Abstract
Films were prepared by casting 2% w/v apple pectin, 0.5% w/v low-acyl gellan and 2.2% w/v glycerol as plasticizer. Bioactive film (BF, films with 3912 International Units (IU) nisin/cm2) and control films (CF, films without nisin) were elaborated. The objective was to analyze the release kinetics of nisin from films to a food model, to determine the period of film bioactivity and potential use as antimicrobial packaging. The release of nisin from BF to a food model was determined at 5 °C and 30 °C. The release kinetics of nisin was fitted to the analytical solution of the Fick’s second law for an infinite plate. The diffusion coefficients of nisin (D) were 5.22 × 10–14 and 7.36 × 10–14 m2/s for 5 °C and 30 °C, respectively. Besides, both films were characterized in their mechanical properties and gas permeabilities [oxygen (PO2) and water vapour permeability (WVP)]. The mechanical properties were reduced by the nisin incorporation, whereas PO2 was increased, and no significant effect on WVP was observed.
Keywords: Biopreservation, Bacteriocins, Diffusion, Permeability, Mechanical properties
Introduction
During the last decades, new food technologies have been developed to meet the significant demands of consumers, namely, minimally processed foods, natural food ingredients and preservative-free food products with a longer shelf life. A current challenge for the food industry is implicated in food packaging innovation. The globalization of the market, which generates longer distribution times for food, as well as changes in the way of life of consumers in urban areas, has accelerated the development of new and improved concepts of food packaging. Active food packaging is an excellent example of an innovation that goes beyond the traditional functions of the package in which it interacts with the food or food environment to extend the shelf life of food systems without compromising their safety or affecting their sensory properties (Realini and Marcos 2014). Different active agents can be added to a food packaging material to enhance its functionality, for example, organic acids, enzymes, antimicrobial substances, antioxidants, ions, ethanol, among others (Realini and Marcos 2014; Appendini and Hotchkiss 2002; Irkin and Esmer 2015; Yildirim et al. 2017). These agents can act either on the surface of solid foods or in the bulk of liquid foods. Antimicrobial packaging is a type of active packaging, which aims at reducing, inhibiting or retarding growth of microorganisms that can contaminate the packaged food (Appendini and Hotchkiss 2002). Currently, bio-based, biodegradable materials, including biopolymer films, are capable carriers of antimicrobials. The incorporation of antimicrobial compounds into a biopolymer film allows a better efficiency in food protection as the film provides better stability of the antimicrobial agent and the control of its release towards food surface can be tailored by careful design of the biopolymer film. In this sense, it is essential to know the release kinetics of the bioactive agent from packaging film to the food matrix in order to design adequate antimicrobial packaging systems.
In the last 20 years, the incorporation of natural antimicrobials into biopolymer films is emerging as a promise of biodegradable and bioactive food packaging technology (Wang et al. 2015b, 2015a; Irkin and Esmer 2015; Imran et al. 2014). Among the natural agents with high potential to be used into bioactive packaging are the bacteriocins, which are peptides ribosomally synthesized by certain bacteria, like lactic acid bacteria (Chandrakasan et al. 2019). Nowadays, nisin and pediocin are the most used bacteriocins as additives for food conservation. Nisin exhibits antimicrobial activity toward a wide range of Gram-positive bacteria, in particular against foodborne pathogens such as Listeria monocytogenes, Staphylococcus aureus and Bacillus cereus (Mills et al. 2011; Galvez et al. 2007). Bacteriocins are ideal for incorporation into biopolymer film packaging where they can be released at known rates to ensure enough antimicrobial activity on the food product surface to inhibit microbial growth of antagonistic bacteria. The implementation of such antimicrobial biopolymer films based on bacteriocins can contribute to satisfying the growing consumer demand for free-chemical preservatives foods and promote to the development of more bio-based and biodegradable plastics for food-packaging applications. According to the market data compiled by European Bioplastics, global bioplastics production capacity is set to increase from around 2.05 million tonnes in 2017 to approximately 2.44 million tonnes in 2022, and the packaging sector remains the largest field of application for bioplastics with almost 60% of the global bioplastics market in 2017 (European Bioplastics and Nova-Institute, 2017). In that sense, the use of innovative biopolymers such as pectin, an important polysaccharide extracted from crops or by-products from the food industry, is expected to grow due to its valuable techno-functional properties such as biodegradability, biocompatibility, antimicrobial activity and physical properties (Espitia et al. 2014; Trejo-González et al. 2018; Calce et al. 2014). However, although studies on bioactive packaging have increased in the last decade, there are still scarce data on diffusion coefficients of natural antimicrobial compounds in bio-based films. Most of the studies have been focused on the evaluation of the in-vitro functionality of bioactive films. To our knowledge, the release kinetics of nisin from pectin-based films has not been reported yet. Since the diffusivity of the antimicrobial molecules through the film defines its antimicrobial activity, the present study aims to investigate the release kinetics of nisin from pectin-gellan films to contribute to the design and characterization of bio-based antimicrobial-packaging films for food applications.
Material and methods
Microorganism
Listeria monocytogenes CFQ-B-104, from the culture collection of the School of Chemistry, UNAM, Mexico, was conserved in 20% (v/v) glycerol at −75 °C.
