Abstract
A large section of the human population relies on legumes as a staple food. Legumes are a rich source of nutrients and possess several health-related beneficial properties. However, the nutritional quality of legumes is challenged by the presence of a considerable amount of antinutrients. Consumption of inadequately processed legumes might affect normal metabolism and cause adverse human health-related effects. Effective processing becomes necessary to reduce these antinutritional factors before consumption. Optimizing the processing variables during preparation of legume-based traditional foods by using response surface methodology could be a valuable option to reduce antinutrients. The present review focuses on the efficacy of traditional household-scale processing unit operations vis-à-vis the reduction of antinutrients. Optimally prepared products should ensure meeting the consumer demand of improved, healthy, and more nutritious and safe foods. Modeling-based optimization approach will be helpful to define best practices at the small-, medium-, and large scale production alike. It should contribute towards effective utilization of legume resources, and to alleviate malnutrition and associated diseases world-wide.
Keywords: Antinutrient, Food legume, Safety, Response surface methodology, Processing
Introduction
Being a rich source of nutritional components, legumes are important in human nutrition, beneficial to human health and, among others, help to prevent ailments including cardiovascular diseases, diabetes, and colon cancer (vaz Patto et al. 2015). However, the consumption constraints of legumes is due to the presence of a considerable amount of antinutrients which limit the availability of various nutrients (Kim et al. 2012; vaz Patto et al. 2015; Sharma et al. 2018). Legume antinutrients include galacto-oligosaccharides, tannins, phytic acid, protease inhibitors, α-amylase inhibitor, saponins, lectins, lathyrogen, cyanogenic glycosides, oxalates, and biogenic amines. Several traditional food processing and preparation methods, like soaking, cooking, germination, and fermentation can be employed to reduce antinutrients and enhance bioavailability of nutritional components in the food system. Such treatments remove or diminish these factors and give acceptable, appetizing, and nutritious products which in turn enhance digestibility, nutrient bioavailability, and safety of the prepared foods. Reduction of antinutrients can also be achieved by selecting plant genotypes with low levels of such factors. Since antinutrients are important to the plants as they function as potent defence compounds against herbivores and pathogens, post-harvest processing has been the strategy for their elimination from seeds. As many of the antinutrients are toxic, unpalatable and/or indigestible for human consumption, the traditional domestic means of their reduction consist mainly of dehulling, soaking, germination, cooking, and fermentation (Shimelis and Rakshit 2008; Shukla et al. 2011; Luo and Xie 2013). Antinutrients and their chemical properties dictate which physical and/or biological process(es) will be more effective in their minimization or removal. It cannot be ignored that during processing a condition may prevail where a complete destruction of these factors may not always be achieved. In practice, a combination of processing parameters (i.e., temperature, time, pH, etc.) prevails. In research, the experimental approach to study one variable at a time is not effective and fails to determine interaction between the combined variables, is time consuming and expensive (Nwabueze 2010). So, an extensive study on the effect of traditional household processing variables on the reduction of antinutritional factors is a timely need.
Various legume-based fermented and non-fermented foods are prepared at household-scale or in small-scale industries using relatively simple available resources and facilities. Fermented products enhance food security and income generation to the livelihood of both rural and peri-urban dwellers residing in different geographical regions (Sharma and Sarkar 2015). Foods are fermented to create or enhance unique flavors, quality, texture, and digestibility (Shimelis and Rakshit 2007). The present review aims to study the strategy that can be applied at the household level for the removal of antinutrients during processing of raw legumes to produce safe and wholesome foods.
Food legumes
Legumes are seeds of dicotyledonous plants belonging to the family Leguminosae. These are globally distributed, and have been effectively manipulated and utilized. According to usage pattern, they are classified as oil-yielding legumes and grain legumes. While soybean, peanut, groundnut, and alfalfa are used for oil extraction, pulses and dry grains of peas, chickpeas, lentils, peas, beans, and lupins are utilized as grain legumes. Important food legumes cultivated and consumed in different regions of the world are shown in Table 1. Legumes show a wide range of adaptability in terms of climate and soil conditions, and are important beneficial components of the agricultural system. The value of legumes as a food source is determined by two factors, i.e., their nutritional potentiality for better health; and their deleterious effects to health due to the presence of antinutrients. Protein digestibility corrected amino acid score (PDCAAS), which has been used as a measure for assessing protein quality. For better health, U.S. labeling regulations indicated that a protein must have a PDCAAS value greater than 20% to qualify as a quality protein for noninfant foods, and greater than 40% to qualify as a quality protein for infants for better health (FAO/WHO 2013). Legumes can be used in innovative food formulation to increase the nutritional value. For example, a low PDCAAS value of cereals could be compensated in cereal-legume combinations (FAO/WHO 2013).
Table 1.
