coefficient of variation values for the fifteen evaluation indicators for R. philippinarum across 7 months was 1.67–43.47%. The CA results revealed that some indicators were correlated to each other within a certain range. Four principal components with eigen-values > 1 were obtained with PCA, and a cumulative contribution of 92.11% was achieved. In addition, four essential quality indicators were extracted using SCA. Using these four indicators, a simple and efficient procedure can be applied for quality control in aquaculture.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04796-6) contains supplementary material, which is available to authorized users.
Keywords: Ruditapes philippinarum, Evaluation indicator, Seasonal variation, Chemometric evaluation
Introduction
Ruditapes philippinarum is an important shellfish both in China and in the rest of the world that is rich in nutrients, including proteins, vitamins, and essential elements, and has become common in the human diet (Zhao and Zhang 2016a). Aquaculture production of R. philippinarum has expanded rapidly in recent decades. In China, R. philippinarum is mainly produced in Shandong province, especially in Jiaozhou Bay (Jiang 2014; Zhao and Zhang 2016b). Jiaozhou Bay is an important shellfish source, located in the northwest of the Yellow Sea (Zheng and Yan 2011). The area is rich in phytoplankton, has ambient water temperature, salinity, and pH, special muddy tidal flats, and many coastal rivers. These rivers carry some sediment and low-salt water into the sea every year, bringing in nutrients that provide good growth conditions for high-quality clams. The unique natural environment provides good conditions for Jiaozhou Bay clams which are famous for their thin shell, delicate meat, and delicious taste (Jiang 2014). However, their habitats include a large number of environmental variables, which follow annual and daily cycles, according to the geographical region, and influence their behavior, feeding, and metabolism (Barrento et al. 2009; Sardia et al. 2020; Shujin et al. 2019). Therefore, seasonal variations may have a significant effect on the nutritional quality and biochemical composition of Jiaozhou Bay clams. The nutritional quality of Jiaozhou Bay clams can be assessed using complex factors, including the proximate chemical composition, fatty acid profiles, flavor-enhancing nucleotides, and organic acid. Different quality factors, which are relatively independent but within a certain range closely related, have increased the challenges of overall evaluation (Bi et al. 2015). Therefore, developing a simpler and efficient method for evaluating the nutritional quality of Jiaozhou Bay clams is of utmost importance. Most studies have applied principal component analysis (PCA) or cluster analysis to select food evaluation indicators. Frau et al. (1999) evaluated the physical properties of Mahon cheese using PCA, and their results showed that parameters from the same group gave similar information. Xu et al. (2011) adopted cluster analysis to simplify apple quality-determining factors. The above studies filtered quality factors only, using PCA or cluster analysis separately.
In this study, R. philippinarum samples were collected from Jiaozhou Bay during seven different months, and their nutritional quality was evaluated using a series of indicators. PCA and system cluster analysis (SCA) were adopted to simplify the quality evaluation indicators of Jiaozhou Bay clams, which provided a scientific basis for further research on the quality evaluation of clams.
Materials and methods
Biological materials
Jiaozhou Bay (Shandong province) clams were sampled between March and September 2018, as they are available in the market in large quantities during this period every year (Zhao and Zhang 2016b). Two kilogram portions of clams were obtained directly from Jiaozhou at the same farm of each month. The obtained clam samples were rinsed with seawater at each wharf, iced, transported to the laboratory within 1 h, and stored in seawater for a day. Every month, ninety individuals of Jiaozhou Bay clams were selected, of which 30 individuals were combined into one testing sample in order to get enough amount of sample for meaningful analysis. For each clam, the weight, carapace width (CW), and carapace length (CL) indices were measured, and the total meat yield (TMY) was calculated. The TMY was calculated as follows: tissue wet weight (g)/body wet weight (g) × 100. For the biochemical analyses, the combined whole soft tissues separated from shells were homogenized separately and stored at − 80 °C until use.
Analytical procedure
Proximate chemical composition
The moisture, ash, protein, glycogen, and lipid contents were determined in each sample according to the Chinese national standards. Briefly, the sample was weight (~ 2 g, m1) and dried in an oven (laboratory heater, Shanghai Xuan Cheng Instrument) at 105 ± 1 °C until a constant weight (m2) was obtained, then moisture content was calculated as follows: (m1 − m2)/m1 × 100 (GB5009.3-2016). The ash content measurement method (GB 5009.4-2016) was similar to the moisture content method according to gravimetric methods, and the sample was quantified after combustion for 4 h at 550 °C ± 25 °C (Shanghai Jingjing Precision Instrument Manufacturing, TYP. MR170). The glycogen content was determined using a glycogen kit (Nanjing Construction). The protein content was determined according to the Kjeldahl method (Kjeltec 8400 automatic Kjeldahl analyzer) with a conversion factor of 6.25 (GB 5009.5-2016). The total lipid content was analyzed using the Folch method by extraction with 2:1 (v/v) chloroform:methanol (Folch et al. 1957), and the total lipid content was determined by gravimetric analysis. All results are expressed in grams per 100 g of wet weight.