Materials
Apple pectin (DE = 70–74%; Fluka Analytical, Switzerland), deacetylated gellan gum (Kelcogel, CP-Kelco, USA) and glycerol (Química Meyer, Mexico) were used to formulate biopolymer films.
A commercial sample of nisin, Nisaplin® was obtained from Danisco, Mexico. According to the manufacturer, Nisaplin formulation contains 1 × 106 International Units (IU) of nisin per gram of formulation. Brain heart infusion (BHI) broth (BD Dibico, Mexico) and nutrient agar (BD Dibico, Mexico) were used for cultivation of L. monocytogenes and the release kinetics of nisin from pectin-gellan films, respectively. The HPLC-grade acetonitrile (ACN) (JT Baker, USA), trifluoroacetic acid (TFA) (Sigma Aldrich, USA) and ultrapure water were used for HPLC analyses.
Determination of the minimal inhibitory concentration of Nisin
Minimum inhibitory concentration (MIC) of nisin required to control the growth of L. monocytogenes was determined using the broth dilution protocol (Wiegand et al. 2008) as a reference method. Microtiter plates (Corning® Costar®) were used to assay by triplicate different nisin concentrations (129–261 IU/mL). A fresh, pure culture of L. monocytogenes was prepared in BHI broth and used for the inoculum, to give 105 colony-forming units (CFU) per millilitre within wells. The plates were incubated at 35 °C, and the density of the cell suspensions was assessed spectrophotometrically at 545–630 nm (Awareness, Stat Fax-2100) at 0, 20 and 24 h. A growth control (well without nisin) and a sterility control (no bacterial inoculum) were also tested. The MIC was the lowest nisin concentration that prevents the growth of L. monocytogenes on any of the broth wells but the growth control.
Preparation of bioactive pectin-gellan films
Bioactive films (BF) were prepared by dissolving pectin and gellan in distilled water under continuous stirring for at least 90 min. The final pectin and gellan concentrations in the film-forming solution (FS) were 2 and 0.5% w/v, respectively. The biopolymer dispersion was heated to ebullition for 10 min to complete biopolymer dissolution. Excessive evaporation of water was reduced using aluminium foil covers. After that, glycerol was added as a plasticizer to obtain a final concentration of 2.2% w/v. The FS was kept at 70 °C and then, a previously defined volume of Nisaplin solution, at 60 °C and pH = 3, was added to obtain a final concentration of 10,275 IU/mLFS equivalent to 3912 IU/cm2 in films. After that, the FS was homogenized under gentle stirring at 70 °C for 10 min; then, 54.5 mL of FS were taken and poured into levelled polystyrene Petri dishes (13.5 cm diameter), which were then dried at 35 ± 2 °C for 11 h in a convection oven (≈ 30% relative humidity (RH)). Control films (CF) were formulated following the above procedure, but excluding the incorporation of Nisaplin solution.
Nisin quantification by HPLC
Before the nisin quantification in the release tests, a calibration curve was developed using a sample of purified nisin. A reversed-phase high-performance liquid chromatography (RP-HPLC) method based on that developed by Wilson-Stanford et al. (2009) was used. A stock solution of Nisaplin (500 mg) was prepared in 35% ACN-0.1% TFA. The solution was sonicated for 20 min (Bransonic, M1800) avoiding overheating of the solution; then, it was centrifuged at 12,000 rpm for 30 min to remove any insoluble material. The supernatant was filtered with a 0.45 µm membrane (nylon syringe filter, Pall Acrodisc), placed into 1.5 mL-HPLC vials and 60 µL were injected to HPLC system (1260 Infinity, Agilent Technologies) equipped with OpenLAB CDS ChemStation software; autosampler; DAD detector (λ = 220 nm) and fraction collector. A C-18 reversed-phase column (250 × 10 mm, 5 µm; Phenomenex Ultremex) was used at 40 °C. A linear ACN-gradient was established by increasing the flow rate of solvent A (99.9% ACN-0.1% TFA) relative to solvent B (99.9% water-0.1% TFA), from 0 to 100%, maintaining a constant flow rate of 0.7 mL/min over a 23-min period. Then a linear gradient of solvent B (0 to 100%) was set at 0.9 mL/min over a 7-min period, and then an isocratic flow of solvent B at 0.9 mL/min was used to complete the elution of nisin. Three fractions were collected which were dried in a Speed-Vac concentrator (ThermoFisher Scientific) at 40 °C and 680 Pa. After that, they were aseptically suspended in a sterile buffer (pH = 6). The antilisterial activity of each collected fraction was evaluated using the critical-dilution method (Calderón-Aguirre et al. 2015). The fraction with antilisterial activity was identified as that containing purified nisin. A nisin calibration curve was prepared by injecting different volumes of a purified nisin solution to the HPLC system. The injected volumes corresponded to 125, 250, 1000, 2000, 3000, 4000 and 5000 IU.