Dominant legume-based traditional foods
| Food | Substrate(s)/ingredients | Principal processing stages | Nature of product/mode of consumption | Area | Reference |
|---|---|---|---|---|---|
| Fermented | |||||
| Adai | Legume seeds and cereal grains | Soaking → grinding → mixing of batters → fermenting → frying | Fried snack food | South India | Ray et al. (2016) |
| Afiyo (okpehe or kpaye) | Mesquite bean (Prosopis sp.) | Boiling → dehulling → boiling → fermenting → sun-drying | Sticky, brown, with a strong odor; condiment | Nigeria | Evans et al. (2013) |
| Aisa | Albizia saman seeds | Boiling → Dehulling → reboiling → rinsing → fermenting | Dark brown with creamish mucilaginous slime, ammoniacal odor; condiment | Nigeria | Ogunshe et al. (2006) |
| Amriti | Black gram dal (Vigna mungo) | Soaking → grinding → fermenting → deep frying | Snack food | India | Roy et al. (2007) |
| Bedvin roti | Black gram dal, opium seed or walnut flour | Dough making → fermenting → baking/frying | Baked or deeply fried; breakfast or snack food | India | Thakur et al. (2004) |
| Chungkokjang (cheonggukjang or jeonkukjang) | Soybean (Glycine max) | Soaking → cooking → fermenting | Alkaline, sticky; condiment, soup | Korea | Shin and Jeong (2015); Kim et al. (2017) |
| Dawadawa (soydawadawa) | Soybean | Soaking → dehulling → cooking → fermenting | Condiments | Nigeria | Sarkar and Nout (2014) |
| Dawadawa, kinda, iru, soumbala | Locust bean (Parkia biglobosa) | Boiling → dehulling → reboiling → fermenting → air-drying | Alkaline, sticky, condiments; flavoring agent in soups | West and Central Africa | Sarkar and Nout (2014) |
| Dhokla | Rice grains and bengal gram dal (Cicer arietinum) | Soaking → grinding → mixing of batters → fermenting → steaming | Mild acidic, spongy, steamed cake; snack food | Western India | Nout et al. (2007) |
| Dosa | Black gram dal and rice grains | Soaking → grinding → mixing of batters → fermenting → frying | Crispy pancake; Snack food | Southern India | Nout et al. (2007) |
| Douchi | Black soybean | Soaking → cooking → inoculating with Aspergillus oryzae → fermenting | Alkaline, paste; condiment, soup | China | Sarkar and Nout (2014) |
| Gochujang /Kochujang | Soybean, red pepper, rice, barley malt powder | Soaking → fermenting | Seasoning agent | Korea | Shin and Jeong (2015) |
| Idli | Black gram dal and rice grains | Soaking → grinding → mixing of batters → fermenting → steaming | Mild acidic, soft, moist, spongy; breakfast food | South India, Sri Lanka | Nout et al. (2007) |
| Kinema, hawaijar, tungrymbai, aakhone, bekang, peruyyan | Soybean | Soaking → cooking → fermenting | Alkaline, sticky; curry soup | Darjeeling hills and North East of India, Bhutan, Nepal | Sarkar and Nout (2014) |
| Maseura (masyaura) | Black gram dal/rice-bean (Vigna umbellate) | Soaking → grinding → molding drying | Dry, ball-like, brittle; condiment | India, Nepal | Chettri and Tamang (2008) |
| Meitauza | Okara (soybean press cake) | Fresh okara → soaking → steaming → squeezing → cooling → shaping → fermenting → sun-drying | Served either fried in oil or cooked with vegetables as flavouring agent | China, Taiwan | Sarkar and Nout (2014) |
| Meju | Soybean | Soaking → cooking → block making → fermenting | Alkaline, paste; seasoning agent | Korea | Shin and Jeong (2015) |
| Natto | Soybean | Soaking → boiling → fermenting | Alkaline, mucilagenous snack food; flavouring agent | Japan, Korea | Sarkar and Nout (2014) |
| Oncom: Hitam (black) and merah (red) | Peanut (Arachis hypogaea) press cake | Soaking → steaming → inoculating with Neurospora spp. or Rhizopus spp. → fermenting | Solid product roasted or fried in oil; used as meat substitute | Indonesia | Nout et al. (2007) |
| Oso | Cathormion altissimum seeds | Boiling → fermenting | Alkaline; condiment | Nigeria | Sarkar and Nout (2014) |
| Otiru | African yam bean (Sphenostylis stenocarpa) | Soaking → dehulling → fermenting | Greyish brown, mucilaginous, with ammoniacal odor; condiment | Nigeria | Sarkar and Nout (2014) |
| Owoh | African yam bean | Boiling → mashing → packing → fermenting | Alkaline; condiment | Nigeria | Sarkar and Nout (2014) |
| Papad or papadam | Black gram, bengal gram, lentil (Lens culinaris), red gram (Cajanus cajan) or green gram (Vigna radiata) flour | Hand-kneading into dough → fermenting → rolling into thin, circular, flat sheets → drying under shade | Circular wafers; condiment; snack food | India | Nout et al. (2007) |
| Pitha (chakuli, enduri, munha, chhuchipatra, podo) | Black gram dal and rice grains | Soaking → grinding → fermenting → frying/steaming | Breakfast or snack food | India | Ray et al. (2016) |
| Sepubari | Black gram dal | Fermenting → frying | Sun-dried /deep-fried solids; side dish | India | Savitri and Bhalla (2007) |
| Soybean paste: Doenjang or jang, miso, tauco, tao chieo | Soybean, wheat or rice grains | Soaking → cooking → adding with rice/wheat koji, salt & water → rough mashing → fermenting | Alkaline, salty, bright yellow to brackish brown; flavoring agent in soups | China, Indonesia, Japan, Korea, Thailand | Sarkar and Nout (2014) |