Fatty acid analysis
Fatty acid methyl esters (FAMEs) were prepared by saponifying approximately 10 mg of lipids. The sample was dissolved in 1 mL of 10% hydrochloric acid–methanol mixture, shaken, and heated (60 °C; 15 min) in a water bath (Ichihara et al. 1996). After cooling, 200 μL of n-hexane was added, followed by vortexing, then an aliquot (1 μL) of n-hexane layer was determined using a 6980 N gas chromatograph (Agilent Technologies, Santa Clara, CA) with a split injector (1:20 ratio), flame ionization detector, and 30-m HP-INNOWax quartz capillary column (30 m × 0.32 mm × 0.25 μm). The inlet temperature was 230 °C, the detector temperature was 250 °C, and the column temperature was increased from 170 to 210 °C at a speed of 3 °C/s and then maintained at 210 °C for 10 min. The entire analytical process took 36 min. Helium was used as the carrier gas at a flow rate of 43 cm/s (Anjum 2013). The FAs were identified by comparing the retention times with those of Sigma (St. Louis, MO) standard FAME mixtures. The relative peak area (%) of each compound was determined, and the results were expressed as FAME%. The total contents of saturated (SFAs), monounsaturated (MUFAs), polyunsaturated (PUFAs), omega-3, and omega-6 fatty acids were used to calculate the atherogenic (AI) and thrombogenic (TI) indices, i.e., the propensity of Jiaozhou Bay clams to influence the incidence of coronary heart disease: TI = (14:0 + 16:0 + 18:0)/(0.5 MUFA + 0.5 n-6 PUFA + 3n-3 PUFA + n-3 PUFA/n-6 PUFA); AI = (12:0 + 4 × 14:0 + 16:0)/(n-6 PUFA + n-3 PUFA + MUFA).
Flavor-enhancing nucleotides and organic acids
To analyze flavor-enhancing nucleotides, each frozen sample (~ 5 g) was weighed and homogenized with 15 mL of 10% perchloric acid (PCA), then centrifugation (12,000g, 15 min). The residue was extracted twice with 10 mL of 5% PCA. The supernatants were combined and neutralized with KOH solutions. The combined supernatants were diluted to 25 mL with distilled water and stored at − 30 °C (Wang et al. 2007). The PCA extract was filtered through a 0.22 µm membrane. A 10 µL portion of the filtrate was injected into a CAPCELLPAK C18 SG column (4.6 × 150 mm, SHISEIDO) equilibrated with a mixture of 20 mM citric acid, 20 mM acetic acid, and 40 mM triethylamine (pH 4.8). The flow rate was 0.8 mL/min and the column temperature was 40 °C. Elution was monitored by UV absorption at 260 nm. The standard compounds (purchased by Sigma): adenosine monophosphate (AMP), inosine monophosphate (IMP), hypoxanthine (Hx), adenosine diphosphate (ADP), guanosine monophosphate (GMP), and inosine (HxR) were analyzed by HPLC in a similar manner.
To determine the organic acid content, approximately 5.00 g of frozen sample was weighed and homogenized with 30 mL of 2.0% acidic dihydrogenamine (pH 2.5) followed by centrifugation at 12,000g for 15 min. The residue was extracted twice with 30 mL of 2.0% acidic dihydrogenamine. The procedure was repeated and the supernatant was combined and placed in a 50 mL volumetric flask. The extracts were stored at 4 °C (Weiss et al. 2001). A 20 µL portion of the filtrate was injected into a C18 column (4.6 × 150 mm, SHISEIDO) equilibrated with 2.0% NH4H2PO4 (PH 2.9). Elution was monitored by UV absorption at 205 nm. The flow rate was 1 mL/min and the column temperature was 40 °C. The standard compounds were purchased from Sigma and analyzed similarly.
For quantization, calibration curves were constructed using the HPLC peaks’ areas of various amounts of standard compounds. ATP-related compounds and organic acid were identified by comparing the retention times of HPLC peaks between samples and standard compounds.
Statistical analyses
All data were processed and analyzed using SPSS 22.0 (IBM, Chicago, IL). Single-factor analysis of variance (ANOVA) was applied to analyze the nutritional quality indicators of the Jiaozhou Bay clams collected over 7 months. The coefficient of variation (CV) of each indicator was calculated, revealing the differences between indicators in Jiaozhou Bay clams from different months. The raw data for each indicator was changed into standardized data within 0–1 by using maximum difference normalization. The main components of the quality assessment factor were based on the cumulative variance contribution rate using PCA. Finally, the characteristics of the nutritional quality of Jiaozhou Bay clams were obtained through SCA.