Release kinetics of nisin from pectin-gellan films
Pectin-gellan films containing nisin were cut into small disks of 1.15 cm of diameter and surface sterilized with UV radiation during 24 h (12 h each side) in a safety cabinet (Purifier™ Delta™ Series Class II, Type A2 Labconco). The sterilized small disks were aseptically placed onto sterile nutrient-agar in Petri dishes (5.2 cm, diameter), which was used as a release medium or food model. Diffusion tests were performed at 30 °C in an incubator (Sheldon, GI6) and at 5 °C in a refrigerator (Tor-Rey, Mexico) to investigate the effect of temperature. Randomly, four films were selected at each time point for each storage temperature; they were carefully removed from the agar, cut in four slices and placed into a vial containing 2 mL of a mixture of 35% ACN, 0.1% TFA and HPLC-grade water, which was sealed and stirred for 48 h. After that, the mixture was filtered with a 0.45 μm syringe filter (Nylon 13 mm × 0.45 μm; Agilent Technologies) before injecting into the HPLC system. The amount of no-released nisin from films to food model (residual nisin) was quantified through RP-HPLC method described in the previous section. Three injections (200 µL each) were carried out for each time point and temperature. Several samplings were carried out until the steady-state condition in the nisin release was reached.
The diffusion coefficient of nisin from the films to food model (D) was determined from the experimental data of release kinetics, using the classical analytical solution of the 2nd Fick’s law for one-dimensional diffusion and the particular case of flow through a membrane, with uniform initial distribution and surface concentrations different, reported by Crank (1975) (Eq. 1). The equation one is based on the following assumptions: (1) the film is initially at a uniform nisin concentration, (2) there is a finite interval of time during which the steady-state condition is set up, (3) initial nisin concentration in food model (nutrient agar) was zero, (4) nisin amount in the mixture: 35%ACN-0.1% TFA was same as the amount of residual nisin in the film.
| 1 |
where Mt and M∞ are the mass of nisin released from the film at time t and at infinite time, respectively; D is the diffusion coefficient, and l is the film thickness. Data were fitted to the Eq. (1), using the software package SigmaPlot® v. 12.5 (Systat Software Inc.). Twenty-five terms (n = 25) were used to minimize the sum of squared errors.
Water vapour permeability
The water vapour permeability (WVP) of the films was determined gravimetrically using a modification of the ASTM E96/E96M procedure (ASTM 2010b). Film disks, previously equilibrated at 53% RH and 23 °C for 48 h, were mounted on permeation cells (aluminium cups with an airtight seal, diameter: 6 cm, height: 2 cm) containing silica gel (approximately 28 g; 0% RH; ≈ 0 Pa water vapour partial pressure). The air gap inside the cup was 0.9 cm. The capped cells were placed in a cabinet that was previously equilibrated at 75 ± 2% RH and 24 ± 2 °C during 24 h prior to WVP tests. The cups were weighed at 60-min intervals to obtain the steady flow of water vapour through the film. Then, the WVP was determined according to the procedure reported previously (Calderón-Aguirre et al. 2015). Four determinations were done for each film type.
Oxygen permeability
The oxygen gas permeability (PO2) through the films was determined by the ASTM D1434-82 (ASTM 1998) using a film-package permeability tester (Labthink™ VAC-V2, China). The tests were performed at 25 °C, using research-grade high-purity oxygen gas (34161, INFRA™ Mexico). The films were conditioned at 53% RH during 48 h before placing them into the chambers of the permeability tester. Taking into account the Fick’s law of diffusion, the film oxygen permeability (PO2; g m s−1 Pa−1 m−2) was determined following the numerical analysis reported before (Aguirre-Loredo et al. 2014). Four determinations were done for each film type.
Mechanical properties
The mechanical tests of the films were conducted on a universal test machine (Instron®, 5943), according to ASTM standard method D882 (ASTM 2010a). The films were cut as rectangular strips (10 cm × 1 cm). Before testing, all samples were equilibrated at 53 ± 2% RH for 48 h in a cabinet using magnesium nitrate saturate solution at 25 °C. Equilibrated films were mounted in the film-extension grips of the testing machine; the gap between grips was 5 cm. The film specimens were stretched at a rate of 50 mm/min until the sample failed. At least twenty replicates of each film formulation were tested. Specimens that failed at the grip contact point were discarded. The stress–strain curves of the samples were obtained through the force-distance data. Young’s modulus was determined through the slope of the linear region of the stress–strain curves. The ultimate mechanical properties of the films, stress (σTmax, MPa) and elongation percentage at break (E; %) were determined at the instant of rupture of the specimen.
Microstructure
Scanning electron microscopy observations of surfaces and cross-sections of the films were carried out using a JEOL JSM 6300 Scanning Microscope. Sample films were frozen at -75 °C and then fractured. After, they were placed on double-sided graphite tape and coated with a thin gold layer. The samples were examined at a magnification of 350 × or 450 × with a voltage of 20 kV.
Statistical analysis
Data are given as the mean ± standard deviation of each treatment and were analyzed for statistical significance through the analysis of variance (ANOVA) followed by Tukey test (p < 0.05) (SigmaPlot® v. 12.5, Systat Software Inc.).
Results and discussion
Minimal inhibitory concentration of Nisin
According to the protocol used, the minimal inhibitory concentration (MIC) of the antimicrobial agent was determined as its lowest concentration that inhibits the growth of the bacterium being investigated (i.e. Listeria monocytogenes). In the 96-well microtiter plate format, the bacterial growth was assessed measuring the absorbance of the cell suspensions after incubation for 20 h. As illustrated in Fig. 1, positive control (Control + , cell suspension without nisin) and the lowest nisin concentration (129.7 IU/mL) did not prevent the growth of L. monocytogenes, nisin concentration of 149.8 IU/mL resulted in partial inhibition, and higher nisin concentrations showed inhibition even until 24 h. Therefore, the MIC of nisin against L. monocytogenes (105 CFU/mL) was 171.5 IU/mL. Then, taking into account this result, the pectin-gellan films were formulated with high enough nisin concentrations to ensure inhibition of antagonist microorganisms and the accurate nisin quantification in the release tests. The final concentration of nisin in the bioactive films, once the drying process was completed, was 3912 IU/cm2.