| Soy sauce: Jiang you, shoyu or tamari shoyu, kanjang, kicap, kecap, taosi, ketjap, inyu | Soybean/black soybean and wheat grains | Soaking → cooking → adding roasted wheat → koji → addiing Aspergillus spores, yeasts → brewing → pressing → filtering to separate sauce → storage | Alkaline syrup, light or dark brown liquid with meat-like salty flavour; condiment, seasoning agent | China, Japan, Korea, Malaysia, Indonesia, Philippines, Indonesia, Taiwan, Hong Kong | Sarkar and Nout (2014) |
| Sufu or furu | Soybean | Dehulling → soaking → grinding, sieving and cooking of water extract → coagulating → making curd cubes → inoculating with mold → fermenting → matruring in brine | Semi-solid flavoring side dish | China, Taiwan | Nout et al. (2007): Guan et al. (2013) |
| Tempeh | Soybean | Dehulling (wet/dry) → soaking → cooking → inoculating (Rhizopus spp.) → fermenting | Mildly alkaline, solid; fried cake; snack food | East Java, Indonesia | Nout et al. 1993; Nout et al. (2007) |
| Thua nao | Soybean | Soaking → boiling → fermenting | Alkaline, paste, dry; soup | Thailand | Sarkar and Nout (2014) |
| Tuong | Soybean | Sieving → roasting → grinding → soaking → fermenting + adding fermented rice → mashing → cooking → fermenting | Seasoning | North and central Vietnam | Nguyen (2015) |
| Vada | Legume and cereal | Soaking → grinding → mixing of batters → fermenting → frying | Deep fried patties; Snack food | India | Ray et al. (2016) |
| Ugba/ukpaka | African oil bean (Pentaclethra macrophylla) | Boiling → dehulling → cooking → washing → cutting into slices → reboiling → adding salt (optional) → fermenting | Condiment | West and Central Africa | Sarkar and Nout (2014) |
| Wadi | Black gram dal | Soaking → batter preparation → fermenting → drying | Ball-like, hollow, brittle; spicy condiment taken with vegetables, legumes, boiled rice | Northern India | Nout et al. (2007) |
| Yandou | Soybean | Soaking → boiling → fermenting → adding salt → sun-drying | Sticky; snacks | China | Sarkar and Nout (2014) |
| Non-fermented | |||||
| Akara | Cowpea (Vigna unguiculata) | Soaking (1st) → dehulling → soaking (2nd) → wet-milling → seasoning → frying | Fried dumpling with seasoning | Nigeria | Bolade (2016) |
| Dhal/dal | Whole/dehulled split seeds | Soaking → cooking | Soup | India | Dahiya et al. (2013) |
| Edamame/mao dou | Immature fresh and frozen soybean | Frying or boiling in water with salt | Sweet, nutty flavoured; ingredient in salad | China and Japan | Miles et al. (2000) |
| Ekuru | Cowpea | Soaking (1st) → dehulling → soaking (2nd) → wet-milling → steaming | Steamed paste | Nigeria | Bolade (2016) |
| Ewa ibeji | Cowpea | Cooking until softened | Cooked and softened product | Nigeria | Bolade (2016) |
| Gachas | Grass pea (Lathyrus sativus) | - | Traditional meal | Spain | Megias et al. (2015) |
| Gbegiri | Cowpea | Soaking → dehulling → pressure-cooking → micronization (overnight tampering of seeds) → seasoning → cooking | Soup | Nigeria | Bolade (2016) |
| Kadi/kadu/kheeru | Buttermilk/dahi (fermented milk) and gram flour | Cooking dahi with spices and besan (gram flour) | Side dish | India | Savitri and Bhalla (2007) |
| Laddu/burfi | Mung bean flour, nuts, ghee | Milling → roasting → mixing → shaping | Round/flat rectangular; sweet | India | Dahiya et al. (2013) |
| Madrah | Kidney beans (Phaseolus vulgaris) | Cooking beans with ghee (clarified butterfat) and yoghurt | Curry with ghee and yoghurt; served with steamed rice | India | Savitri and Bhalla (2007) |
| Moin-Moin | Cowpea | Soaking → dehulling → resoaking → wet-milling → seasoning → steaming | Steamed paste with seasoning | Nigeria | Bolade (2016) |
| Okara | Soybean | Residual solid from soymilk extraction | Prepared to various food items | China, Japan and Korea | He and Chen (2013) |
| Sang | Wheat grain, pea (Pisum sativum), horse gram (Macrotyloma uniflorum) etc | Boiling in water | Soup | India | Savitri and Bhalla (2007) |
| Soynuts | Fried, baked or roasted soaked soybeans | Soaking → frying/baking/roasting | Snack | Europe | He and Chen (2013) |
| Teliye mah | Blackgram | – | Semi solid product | India | Savitri and Bhalla (2007) |
| Tofu | Soybean | Soaking → boiling → sieving → coagulating | Coagulation of soy protein curd; side dish | China | Nout et al. (2007) |
Nutritional and health benefits
Legumes, being considered as poor man’s meat, play an important role in human nutrition as a source of good-quality protein, calories, minerals, vitamins, and fiber (vaz Patto et al. 2015). The major proteins found in most legumes are globulins (legumin, 11S, vicilin, and 7S) and albumins (protease inhibitors, amylase inhibitors, and lectins). Minor proteins include prolamins and glutelins (vaz Patto et al. 2015). Legumes contain (per 100 g; as such basis): 0–65 g carbohydrates, 18–32 g total dietary fiber, 2–21 g fat, and minerals, such as calcium, copper, iron, magnesium, phosphorus, potassium, and zinc (Venter and Eyssen 2001). Legumes are a good source of water-soluble vitamins, especially thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), pyridoxine (vitamin B6), and folate (Venter and Eyssen 2001). Whereas, the PDCAAS value for soybean is 100%, the same for other legumes is 28–75% (Annor et al. 2014). Recently, and expert panel of the Food and Agriculture Organization of the United Nations (FAO) introduced an alternative measurement of protein quality, named as Digestible Indispensable Amino Acid Score (DIAAS) (FAO/WHO 2013).