Results and discussion
To achieve a comprehensive quality evaluation of Jiaozhou Bay clams, overlapping and erroneous evaluation indicators were removed through the statistical methods of PCA, CA, and SCA to obtain relatively independent indicators; moreover, the data should obtain variation degree between varieties. The differences among the evaluation indicators and comprehensive evaluations between variables were identified using the CVs and ANOVA.
Analysis of variance (ANOVA) of seasonal variation of Jiaozhou Bay clam nutritional quality
The measured values of all the evaluation indicators of Jiaozhou Bay clams collected during seven different months are shown in Tables s 1–5. ANOVA results show that the nutritional quality of the clams varied significantly during the seven different months (P < 0.05) (Ginson et al. 2020).
Proximate composition of Jiaozhou Bay clams
The biometric data showed differences in Jiaozhou Bay clams across the seasons (Table s.1). The weight, CW, CL, and TMY, ranging from 3.83–8.67 g (5.58 ± 1.87 g, mean ± SD), 1.84–2.71 cm (2.20 ± 0.28 cm), 2.91–3.87 cm (3.32 ± 0.37 cm), and 24.74–35.86% (30.81 ± 4.41%), respectively, were the highest in June, and decreased thereafter. These seasonal differences may result from changes in nutrient supply, temperature, migration behavior, and reproductive strategies (Beninger and Lucas 1984; Qi et al. 2006). Significant differences were also detected in the approximate chemical composition of edible tissues throughout the season (Table s.2). The average contents of moisture, protein, ash, fat, and glycogen in Jiaozhou Bay clams were 77.35 ± 1.39 g/100 g, 10.90 ± 1.39 g/100 g, 3.24 ± 0.31 g/100 g, 1.61 ± 0.43 g/100 g, and 2.75 ± 0.52 g/100 g, respectively. In general, when food is abundant, the reserves accumulate in the form of glycogen, lipids, and protein substrates, which then are used for gamete production when metabolic requirements are high (Michel and Pierre 2011). Generally, the spawning period of Jiaozhou Bay clams is between July and September (Jiang 2014). As seen in Table s 2, the contents of protein and glycogen peaked in June, declined in July, rose in August, and fell again in September. The fat content peaked in April, then fell, and rose again in August. Therefore, it can be speculated that the substance consumed during gametogenesis is fat, whereas the substances consumed during the spawning period include protein and glycogen. Similar variations among tissues and seasons have been previously reported for the chemical composition of other seafood, such as pink and red shrimps (Cartes et al. 2008) and Atlantic spider crab (Marques et al. 2010).
Fatty acid (FA) content of Jiaozhou Bay clams
The main FAs are presented in Table s 3, along with the statistical differences across months. Among SFAs, the contents of C14:0, C16:0, and C18:0 were high, whereas among MUFAs, the contents of C16:1, C18:1, and C20:1 were relatively high. For PUFAs, the contents of C18:2n-6, C20:4 (n-6), C20:5 (n-3), and C22:6 (n-3) were high. The contents of SFAs rise from March to August, with the highest content in August and the lowest in March and September. The contents of MUFAs were very low in July and September, with the highest being in June. Similarly, the contents of PUFAs were very low in July and September, but peaked in March. As a result, relatively high levels of AI and TI occurred in July and September. The main n-3 PUFAs are EPA (20:5n-3) and DHA (22:6n-3); the content of EPA is the lowest in May, whereas the content of DHA began to rise in March, peaked in May, and then fell, similar to the trend for total fat. Furthermore, the n-3/n-6 content was higher in March and April. Seasonal changes in SFAs and MUFAs in clams have been reported in previous studies, indicating that these FAs might play an important role in embryonic/early larval development and sexual maturation. This fact may be related to the mobilization of important lipids from muscle to gonads during the summer breeding season to promote maturation and reproduction (Teshima et al. 1989).
Flavor-enhancing nucleotides and organic acids of Jiaozhou Bay clams
As the physical and chemical components of muscles in aquatic animals vary with the seasons, their taste also has seasonal differences. In shellfish, the composition and content of substances with different tastes are quite different and account for their different taste characteristics. The main taste nucleotides in Jiaozhou Bay clams are AMP, IMP, Hx, ADP, GMP and HxR (Liu et al. 2014), as listed in Table s 4. Among them, the contents of IMP and AMP were the highest. The total taste-related nucleotide content was lowest in May and highest in April and August. The IMP content was higher during June–September. The Hx content was higher in May and July, and lowest in August and September. The HxR content was higher in April and July.