Fig. 1.
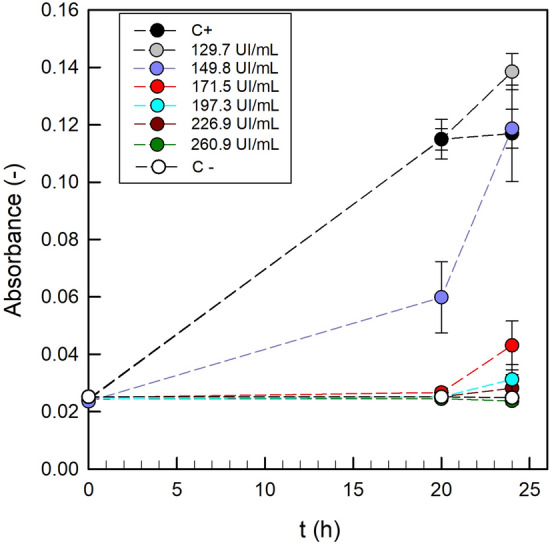
Optical density (λ = 545–630 nm) of the cell suspensions of Listeria monocytogenes during incubation under different concentrations of nisin. Mean values (error bars represent standard deviations) of 12 measurements
Release kinetics of Nisin from pectin-Gellan films
The release of nisin from BF was analyzed at two temperatures, 30 °C and 5 °C. The release kinetics are shown in Fig. 2. The Crank equation was found to be suitable for the nisin diffusion experiments (R2 = 0.91). The diffusion coefficients D for BF at 30 and 5 °C were 7.36 × 10–14 and 5.22 × 10–14 m2/s, respectively. As it was expected, the higher temperature increased D-value. At both temperatures, the release of more than 75% of nisin takes place mainly in the first 72 h, and it continues until equilibrium is achieved (i.e. Mt/M∞ ≲1). At that time point, the values Mt = 72 h/Mt = 0 were 0.78 and 0.84 for 5 and 30 °C, respectively.
Fig. 2.
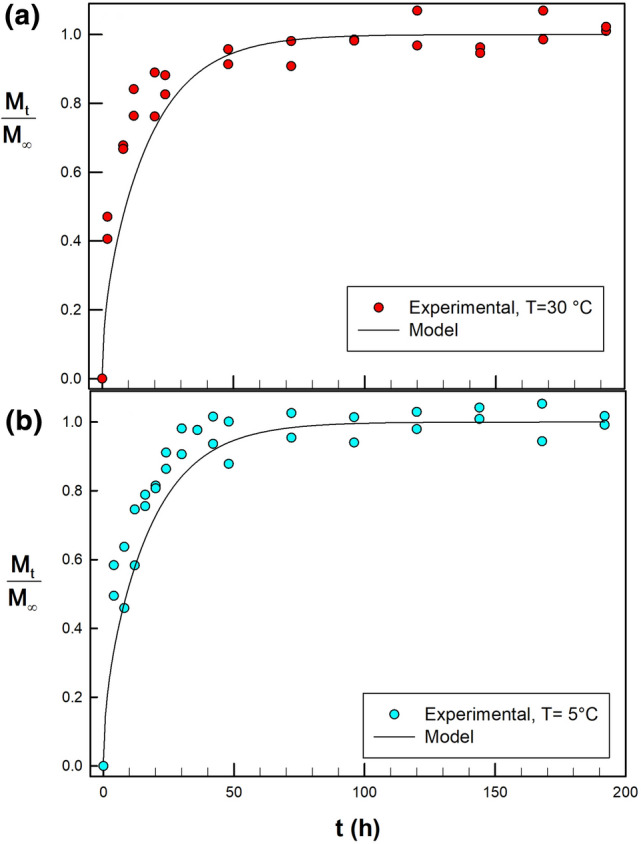
Fractional release of nisin from pectin-gellan films (BF) on nutrient agar (release medium or food model). Diffusion tests performed at: a 30 °C and b 5 °C. Lines show the best fit to Crank equation, using 25 terms (R2 = 0.91)
It is generally discussed that the release of bioactive molecules from biopolymeric networks occurs in two stages. Firstly, water molecules from food, food-simulant or release medium, penetrate and diffuse into the film; thus, the relaxation of the biopolymer network occurs. Secondly, bioactive molecules diffuse through film; after that, they release into outer medium, which continues until a thermodynamic equilibrium between the two phases is reached (Imran et al. 2014; Buonocore et al. 2003). Taking into consideration the before-mentioned theories and the notorious swelling of the pectin-gellan films during the first two hours in contact with the agar surface (Fig. 3), which could have facilitated the diffusion of nisin, we can assume that nisin release takes place on the following events: (i) penetration/diffusion of water from agar (food-simulant) to the film, thereby enabling the swelling of the film, (ii) pectin-gellan network relaxation, (iii) diffusion of nisin through the swelled film, (iv) nisin releasing from the film to the agar surface. Similar findings were reported for natamycin release from single and composite pectin and alginate films (Bierhalz et al. 2012). Pectin and pectin-alginate films reached swelling degrees of 4.31 and 1.25 gwater/gfilm for pectin and pectin-alginate films, respectively; and those swelling degrees correlated well with a higher D-value obtained for pectin films (D = 3.22 × 10–13 m2/s) compared to that of pectin-alginate films (D = 2.80 × 10–14 m2/s).