Legumes due to their low glycemic index and high fiber content, contribute to diets lowering the risks of obesity, diabetes, cardiovascular diseases, cancer, and elevated serum cholesterol levels. According to National Health and Nutrition Examination Survey (NHANES) reports on the management of obesity, adults who consumed a variety of legumes had significantly lower body weights compared to those who did not consume. The ability of legume protein to bind with bile acids in the gastrointestinal tract was reported to increase cholesterol metabolism and to help reducing cholesterol levels in blood (vaz Patto et al. 2015).
Legume-based traditional foods
Traditional food fermentation involves the activity of several microorganisms either present originally in raw materials or occur as contaminants or added as inoculums (Sharma and Sarkar 2015). Microorganisms possessing enzymic activity for fermentation might be endogenously present in substrates or may be added as a starter culture where substrates are transformed biochemically and organoleptically by these organisms into upgraded edible products (Ray et al. 2016). Diverse groups of microorganisms such as yeasts, molds and bacteria are involved in the production of fermented foods. They act alone or in combination for the production of particular foods (Nout et al. 2007; Sharma and Sarkar 2015). When yeasts are abundant, alone or in stable mixed populations with mycelial fungi or with lactic acid bacteria (LAB), they have a significant impact on food quality parameters such as taste, texture, odor and nutritive value (Aidoo et al. 2006). Japanese natto, Nigerian dawadawa or iru, Nepalese kinema and Thai thua nao are fermented by Bacillus, and each product has a unique distinct flavor and aroma which are used as a meat substitute and as a flavoring agent in soups or consumed directly. Molds are predominant in yukiwari-natto and hama-natto, while the more common itohiki-natto is a Bacillus-fermented product. Molds are also predominant in meitauza, oncom and sufu. Indian papad/papadam is yeast-fermented. Tempe is prepared by the combine fermentative activity of molds and LAB. Popular indigenous fermented foods of India such as wadi, idli and dhokla are predominantly fermented by LAB and yeasts. Inyu, kecap asin, kecap manis, meju, miso, soy sauce and tauco are the products prepared from combined fermentative activity of molds, yeasts and LAB (Aidoo et al. 2006; Nout et al. 2007). In addition, various legumes are processed and consumed as non-fermented products (Table 1).
Legume antinutrients: chemical structure and properties, dietary intake, and biochemical effects on human health
The antinutrients present in raw legumes or improperly processed legumes reduce digestibility and bioavailability of nutrients in foods, and are also responsible for various physiological abnormalities in humans. Nutritional deficiency is a major problem; poverty, hunger, and malnutrition are highest in South and Southeast Asia. The chemical structure and properties of antinutrients, their interactions with food matrix, dietary intake, and biochemical effects on human health are discussed below.
Oligosaccharides
Raffinose family oligosaccharides (RFO) predominate in most legumes. These are stored during seed ripening (Guillon and Champ 2002). Raffinose is the first member of the series followed by stachyose, verbascose, and ajugose. Oligosaccharides are considered as antinutrients because they are a major producer of flatulence. As human beings are devoid of α-galactosidase to cleave or break α-galactosyl linkages, the intact oligosaccharides are not absorbed by the digestive tract. The undigested oligosaccharides arrive in the cecum and are rapidly degraded by cecal and colonic microbiota having α-galactosidase activity, and subsequent anaerobic fermentation results in the production of gases, such as carbon dioxide, hydrogen, and methane (Guillon and Champ 2002). Flatulence is usually accompanied by abdominal pain, nausea, cramps, diarrhea, and discomfort (Rakshit et al. 2015). The oligosaccharide contents of different raw legumes are shown in Table 2.
Table 2.