Common organic acids in fish and shellfish include lactic acid, fumaric acid, succinic acid, citric acid, oxalic acid, and other acids. In addition to regulating pH, these organic acids also contribute to flavor. The main organic acids associated with the taste of fish and shellfish are lactic acid and succinic acid (Kilinc and Cakli 2005), as listed in Table s 5. The total content of taste-enhancing organic acids was the highest in July and lowest in June. The content of succinic acid was the highest in August and lowest in March and June, whereas that of lactic acid was the highest in July and lowest in June.
The characteristics of the fifteen evaluation indicators of Jiaozhou Bay clams are shown in Table 1. Of the evaluation indicators, the CV of flavor-enhancing organic acids was the largest (43.47%). Weight, which ranged from 3.83 to 8.67 g, had the next highest CV (30.99%). By contrast, the CVs of water, protein, ash, and SFA were all less than 10% (1.67%, 5.54%, 8.82%, and 9.81%, respectively) and the degrees of dispersion were also small. These results provide an effective basis for screening the assessment indicators (Bi et al. 2015).
Table 1.
Descriptive statistics of Ruditapes philippinarum nutritional characterization
| Quality parameter | Luffinga | Mean | Standard deviation | CV (%)b |
|---|---|---|---|---|
| X1 | 3.83–8.67 | 5.58 | 1.73 | 30.99 |
| X2 | 2.91–3.87 | 3.32 | 0.34 | 10.39 |
| X3 | 1.84–2.71 | 2.20 | 0.26 | 11.82 |
| X4 | 24.74–35.86 | 30.81 | 4.08 | 13.24 |
| X5 | 76.13–79.9 | 77.36 | 1.29 | 1.67 |
| X6 | 9.57–11.54 | 10.90 | 0.60 | 5.54 |
| X7 | 2.72–3.63 | 3.24 | 0.29 | 8.82 |
| X8 | 1.21–2.29 | 1.61 | 0.39 | 24.49 |
| X9 | 3.26–5.07 | 3.90 | 0.61 | 15.68 |
| X10 | 2.21–3.57 | 2.75 | 0.49 | 17.66 |
| X11 | 138.01–235.53 | 156.94 | 32.50 | 20.71 |
| X12 | 28.36–153.8 | 84.60 | 36.78 | 43.47 |
| X13 | 24.94–32.87 | 28.53 | 2.80 | 9.81 |
| X14 | 7.33–15.11 | 10.68 | 3.11 | 29.15 |
| X15 | 18.37–34.12 | 26.77 | 7.08 | 26.43 |
X1–X15: weight (g), carapace width (cm), carapace length (cm), total meat yield (%), moisture content (g/100 g), crude proteins (g/100 g), ash (g/100 g), crude fat (g/100 g), water-soluble proteins (g/100 g), glycogen (g/100 g), main flavor-enhancing nucleotides, main flavor-enhancing organic acids, saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs)
aRange of quality parameters between the minimum and maximum values
bCoefficient of variation
Correlation analysis (CA) of Jiaozhou Bay clam quality indicators
Through the CA method, the relationship between different nutritional quality evaluation indicators of Jiaozhou Bay clams was determined. Prior to CA, raw data were converted to standardized data, ranging between 0 and 1. CA quantifies the relationship between two variables and measures the correlation coefficient (r) (-1 to +1). As shown by the CA results presented in Table 2, the individual sizes were positively correlated with glycogen (r = 0.639), TMY was positively correlated with PUFAs (r = 0.921) and with fat (r = 0.932), and protein was negatively correlated with water-soluble proteins water (r = − 0.807). The information provided by each quality trait overlapped, and the effect of each individual indicator on the quality of clams was not the same; therefore, direct use of these indicators cannot accurately evaluate the comprehensive quality of clams.
Table 2.