Fig. 3.
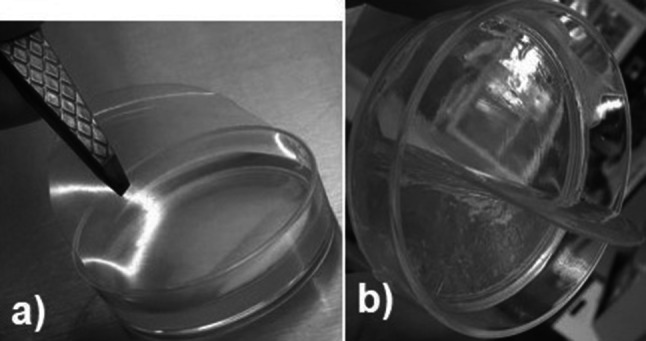
The aspect of a representative bioactive film (BF) before (a) and after (b) contact with the agar surface. A notorious swelling of the BF was evident during the first two hours in contact with the agar surface
According to the main findings of the release kinetics of bioactive compounds from biopolymer films, the diffusion of them depends on several parameters, namely the physicochemical characteristics of the active molecules (size, charge and conformation), temperature, pH, physicochemical properties of biopolymer matrix (crosslinking degree, main functional groups, viscoelastic properties), interaction forces between bioactive molecules and biopolymer chains, among others (Imran et al. 2014). In the case of pectin-gellan films, as expected, the higher temperature resulted in higher desorption rate of nisin. At the end of the experiments (t = 192 h) when the steady-state condition was reached, 83% of initial nisin was released at 30 °C while it was 81% at 5 °C. Due to the lack of experimental data of diffusivity of nisin in pectin or gellan films, some data of diffusivity of nisin in other hydrophilic biopolymer films were used to compare our results. For instance, Imran et al. (2014) reported nisin diffusion coefficients (D) ranged between 1.97 × 10–17 and 97.8 × 10–17 m2/s for hydroxypropyl methylcellulose (HPMC), chitosan (CTS), sodium caseinate (SC) and polylactic acid (PLA) active films at 4 °C and 40 °C, using water–ethanol as release medium. The differences were explained due to likely interactions between nisin and biopolymers. The most hydrophilic biopolymer with no electrical charge (HPMC), caused the higher nisin release; otherwise, lower D values were presented in CTS, PLA and SC films due to different charge affinity between polymer and aminoacids of nisin. Other works have reported higher D values and some of them close to those obtained in our work. Diffusion coefficients of nisin from chitosan-alginate films, determined at 21 °C, ranged from 8.72 × 10−14 to 8.03 × 10−13 m2/s (Chandrasekar et al. 2016). Based on the highly hydrophilic character of both biopolymers used in this study and the chemical features of nisin, namely an amphipathic polypeptide with an overall positive charge; we can assume that the nisin diffusion into pectin-gellan-films is affected mainly for the hydrogel network organization and the limited interactions between nisin and the polysaccharides (pectin and gellan). Our protocol for film elaboration did not use divalent ions as crosslinkers for the gellan network formation; thus, both the low degree of crosslinking of the hydrogel network and the proteins presents in the Nisaplin formulation could have yielded a more plasticized film structure, allowing that the diffusion of nisin through the swelled film was not significantly retarded.
Mechanical and barrier properties
Table 1 shows the mechanical properties of CF and BF. The inclusion of Nisaplin in BF films had a significant effect on mechanical properties. Young’s modulus and stress at break for BF were at least three times lower than those for CF, and the elongation of BF was decreased by 11%. Young’s modulus accounts for film stiffness, the larger the stiffness, the higher the force or stress required to cause a given deformation or strain. Stress at breakpoint or ultimate strength is the highest stress reached in the true stress–strain curve before the fracture of the film occurs; it is referred to the film’s mechanical resistance due to cohesion forces; on the other hand, elongation at break measures the film plasticity or the capacity of a film to extend before breaking. Figure 4 shows the representative stress–strain curves of CF and BF where the differences in their mechanical behaviour are evident. Probably, both proteins and nisin present in Nisaplin would work as plasticizers. Their small size and molecular conformation could have led to a less ordered biopolymer matrix, reducing the cohesion forces between biopolymer chains, which are formed by low-energy physical bonds (for instance, Van der Waals, hydrogen, and hydrophobic), since no divalent cations were used for crosslinking the hydrogel. The mechanical properties of this kind of materials are strongly sensitive to experimental conditions such as deformation rate (generally, the faster the rate, the higher the stress), film specimen size, ambient conditions (temperature and relative humidity) and data analysis. A wide range of tensile test conditions has been reported for biopolymer films; therefore, it is not suitable to compare our results with others reported for pectin or gellan films.
Table 1.