Oligosaccharide contents of different legumes
| Legumes | Oligosaccharide (mg/g dry wt) | References | |||
|---|---|---|---|---|---|
| Raffinose | Stachyose | Verbascose | Ajugose | ||
| Bengal gram | 4–12 | 20–36 | 6–42 | Guillon and Champ (2002) | |
| Black gram | 1.26 | 5.4 | 31 | 4.1 | Rakshit et al. (2015) |
| Cowpea | 7–8 | 42–52 | 7–8 | Madodé et al. (2013) | |
| Faba bean | 1–4 | 8–16 | 25–34 | Jezierny et al. (2010) | |
| Kidney bean | 2.4–3.4 | 12.4–18.4 | Shimelis and Rakshit (2007) | ||
| Lupin seed | 5.4–13.6 | 34.5–41.8 | 4.0–24.9 | 0.4–1.6 | Ruiz-lopez et al. (2000) |
| Soybean | 5–13 | 22–43 | 0–3 | Guillon and Champ (2002) | |
Tannins
Tannins (MW ≥ 500 Da) are well-distributed among a large variety of plants used as a source of food. On the basis of chemical structure, tannins are divided into two major groups, hydrolyzable tannins and condensed tannins. Hydrolyzable tannins possess a central core of carbohydrate and hydroxyl groups which are esterified by phenolic acid, such as gallic acid (gallotannins) or hexahydroxydiphenic acid (ellagitannins). A large number of hydrolyzable tannins exist in nature and exhibit a diverse variation in their structure. Such a variation is due to oxidative coupling of neighboring gallic acid units or aromatic rings in their structures (Schofield et al. 2001). On the other hand, condensed tannins, also called proanthocyanidins, having molecular weights higher than hydrolyzable ones, are structurally more complex. Condensed tannins are oligo- or poly-mers of a polyhydroxy flavanol. The monomeric flavanols differ in their hydroxylation pattern in ring A (first ring left) and B (last ring right) and in the stereochemistry of C-3 (middle ring). The most common monomers are the diastereomers ( +)-catechin/(−)-epicatechin, the respective oligo- and poly-mers are called procyanidins. Next common monomers are the diasteromers (−)-gallocatechin/(−)-epigallocatechin, the respective oligo- and poly-mers are called prodelphinidins. Diasteromers ( +)-afzelechin/(−)-epiafzelechin with respective oligo- and polymers are called propelargonidins.The flavanol monomers are usually linked by carbon–carbon bonds in the 4 → 6 or the 4 → 8 position in case of B-type condensed tannins. A-type condensed tannins also occur in some plant compounds with an additional C2 → C7 ether-linkage (Serrano et al. 2009). Tannins exhibit positive human health-related properties, e.g., anticarcinogenic, antimutagenic, antimicrobial, and antioxidant. In spite of having these beneficial properties, tannins have been considered as antinutrients. This is related to the high level of hydroxylation and property of forming insoluble complexes with protein, carbohydrate, metal ions, and polysaccharides. The complex formation is associated with reversible interaction of hydrogen and hydrophobic interactions, and irreversible interactions of ionic and covalent bonds (Schofield et al. 2001). The complex of tannins and proteins causes inhibition of enzymes and affects gastrointestinal digestion and absorption of nutrients, damages the intestinal tract, and lowers nutrient availability (Serrano et al. 2009). Tannins reduce protein digestibility, making these partially unavailable for absorption and inhibit proper functioning of proteins and other digestive enzymes. These damage the mucosal lining of the gastro-intestinal tract and increase excretion of proteins and essential amino acids. Amino acid availability becomes low in diets rich in tannins. Food composition survey data provided a good account of dietary intake of tannins among different human populations. The intake is roughly ranged from several tens to several hundreds mg/day in Spanish population (Santos-Buelga and Scalbert 2000). Another study reported an intake of 440 mg condensed tannins/person/day by Spanish population (Saura-Calixto et al. 2007). Limited data is available on dietary intake of hydralyzable tannins. Intake of hydrolysable polyphenols in the Spanish populations is around 1250 mg/person/day (Saura-Calixto et al. 2007). Dietary dose is essential in order to determine the significance of tannins for human health. Tannins exert their biological effects in two different ways, i.e. as an insoluble complex structure having binding properties which produce local effects in the gastrointestinal tract and as absorbable form which may produce systemic effects in various organs (Serrano et al. 2009). Therefore, it is advisable to reduce the excess intake of tannin-rich foods. Plants possess polyphenol oxidase which hydrolyzes various phenolic compounds (Sandberg 2002).