Correlation coefficient matrix of the nutritional quality evaluation indicators of Ruditapes philippinarum
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | X12 | X13 | X14 | X15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | 1 | ||||||||||||||
| X2 | 0.914 | 1 | |||||||||||||
| X3 | 0.836 | 0.925 | 1 | ||||||||||||
| X4 | − 0.087 | 0.111 | 0.038 | 1 | |||||||||||
| X5 | 0.518 | 0.545 | 0.437 | − 0.494 | 1 | ||||||||||
| X6 | 0.39 | 0.3 | 0.44 | − 0.764 | 0.391 | 1 | |||||||||
| X7 | − 0.03 | − 0.044 | 0.141 | − 0.827 | 0.329 | 0.834 | 1 | ||||||||
| X8 | − 0.279 | − 0.029 | − 0.045 | 0.932 | − 0.6 | − 0.715 | − 0.615 | 1 | |||||||
| X9 | − 0.653 | − 0.445 | − 0.32 | 0.676 | − 0.807 | − 0.496 | − 0.31 | 0.821 | 1 | ||||||
| X10 | 0.639 | 0.374 | 0.501 | − 0.434 | 0.045 | 0.623 | 0.349 | − 0.466 | − 0.476 | 1 | |||||
| X11 | − 0.396 | − 0.07 | 0.12 | 0.46 | − 0.432 | − 0.11 | 0.101 | 0.682 | 0.809 | − 0.34 | 1 | ||||
| X12 | − 0.317 | − 0.19 | − 0.242 | − 0.368 | 0.407 | 0.306 | 0.371 | − 0.369 | − 0.103 | − 0.51 | 0.021 | 1 | |||
| X13 | 0.31 | 0.233 | 0.364 | − 0.346 | 0.335 | 0.583 | 0.249 | − 0.539 | − 0.307 | 0.273 | − 0.192 | 0.431 | 1 | ||
| X14 | 0.185 | 0.337 | 0.435 | 0.71 | − 0.257 | − 0.489 | − 0.469 | 0.714 | 0.415 | 0.009 | 0.464 | − 0.701 | − 0.304 | 1 | |
| X15 | 0.198 | 0.32 | 0.232 | 0.921 | − 0.434 | − 0.657 | − 0.791 | 0.848 | 0.467 | − 0.114 | 0.306 | − 0.648 | − 0.435 | 0.797 | 1 |
X1–X15: weight (g), carapace width (cm), carapace length (cm), total meat yield (%), moisture content (g/100 g), crude proteins (g/100 g), ash (g/100 g), crude fat (g/100 g), water-soluble proteins (g/100 g), glycogen (g/100 g), main flavor-enhancing nucleotides, main flavor-enhancing organic acids, saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs)
Principal component analysis (PCA) of Jiaozhou Bay clam quality indicators
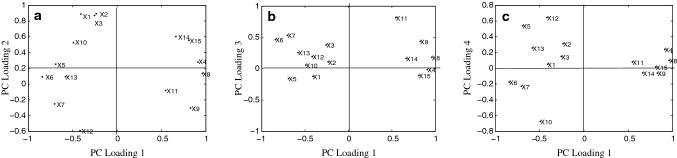
PCA not only reduces the dimensions, but also highlights the relationship between the elements (Najafi et al. 2019; Wang and Liu 2010). The gravel map (Fig. 1) can be used to determine the optimal number of principal components (PCs). In the gravel diagram, the abscissa indicates the number of PCs and the ordinate indicates the eigen-values, and the eigen-vales of the PCs were only taken from the steep portion of the curve. In this experiment, each eigen-value λ > 1 was considered, and the optimal PC number was determined by considering the gravel map and the variance contribution rate (Shin et al. 2010). As seen from Fig. 1, the eigen-values of the first four PCs are large (λ > 1) and the connection was steep, i.e., the first four PCs contribute the most to the explanatory variables. The cumulative variance contribution rate was 92.11% (Table 3), which combined most of the information for Jiaozhou Bay clams. The first four principal components were explained separately 43.74%, 26.63%, 11.64%, and 10.11% of the total data (Table 3). The PC1 represents the biggest change (Nowicka et al. 2019). The Varimax rotated factor loadings of the first five PCs are shown in Table 3 and Fig. 2. From Table 3, it was clear that the first PC mainly reflects the TMY, protein, fat, and water-soluble protein, reflecting its basic index factor. The second PC includes mainly the weight, length, and width of the individual, mainly reflecting its appearance quality. The third PC was determined to correspond to total nucleotides, whereas the main components of the fourth PC were determined to be organic acids and glycogen. The third and fourth PCs together reflected the taste factor. As shown in Fig. 2, the distance between the plots of weight, CL, CW, fat content, and moisture was very small, indicating an overlap of these indicators. Therefore, it was reasonable to classify them into four categories using SCA.
Fig. 1.
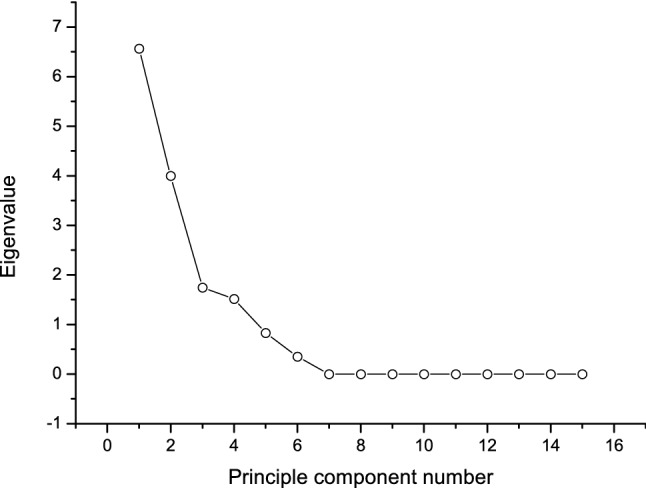
Screen plot of principal component analysis (PCA)
Table 3.