Barrier and mechanical properties of bioactive (BF) and control (CF) films
| CF | BF | |
|---|---|---|
| Young’s modulus (MPa) | 270.67 ± 15.99 a | 73.66 ± 6.91 b |
| Elongation at break (%) | 41.32 ± 1.44 a | 36.73 ± 3.63 b |
| Stress at break (MPa) | 27.01 ± 1.42 a | 8.76 ± 0.67 b |
| WVP (× 10–10, g m Pa−1 s−1 m−2) | 1.61 ± 0.14 a | 1.41 ± 0.23 a |
| PO2 (× 10–12, g m Pa−1 s−1 m−2) | 0.95 ± 0.14 b | 3.44 ± 0.13 a |
Mean values ± standard deviation. Different letters in the same row indicate significant differences (P < 0.05) between samples
Fig. 4.
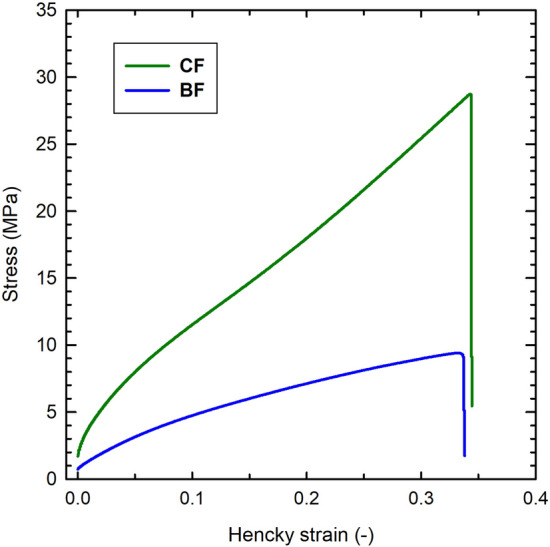
Representative stress-Hencky strain curves obtained in tensile tests on control films (CF) and bioactive films (BF). A crosshead speed of 50 mm/min, samples conditioned at 25 °C and 53% RH
Water vapour and oxygen permeabilities of CF and BF are showed in Table 1. The water vapour permeability (WVP) was not significantly affected by the Nisaplin inclusion in the films. Both films, CF and BF, were formulated with the same content of hydrophilic plasticizer (glycerol), however, although the proteinaceous moieties from Nisaplin could be rendering a less ordered polymer matrix, which would favour the diffusion of any gaseous molecule throughout the film, the hydrophobicity of that proteinaceous plasticizer seems to have a counteracting effect in the water diffusion. Besides, the inclusion of Nisaplin into the films led to more oxygen-permeable films. The oxygen permeability (PO2) of BF was up to three times higher than that of CF whose behaviour can be assigned to the non-polar nature of oxygen, which allows it a higher solubility and diffusion in proteinaceous zones of BF. It is well recognized that the polysaccharide-based films, due to hydrophilic nature, offer a better barrier to oxygen than to water vapour, our results agree with this statement, PO2 values were two orders of magnitude lower than WVP ones. The WVP values of CF and BF were similar to those reported for sodium alginate/pectin films (0.84–1.73 × 10–10 g m−1 s−1 Pa−1 (Galus and Lenart 2013), for Alyssum homolocarpum seed gum-polyvinyl alcohol films (5.09–13.5 × 10–10 g m−1 s−1 Pa−1) (Marvdashti et al. 2019), for whey protein concentrate (WPC)-based edible films, with various glycerol contents (1.25–1.62 × 10–10 g m−1 s−1 Pa−1) (Ramos et al. 2013), and chitosan-oleic acid (5–8.81 × 10–10 g m−1 s−1 Pa−1) (Aguirre-Loredo et al. 2014), among others. Otherwise, data concerning the oxygen permeability of biopolymer films are less numerous than those of WVP. The PO2 values obtained in this work are in good agreement with the earlier reported results for pectin-gellan films (Trejo-González et al. 2018; Jiménez-Villeda et al. 2018).
Film microstructure
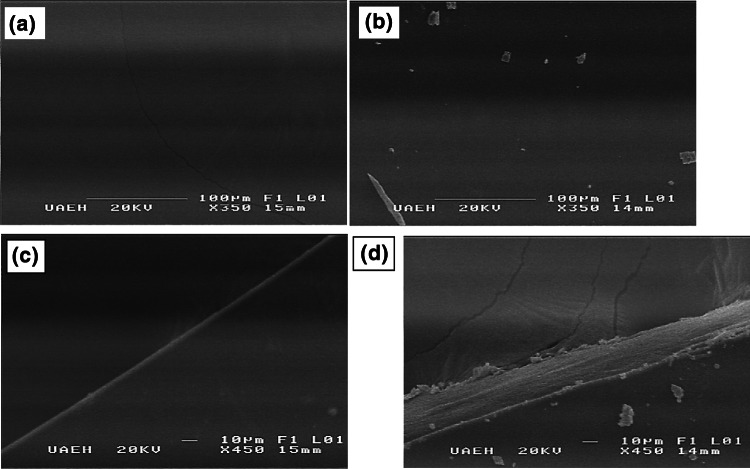
Scanning electron microscopy observations of the surface of the control film (CF) and the bioactive film (BF) are shown in Fig. 5. The surface of CF was smooth and homogeneous; in contrast, some crystals were observed on the surface of BF which may arise from the sodium chloride and other salts contained in the commercial nisin preparation; but even so, the surface was more uniform than that seen in other antimicrobial films as those formulated with natamycin (Bierhalz et al. 2012; da Silva et al. 2012). The cross-section of CF was also homogeneous (Fig. 4c), but the inclusion of Nisaplin into the gellan-pectin films resulted in a fibrous-like cross-section with small crystals on the borders. According to the microscopical morphology, the nisin formulation could have been well distributed into the polymeric matrix, but the changes observed in the BF microstructure corroborate the trends found for mechanical properties. Nisin formulation that contains 2.5% nisin and the rest predominantly salts, proteins and carbohydrates, acted as plasticizer but it did not significantly modify the water vapour permeability of BF.