Phytic acid
Phytic acid, also called myo-inositol hexaphosphoric acid or 1,2,3,4,5,6-hexakis (dihydrogen phosphate) myo-inositol, or phytate in salt form, is the principal storage form of phosphorus in many legume seeds (Kumar et al. 2010). The antinutritional property of phytic acid lies in the fact that it forms an insoluble complex with minerals. Phytic acid also reduces the availability of divalent and trivalent metal ions such as Zn2+, Fe2+, Fe3+, Ca2+, Mg2+, Mn2+, and Cu2+. Low bioavailability of Zn2+causes the most adverse effect on human health; a reduction of Zn2+ absorption results in dwarfism and hypogonadism (Kumar et al. 2010). Inositol tri-, tetra-, and penta-phosphates are also responsible for diminution of iron and zinc absorption (Sandberg 2002). Phytic acid forms complexes with proteins and minerals at a wider range of pH. Chelating effect of phosphate groups of phytic acid bind to mineral cations, especially Cu2+ and Zn2+ for all IP3 is pH 3.0 and those of IP6 is 7.0. The phytic acid-protein complex is not readily absorbed in the gastro-intestinal tract and small intestine of humans, because humans are devoid of phytic acid-degrading enzyme. This hampers enzymic activity, protein solubility, and protein digestibility (Sandberg 2002). With carbohydrates, it reduces solubility and digestibility of glucose (Kumar et al. 2010). Phytic acid can be degraded by phytase (myo-inositol hexakisphosphate phosphohydrolase) enzyme. Various possible sources of phytase are plants, microorganisms (fungi and bacteria), small intestinal mucosa, and gut-associated microbiota. Phytase activity of small intestine is very low, and the activity in the gut is associated with a large number of bacteria present in colon (Kumar et al. 2010). However, the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Biochemistry and Molecular Biology (IUBMB) categorized phytase into two types, 3-phytase (myo-inositol hexakisphosphate 3-phosphohydrolase) and 6-phytase (myo-inositol hexakisphosphate 6-phosphohydrolase).
Lathyrogens
Seeds of Lathyrus spp. contain lathyrogens responsible for neurotoxic diseases in humans and animals, called lathyrism (Hanbury et al. 2000). Chemically, lathyrogen is a non-protein amino acid, named as β-(-N-oxalyl)-L-α,β-diamino propionic acid (β-ODAP) or β-N-oxalylamino-L-alanine (BOAA). The cause of lathyrism has been associated with consumption of daily dose of approximately 300 g kesari (Lathyrus sativus L.) and a diet devoid of adequate amount of cereals (Bora 2014). The disease occurs in the form of neurolathyrism, osteolathyrism, and angiolathyrism (Grela et al. 2001; Hanbury et al. 2000). Neurolathyrism leads to spastic paralysis of the legs, weakening of skeletal muscles with pronounced stiffness, and chronic crippling syndrome. In osteolathyrism, a disturbance in the synthesis of elastic components of mesenchymal tissues leads to skeletal deformities together with disorders in the growth of cartilages and bones. Increased stiffness sometimes causes sudden death due to rupture of aorta, called angiolathyrism. Lathyrogen also affects the vascular system by disturbing vascular wall formation, increasing the fibroblast proliferation and irregularly arranged collagenous fibers, thereby decreasing the resistance of vascular wall to stretching (Grela et al. 2001). Other symptoms of lathyrism include skeletal deformities, initial painful spasms in lower limb muscle followed by weakness, and chronic spastic paraplegia occurs ultimately leading to total paralysis (Hanbury et al. 2000).
Cyanogenic glycosides
Chemically, these are glycosides of α-hydroxynitriles belonging to secondary metabolites in plants. Major plant cyanogenic glycosides include dhurrin in sorghum, prunasin in ferns, taxiphyllin in bamboo shoot, sambunigrin in elderberries, neolinustatin in flax seed, linustatin in flax seed and cassava, amgydalin in almond, peach, apple kernels, apricot, and the legumes. In legumes, the predominant ones are linamarin and lotaustralin in lima bean, and vicianin in seeds of several species of Vicia. Cyanogenic glycoside are basically amino acid-derived plant constituents. By enzymic hydrolysis, they produce aglycone (α-hydroxynitrile) and a sugar moiety, mainly D-glucose and are grouped according to aliphatic, aromatic substitution, and also into glycosides with a free α-hydroxynitrile. These are considered as antinutrients because of the production of hygrogen cyanide (HCN) after their hydrolysis either spontaneously or by enzyme-regulated reactions (Vetter 2000). The hydrolyzed products of cyanogenic glycosides, i.e. HCN and β-glucosidase activity have toxic effect on humans and animals (Bora 2014).
Saponins
Saponins are low molecular weight compounds of active sterol glycosides or triterpene glycosides containing mono- or oligo-saccharides attached to the core. The common legume saponins include soy saponins which are classified into groups A, B, and E saponins on the basis of the chemical structure of the aglycone (Champ 2002). Saponins possess health promoting effects as these are involved in the reduction of cell fission of mucous membrane and have an ability to prevent cancer. These readily react with cholesterol in the erythrocyte wall and exhibit hemolytic activity, during which a high affinity of saponins to blood cell membrane sterols causes blood cells to burst by changing their membrane permeability (Campos-Vega et al. 2010).
Oxalates
In plants, oxalates exist in both soluble and insoluble forms, and each form has a deleterious impact on human health. The soluble form occurs as its sodium salt where it is directly absorbed from the diet and causes renal stone disease (Judprasong et al. 2006), whereas majority of all kidney stones contain insoluble calcium oxalate which tends to precipitate and gets accumulated in renal glomeruli and results in renal disorders leading to the risk of kidney stone (Judprasong et al. 2006). Consumption of a high dietary oxalate results in increased urinary oxalate excretion, and during such process of excretion process oxalate precipitates with calcium leading to the formation of calcium oxalate kidney stones. A number of biosynthetic pathways have been reported where isocitrate, glycolate, glyoxylate, oxaloacetate, and ascorbate have all been suggested as possible precursors in the biosynthesis of oxalate.