Varimax rotated factor loadings of the four principal components
| Quality parameter | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| X1 | − 0.409 | 0.89 | − 0.121 | 0.055 |
| X2 | − 0.234 | 0.895 | 0.11 | 0.313 |
| X3 | − 0.255 | 0.873 | 0.382 | 0.152 |
| X4 | 0.91 | 0.282 | − 0.013 | 0.242 |
| X5 | − 0.692 | 0.255 | − 0.155 | 0.541 |
| X6 | − 0.842 | 0.094 | 0.467 | − 0.173 |
| X7 | − 0.704 | − 0.249 | 0.536 | − 0.227 |
| X8 | 0.961 | 0.135 | 0.183 | 0.102 |
| X9 | 0.828 | − 0.302 | 0.44 | − 0.059 |
| X10 | − 0.491 | 0.527 | 0.059 | − 0.673 |
| X11 | 0.546 | − 0.079 | 0.817 | 0.082 |
| X12 | − 0.417 | − 0.588 | 0.197 | 0.645 |
| X13 | − 0.581 | 0.094 | 0.258 | 0.26 |
| X14 | 0.664 | 0.606 | 0.165 | − 0.057 |
| X15 | 0.811 | 0.555 | − 0.106 | 0.02 |
| Characteristic root | 6.56 | 3.994 | 1.746 | 1.517 |
| % of variance | 43.735 | 26.628 | 11.64 | 10.111 |
| Cumulative % | 43.735 | 70.363 | 82.003 | 92.114 |
X1–X15: Weight (g), carapace width (cm), carapace length (cm), total meat yield (%), moisture content (g/100 g), crude proteins (g/100 g), ash (g/100 g), crude fat (g/100 g), water-soluble proteins (g/100 g), glycogen (g/100 g), main flavor-enhancing nucleotides, main flavor-enhancing organic acids, saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs)
Fig. 2.
Varimax rotated principal component loadings. a PC loading 1 versus PC loading 2; b PC loading 1 versus PC loading 3; and c PC loading 1 versus PC loading 4
System cluster analysis (SCA) of Jiaozhou Bay clam quality indicators
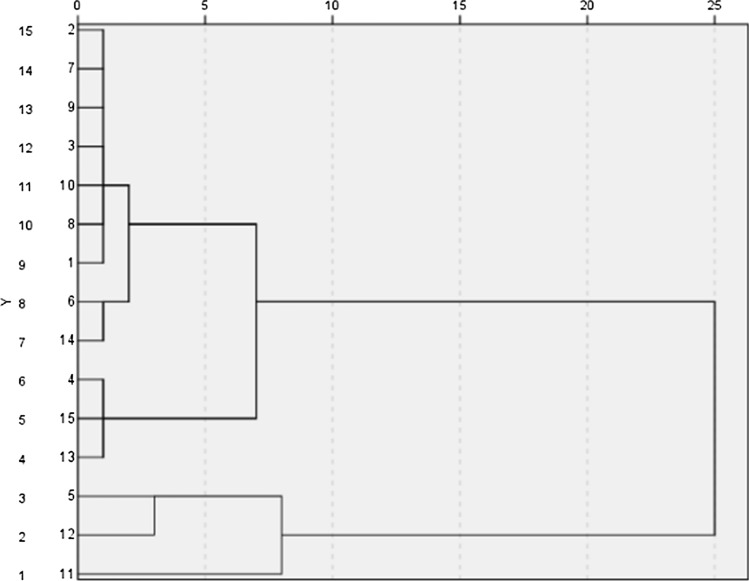
SCA is one of the most used unsupervised pattern recognition techniques. It is a method involving hierarchical grouping of samples based on similarity without using prior information (Bi et al. 2015). A SCA tree diagram is shown in Fig. 3. The following four groups were obtained from SCA when the cluster distance was 5.
Group 1: individual length, ash, water soluble protein, individual width, glycogen, fat, individual weight, protein, and MUFA.
Group 2: TMY, PUFA, and SFA.
Group 3: moisture and flavor-enhancing organic acids.
Group 4: flavor-enhancing nucleotides.
Fig. 3.
Dendrogram of system cluster analysis for 15 evaluation indicators
Because there were correlations between evaluation indicators within each group, the representative indicator should be chosen. In group 1, based on the ANOVA results, the CVs of protein, ash, and SFA were all smaller than 10% (5.54%, 8.82%, and 9.81%, respectively), as fat with a CV value of 24.49% had a greater impact on the nutritional quality of Jiaozhou Bay clams. In addition, the measurement of fat was relatively easy compared to other indicators in group 1, and thus the fat content was selected as a representative indicator in group 1. In group 2, based on the above results, PUFAs had the largest CV value (26.43%), which indicates that these molecules had the most significant effect on the nutritional quality of Jiaozhou Bay clams, and could be screened out from group 2. In group 3, according to the CV values (Table 1), ash was less than the flavor-enhancing organic acids (43.47%). At the same time, the flavor-enhancing organic acids were also an important contribution to the flavor of clams, so the representative indicator in group 3 was flavor-enhancing organic acids. As the total taste nucleotides stood alone in group 4, which was also the characteristic indicator.