Fig. 5.
Scanning electron micrographs of the surfaces and cross-sections of gellan-pectin films (after fracture); a surface of the control film, b surface of the bioactive film, c cross-section of the control film, and d cross-section of the bioactive film. Magnifications and scale bars are shown on the photographs
Conclusion
The Crank equation satisfactorily described the release kinetics of nisin from pectin-gellan films. The diffusion coefficients (D) at 30 and 5 °C were 7.36 × 10–14 and 5.22 × 10–14 m2/s, respectively. The macromolecular network organization and the swelling observed for the pectin-gellan film could have allowed the release of more than 75% of nisin in the first 72 h; after that, the nisin release achieved equilibrium. At equilibrium time, 83% of initial nisin was released at 30 °C while it was 81% at 5 °C. Release patterns of nisin from the pectin-gellan films indicate that these materials can be used as antilisterial films for prolonging shelf-life of packed food systems. The incorporation of nisin formulation into the films led to more plasticized films, whereas PO2 was increased and no significant effect on WVP was observed.
Acknowledgements
LRH acknowledges CONACyT MSc. Scholarship (CVU/Becario: 699123/416247). Donation of Listeria monocytogenes strain is acknowledged to Dr Díaz-Ruiz (FQ-UNAM). Support and advice are acknowledged to Dr Zepeda-Bastida (MVZ-UAEH) and Dr Hernández-Ávila (SEM, ICBI-UAEH).
Author contributions
Data curation, LRH, AIRH and NCH; Investigation, LRH, MRLC, VMMJ, NCH and AIRH; Methodology, LRH; Project administration, AIRH and NCH; Writing original draft, AIRH and NCH.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Norberto Chavarría-Hernández, Email: norberto@uaeh.edu.mx.
Adriana-Inés Rodríguez-Hernández, Email: inesr@uaeh.edu.mx.
References
- Aguirre-Loredo RY, Rodríguez-Hernández AI, Chavarría-Hernández N. Physical properties of emulsified films based on chitosan and oleic acid. CyTA J Food. 2014;12(4):305–312. doi: 10.1080/19476337.2013.853207. [DOI] [Google Scholar]
- Appendini P, Hotchkiss JH. Review of antimicrobial food packaging. Innov Food Sci Emerg Technol. 2002;3(2):113–126. doi: 10.1016/S1466-8564(02)00012-7. [DOI] [Google Scholar]
- ASTM (1998) Standard test method for determining gas permeability characteristics of plastic film and sheeting. vol D1434. American Society for Testing and Materials, Philadelphia, EE.UU.
- ASTM (2010a) Standard Test Method for Tensile Properties of thin plastic sheeting, D882–10. In, vol D882-10. American Society for Testing and Materials, Philadelphia, EE.UU.
- ASTM (2010b) Standard Test Methods for Water Vapor Transmission of Materials. In: ASTM (ed) E96/E96M-10. American Society for Testing and Materials, Philadelphia, EE.UU.
- Bierhalz ACK, da Silva MA, Kieckbusch TG. Natamycin release from alginate/pectin films for food packaging applications. J Food Eng. 2012;110(1):18–25. doi: 10.1016/j.jfoodeng.2011.12.016. [DOI] [Google Scholar]
- European Bioplastics, Nova-Institute (2017) Bioplastics market data 2017. Nova Institute. www.european-bioplastics.org/market
- Buonocore GG, Del Nobile MA, Panizza A, Corbo MR, Nicolais L. A general approach to describe the antimicrobial agent release from highly swellable films intended for food packaging applications. J Control Release. 2003;90(1):97–107. doi: 10.1016/S0168-3659(03)00154-8. [DOI] [PubMed] [Google Scholar]
- Calce E, Mignogna E, Bugatti V, Galdiero M, Vittoria V, De Luca S. Pectin functionalized with natural fatty acids as antimicrobial agent. Int J Biol Macromol. 2014;68:28–32. doi: 10.1016/j.ijbiomac.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Calderón-Aguirre Á-G, Chavarría-Hernández N, Mendoza-Mendoza B, Vargas-Torres A, García-Hernández E, Rodríguez-Hernández A-I. Antilisterial activity and physical-mechanical properties of bioactive caseinate films. CyTA J Food. 2015;13(4):483–490. doi: 10.1080/19476337.2014.1003200. [DOI] [Google Scholar]
- Crank J. The mathematics of diffusion. 2. Bristol: Oxford University Press; 1975. [Google Scholar]
- Chandrakasan G, Rodríguez-Hernández AI, López-Cuellar MdR, Palma-Rodríguez HM, Chavarría-Hernández N. Bacteriocin encapsulation for food and pharmaceutical applications: advances in the past 20 years. Biotechnol Lett. 2019;41:453–469. doi: 10.1007/s10529-018-02635-5. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Coupland JN, Anantheswaran RC. Release kinetics of nisin from Chitosan-Alginate complex films. J Food Sci. 2016;81(10):E2503–E2510. doi: 10.1111/1750-3841.13443. [DOI] [PubMed] [Google Scholar]