Lectins
Hemagglutinins or lectins are abundant in legumes (van Buul and Brouns 2014). These are glycoproteins that have an ability to reversibly bind specific carbohydrates without altering their covalent structure. Lectins are known to protect plants from pathogens, such as microorganisms, pests, and insects. Lectins are also categorized according to sugar-binding specificities into polyspecific or monospecific. Their interactions with galactose, mannose, mannose-containing glycan, and glucose have also been reported. Lectins have an important function in many areas of research. The hemagglutinating activity (HA) of lectins is exploited for blood typing. They also play an important role in immune function, cell growth, cell death, body fat regulation, glycoconjugate studies, cell characterization, and cell separation. The molecular weight of lectins ranges from 25 to 400 kDa. Hemagglutinin or lectin, due to its globular tertiary structure, remains undegraded by digestive enzymes and causes several health-related problems (van Buul and Brouns 2014). Lectins interfere with normal gastric secretion for nutrient absorption by enhancing the shedding of bush border membrane and decreasing villus length of the small intestine. They are also found to bind with glycan receptors in the intestinal tract causing discomfort. Lectins interact with enterocytes and lymphocytes, causing persistent antigenic responses. When present in the blood stream, lectins bind to cell membrane, arteries, and organs, such as joints, kidney, pancreas, and brain, causing auto-immune disorders. Other toxic manifestations, caused by the intake of a high dose of dietary lectin, include nausea, bloating, vomiting, and diarrhea (van Buul and Brouns 2014).
Biogenic amines
Biogenic amines are nitrogenous, low molecular weight compounds which usually get accumulated in processed and stored food products as a result of microbial decarboxylation of amino acids (Gardini et al. 2016; Naila et al. 2010). These are either aliphatic (putrescine, cadaverine, spermine, and spermidine), aromatic (tyramine and phenylethylamine), or heterocyclic (histamine and tryptamine) (Gardini et al. 2016). Biogenic amine formation depends on the availability of free amino acids, the presence of decarboxylase-positive microorganisms, and conditions suitable for microbial growth, decarboxylase synthesis, and decarboxylase activity (Gardini et al. 2016). Raw material, manufacturing process, pH variation, salt concentration, and temperature are the influencing factors for their formation (Shukla et al. 2011; Gardini et al. 2016). Consumption of foods rich in biogenic amines leads to several human health-related abnormalities. The doses of biogenic amines have been reviewed by Kim et al. (2012). A level of ≥ 1000 μg amines/g food is considered hazardous to human health. The value of 100 μg histamine/g food has been suggested as an upper limit for human consumption and 100–800 μg tyramine/g and 30 μg β-phenylamine/g food is considered as toxic. When intake of amines is high, the detoxification system present in our body becomes unstable and fails to detoxify these amines effectively. Among the amines, putrescine and cadaverine are considered as a precursor to the formation of carcinogenic N-nitrosamines, but the toxicity effect is low. However, reports on colon tumor development upon consumption of a higher dose of putrescine cannot be ignored. Spermine and spermidine decrease blood pressure, cause respiratory disorder, inhibit blood clotting, and have neurotoxic effect. Tyramine has a vasoconstrictor effect, and may cause migraine, increased cardiac input, nausea, vomiting, and respiratory disorder. Phenylethylamine is known to trigger migraine and increase blood pressure, and histamine is associated with food poisioning (Gardini et al. 2016).
The levels of tannins, phytic acid, saponins, oxalates, HCN, lathyrogens, and total biogenic amines in raw legumes have been shown in Table 3.
Table 3.