Conclusion
In this study, the nutritional quality was comprehensively evaluated by ANOVA, CA, PCA, and SCA to select characteristic evaluation indicators for the nutritional quality of R. philippinarum during the selected 7 months. The ANOVA results showed that the nutritional quality of R. philippinarum had significant differences (P < 0.05), and the CV values of the fifteen evaluation indicators were within 1.67–43.47%. The CA results showed that there were positive or negative correlations between some evaluation indicators. Finally, four characteristic indicators were obtained from four PCs using PCA and SCA, namely, fat, PUFAs, flavor-enhancing organic acids, and flavor-enhancing nucleotides. Screening the four characteristic indicators simplified the quality evaluation process and improved its efficiency, thereby making it applicable in aquaculture and marketing for quality control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC0311201), the National Natural Science Foundation of China (No. 31871867), and earmarked fund for Modern Agro-industry Technology Research System (CARS-49).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anjum FM. Impact of extruded flaxseed meal supplemented diet on growth performance, oxidative stability and quality of broiler meat and meat products. Lipids Health Dis. 2013;12(1):1–12. doi: 10.1186/1476-511X-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrento S, Marques A, Teixeira B, Anacleto P, Vaz-Pires P, Nunes ML. Effect of season on the chemical composition and nutritional quality of the edible crab Cancer pagurus. J Agric Food Chem. 2009;57(22):10814–10824. doi: 10.1021/jf9025597. [DOI] [PubMed] [Google Scholar]
- Beninger PG, Lucas A. Seasonal variations in condition, reproductive activity, and gross biochemical composition of two species of adult clam reared in a common habitat: Tapes decussatus L. (Jeffreys) and Tapes philippinarum (Adams and Reeve) J Exp Mar Biol Ecol. 1984;79(1):19–37. doi: 10.1016/0022-0981(84)90028-5. [DOI] [Google Scholar]
- Bi JF, Wang X, Chen QQ, Liu X, Wu XY, Wang Q, Lv J, Yang AJ. Evaluation indicators of explosion puffing Fuji apple chips quality from different Chinese origins. LWT Food Sci Technol. 2015;60(2):1129–1135. doi: 10.1016/j.lwt.2014.10.007. [DOI] [Google Scholar]
- Cartes JE, Papiol V, Guijarro B. The feeding and diet of the deep-sea shrimp Aristeus antennatus off the Balearic Islands (Western Mediterranean): influence of environmental factors and relationship with the biological cycle. Prog Oceanogr. 2008;79(1):37–54. doi: 10.1016/j.pocean.2008.07.003. [DOI] [Google Scholar]
- Folch J, Lees M, Sloane GS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- Frau M, Simal S, Femenia A, Sanjuán E, Rosselló C. Use of principal component analysis to evaluate the physical properties of Mahón cheese. Eur Food Res Technol. 1999;210(1):73–76. doi: 10.1007/s002170050536. [DOI] [Google Scholar]
- Ichihara K, Shibahara A, Yamamoto K, Nakayama T. An improved method for rapid analysis of the fatty acids of glycerolipids. J Food Lipids. 1996;31(5):535–539. doi: 10.1007/BF02522648. [DOI] [PubMed] [Google Scholar]
- Jiang X. Analysis of Hongdao clam industrial structure based on industry chain. Anim Husb Feed Sci. 2014;4:221–224. [Google Scholar]
- Joseph G, Remya Kumari KR, Kamalakanth CK, Bindu J, Asha KK. Changes in functional properties of protein in high pressure-treated Indian White Prawn (Fenneropenaeusindicus) during chilled storage. J Aquat Food Prod Technol. 2020;29(6):531–543. doi: 10.1080/10498850.2020.1772431. [DOI] [Google Scholar]
- Kilinc B, Cakli S. Chemical, enzymatical and textural changes during marination and storage period of sardine (Sardina pilchardus) marinades. Eur Food Res Technol. 2005;221(6):821–827. doi: 10.1007/s00217-005-0114-y. [DOI] [Google Scholar]
- Liu Y, Gong X, Ying XU, Zhang J, Hong AN, Zhang X. Determination and comparative analysis of flavor-enhancing nucleotides and amino acids in three common shellfish from offshore Yantai. J Fish Sci China. 2014;21(2):351–360. [Google Scholar]