- da Silva MA, Bierhalz ACK, Kieckbusch TG. Modelling natamycin release from alginate/chitosan active films. Int J Food Sci Technol. 2012;47(4):740–746. doi: 10.1111/j.1365-2621.2011.02902.x. [DOI] [Google Scholar]
- Espitia PJP, Du W, Avena-Bustillos RJ, Soares NDFF, McHugh TH. Edible films from pectin: physical-mechanical and antimicrobial properties—a review. Food Hydrocoll. 2014;35:287–296. doi: 10.1016/j.foodhyd.2013.06.005. [DOI] [Google Scholar]
- Galus S, Lenart A. Development and characterization of composite edible films based on sodium alginate and pectin. J Food Eng. 2013;115(4):459–465. doi: 10.1016/j.jfoodeng.2012.03.006. [DOI] [Google Scholar]
- Galvez A, Abriouel H, Lopez RL, Ben Omar N. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007;120(1–2):51–70. doi: 10.1016/j.ijfoodmicro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Imran M, Klouj A, Revol-Junelles A-M, Desobry S. Controlled release of nisin from HPMC, sodium caseinate, poly-lactic acid and chitosan for active packaging applications. J Food Eng. 2014;143:178–185. doi: 10.1016/j.jfoodeng.2014.06.040. [DOI] [Google Scholar]
- Irkin R, Esmer OK. Novel food packaging systems with natural antimicrobial agents. J Food Sci Technol. 2015;52(10):6095–6111. doi: 10.1007/s13197-015-1780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Villeda P-Y, Rodríguez-Hernández A, López-Cuellar Md-R, Franco-Fernádez M-J, Chavarría-Hernández N. Elaboration and characterization of pectin-gellan films added with concentrated supernatant of Streptococcus infantarius fermentations, and EDTA: effects on the growth of Escherichia coli, Staphylococcus aureus and Listeria monocytogenes in a Mexican cheese medium, and physical-mechanical properties. Food Sci Technol. 2018;52(10):436–443. doi: 10.1590/fst.32717. [DOI] [Google Scholar]
- Marvdashti LM, Yavarmanesh M, Koocheki A. Controlled release of nisin from polyvinyl alcohol-Alyssum homolocarpum seed gum composite films: Nisin kinetics. Food Biosci. 2019;28:133–139. doi: 10.1016/j.fbio.2019.01.010. [DOI] [Google Scholar]
- Mills S, Stanton C, Hill C, Ross RP. New developments and applications of bacteriocins and peptides in foods. Annu Rev Food Sci Technol. 2011;2:299–329. doi: 10.1146/annurev-food-022510-133721. [DOI] [PubMed] [Google Scholar]
- Ramos OL, Reinas I, Silva SI, Fernandes JC, Cerqueira MA, Pereira RN, Vicente AA, Poças MF, Pintado ME, Malcata FX. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013;30(1):110–122. doi: 10.1016/j.foodhyd.2012.05.001. [DOI] [Google Scholar]
- Realini CE, Marcos B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014;98(3):404–419. doi: 10.1016/j.meatsci.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Trejo-González L, Rodríguez-Hernández AI, López-Cuellar MR, Juárez-Martínez VM, Chavarría-Hernández N. Antimicrobial pectin-gellan films: effects on three foodborne pathogens in a meat medium, and selected physical-mechanical properties. CyTA - J Food. 2018;16(1):469–476. doi: 10.1080/19476337.2017.1422278. [DOI] [Google Scholar]
- Wang H, Liu H, Chu C, She Y, Jiang S, Zhai L, Jiang S, Li X. Diffusion and antibacterial properties of nisin-loaded Chitosan/Poly (L-Lactic Acid) towards development of active food packaging film. Food Bioproc Tech. 2015;8(8):1657–1667. doi: 10.1007/s11947-015-1522-z. [DOI] [Google Scholar]
- Wang H, Zhang R, Zang H, Jiang S, Liu H, Sun M. Kinetics and functional effectiveness of nisin loaded antimicrobial packaging film based on chitosan/poly(vinyl alcohol) Carbohydr Polym. 2015;127:64–71. doi: 10.1016/j.carbpol.2015.03.058. [DOI] [PubMed] [Google Scholar]
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Wilson-Stanford S, Kalli A, Hakansson K, Kastrantas J, Orugunty RS, Smith L. Oxidation of lanthionines renders the lantibiotic nisin inactive. Appl Environ Microbiol. 2009;75(5):1381–1387. doi: 10.1128/AEM.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim S, Röcker B, Pettersen MK, J, Z, R, Radusin T, Suminska P, Marcos B, Coma V, MK, Nilsen-Nygaard J, Ayhan Z, Rutkaite R, Radusin T, Suminska P, Marcos B, Coma V. Active packaging applications for food. Compr Rev Food Sci Food Saf. 2017;17:165–199. doi: 10.1111/1541-4337.12322. [DOI] [PubMed] [Google Scholar]