- Marques A, Teixeira B, Barrento S, Anacleto P, Carvalho ML, Nunes ML. Chemical composition of Atlantic spider crab Maja brachydactyla: human health implications. J Food Compos Anal. 2010;23(3):230–237. doi: 10.1016/j.jfca.2009.10.007. [DOI] [Google Scholar]
- Michel M, Pierre L. Storage tissue metabolism and reproduction in marine bivalves—a brief review. Int J Invertebr Reprod. 2011;23(2–3):123–129. [Google Scholar]
- Najafi MBH, Leufven A, Dovom MRE. Probing the interactions between hardness and sensory of pistachio nuts during storage using principal component analysis. Food Sci Nutr. 2019;7:2684–2691. doi: 10.1002/fsn3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka P, Wojdyło A, Laskowski P. Principal component analysis (PCA) of physicochemical compounds’ content in different cultivars of peach fruits, including qualification and quantification of sugars and organic acids by HPLC. Eur Food Res Technol. 2019;245:929–938. doi: 10.1007/s00217-019-03233-z. [DOI] [Google Scholar]
- Qi L, Liu W, Shirasu K, Chen W, Jiang S. Reproductive cycle and biochemical composition of the Zhe oyster Crassostrea plicatula Gmelin in an eastern coastal bay of China. Aquaculture. 2006;261(2):752–759. doi: 10.1016/j.aquaculture.2006.08.023. [DOI] [Google Scholar]
- Sardia AE, Sandrini-Neto L, da Cunha P, Lana LC. Seasonal variation of oxidative biomarkers in gills and digestive glands of the clam Anomalocardia flexuosa and the mangrove oyster Crassostrea rhizophorae. Mar Pollut Bull. 2020;156:111193. doi: 10.1016/j.marpolbul.2020.111193. [DOI] [PubMed] [Google Scholar]
- Shin EC, Pegg RB, Phillips RD, Eitenmiller RR. Interrelationships among tocopherols of commercial Runner market type peanuts grown in the United States. Int J Food Sci Technol. 2010;45(12):2622–2628. doi: 10.1111/j.1365-2621.2010.02435.x. [DOI] [Google Scholar]
- Shujin G, Mingliang Z, Zengxia Z. Spatial-temporal variation of phytoplankton community structure in Jiaozhou Bay, China. J Oceanol Limnol. 2019;037(005):1611–1624. doi: 10.1007/s00343-019-8249-z. [DOI] [Google Scholar]
- Teshima SI, Kanazawa A, Koshio S, Horinouchi K. Lipid metabolism of the prawn Penaeus japonicus during maturation: variation in lipid profiles of the ovary and hepatopancreas. Comp Biochem Physiol Part B Comp Biochem. 1989;92(1):45–49. doi: 10.1016/0305-0491(89)90311-8. [DOI] [Google Scholar]
- Wang HW, Liu YQ. Evaluation of trace and toxic element concentrations in Paris polyphylla from China with empirical and chemometric approaches. Food Chem. 2010;121(3):887–892. doi: 10.1016/j.foodchem.2010.01.012. [DOI] [Google Scholar]
- Wang Q, Xue C, Li Z, Fu X, Xu J, Xue Y. Changes in the contents of ATP and its related breakdown compounds in various tissues of oyster during frozen storage. J Ocean Univ China. 2007;6(4):407–412. doi: 10.1007/s11802-007-0407-9. [DOI] [Google Scholar]
- Weiss R, Molinelli A, Jakusch M, Mizaikoff B. Molecular imprinting and solid phase extraction of flavonoid compounds. Bioseparation. 2001;10(6):379–387. doi: 10.1023/A:1021554106297. [DOI] [PubMed] [Google Scholar]
- Xu J-H, Zhao ZY, Wang LC, Gao H, Liu ZZ, Fan HK. Selection of factors for apple fruit quality evaluation. Agric Res Arid Areas. 2011;29(6):269–274. [Google Scholar]
- Zhao H, Zhang S. Identification of Jiaozhou Bay Clams (Ruditapes philippinarum) by multi-element fingerprinting technique. Food Anal Method. 2016;9(9):1–9. doi: 10.1007/s12161-016-0461-2. [DOI] [Google Scholar]
- Zhao H, Zhang S. Effects of sediment, seawater, and season on multi-element fingerprints of Manila clam (Ruditapes philippinarum) for authenticity identification. Food Control. 2016;66:62–68. doi: 10.1016/j.foodcont.2016.01.045. [DOI] [Google Scholar]
- Zheng K, Yan C. Application of ICP-AES with microwave digestion to detect trace elements in oysters from Jiaozhou Bay, China. J Ocean Univ China. 2011;10(3):301–304. doi: 10.1007/s11802-011-1790-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.