Antinutrient contents of some raw legumes
| Legume | Antinutrient* (mg/g dry wt) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| TC | PAC | Saponins | Oxalates | HCN | β-ODAP | TBAC | ||
| Bengal gram | 2.2–4.9 | 1.2–1.7 | 0.9 | 0.7 | 0.423 | Champ (2002); Alajaji and El-Adawy (2006); Sharma et al. (2018) | ||
| Black gram | 0.5 | 5.1 | 0.295 | Sharma et al. (2017) | ||||
| Faba bean | 6.5 | 8.4 | 4 | Champ (2002); Luo and Xie (2013) | ||||
| Grass pea | 2.12 | 3.03 | 0.948 | Grela et al. (2001) | ||||
| Kidney bean | 5.4–28.8 | 17.3- 24.1 | 9–13 | 1–5 | Champ (2002); Shimelis and Rakshit (2007) | |||
| Pea nut | 0.0009 | 14.7 | Ejigui et al. (2005) | |||||
| Soybean | 1.76 | 8.41 | 6 | 670–3500 | 0.158 | Massey et al. (2001); Champ (2002); Egounlety and Aworh (2003); Sharma et al. (2015) | ||
*TC tannins content, PAC phytic acid content, HCN hydrogen cyanide, β-ODAP β-(-N-oxalyl)-L-α,β-diamino propionic acid, TBAC total biogenic amines content
Trypsin inhibitors
Legume protease inhibitors gained much attention during 1970s and 1980s due to their interference with digestion and growth in animals (Champ 2002). Mainly two types of inhibitors, namely Kunitz and Bowman-Birk, exist. Trypsin inhibitors function as storage of sulfur amino acids during dormancy. It has a potential to protect against predators, such as plant pathogens and pests (Champ 2002). The negative effect of trypsin inhibitor lies in the fact that it is resistant to pepsin and acidic pH of the human digestive tract and stomach, and disturbs normal functioning of the digestive enzymes. The inactivation of trypsin in the digestive tract by trypsin inhibitor induces the release of a pancreozymin-cholecystokinin from the intestinal mucosa. This hormone then stimulates pancreatic acinar cells to produce more trypsin. When synthesis of trypsin continues in the pancreas, an increased requirement for amino acid cysteine takes place, leading to an important loss of sulfur-rich amino acids. This negative feedback leads to pancreatic hypertrophy/hyperplasia, growth depression, and other carcinogenic effects. Like trypsin inhibitor, chymotrypsin inhibitor and α-amylase inhibitor limit the usage of nutritional value of foods (Champ 2002; Ejigui et al. 2005). The enzyme inhibitors in different legumes are shown in Table 4.
Table 4.
Enzyme inhibitors in different food legumes
| Legume | Antinutrient* (kU/g dry wt) | Reference | |||
|---|---|---|---|---|---|
| TIA | CTIA | AAIA | HA | ||
| Bengal gram | 1–16 | 5.0–6.2 | Alajaji and El-Adawy (2006); Champ (2002); Sharma et al. (2018) | ||
| Black gram | 120 | 0.2 | Sharma et al. (2017) | ||
| Cow pea | 12.4 | Uwaegbute et al. (2000) | |||
| Faba bean | 6.7 | 0.38–0.77 | Champ (2002) | ||
| Grass pea | 133–174 | 0–23 | 0.004–0.09 | Hanbury et al. (2000) | |
| Kidney bean | 4.6–29.3 | 0.448 | 2450–3560 | Shimelis and Rakshit (2007); Ejigui et al. (2005); Champ (2002) | |
| Pea nut | 16.3 | 0.063 | Ejigui et al. (2005) | ||
| Soybean | 40 | 2.34 | Sharma et al. (2015) | ||
*TIA trypsin inhibitor activity, CTIA chymotrypsin inhibitor activity, AAIA α-amylase inhibitor activity, HA hemagglutinating activity
Ways and means to reduce the level of antinutrients in legume foods
Processing of food legumes enhances physical and biochemical properties of raw ingredients to produce more useful, value-added, safe, palatable, and economically viable products (Sarkar and Nout 2014). Traditional processing steps of food preparation are mostly followed in households in order to enhance the bioavailability of nutrients in foods by reducing the level of antinutrients (Luo and Xie 2013; Shimelis and Rakshit 2008). During processing, it is important that toxic components should be reduced to a minimum level so that these pose no threat to the health of the potential consumers. Inadequate processing and presence of antinutrients limit edibility of legumes. Therefore, several approaches may be considered for their removal. Various legume-based traditional fermented as well as non-fermented foods are prepared and consumed throughout the world (Table 1). Therefore, some of the traditional processing strategies or treatments for the reduction of antinutrients of legumes used for the preparation of some of these foods are presented in Table 5. The processing treatments can broadly be categorized into physical processing and bio-processing.
Table 5.
Effect of traditional processing conditions on the contents of antinutrients of some edible legumes
| Legume | Processing condition | Antinutrient* (% reduction) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RFO | TC | PAC | Saponins | Oxalates | TIA | AAIA | HA | BOAA | |||
| Bengal gram | Boiling: Soaked seeds-water, 1:10 w/v; 90 min | 46 | 48 | 29 | 52 | 82 | 100 | Alajaji and El-Adawy (2006) | |||
| Autoclaving: Soaked seeds-water, 1:10 w/v; 1.1 kg/cm2; 35 min | 46 | 50 | 41 | 44 | 84 | 100 | |||||
| Microwave cooking: Soaked seed-water, 1:10 w/v; 15 min | 48 | 48 | 38 | 47 | 81 | 100 | |||||
| Black gram | Cooking | 24 | 33 | Rehman and Shah (2005) | |||||||
| Raw seed-water, 1:5 w/v, Temp., 121 °C; Time, 10, 20, 40, 60, 90 min | 35–41 | 33–51 | |||||||||
| Cow pea | Soaking (raw seed): 15 min, 30 ± 2 °C | 10–16 | 15–21 | 6–11 | Batista et al. (2010); Bolade (2016) | ||||||
| Dehulling: Manually | 58 | 51–61 | 16–21 | ||||||||
| Cooking (raw seed): Time, 45 min; Temp., 105 °C | 88–90 | 82–86 | 100 | ||||||||
| Extrusion cooking: Single-screw extruder; Temp., 150 °C; Screw speed, 150 rpm; Feed moisture, 20% | 26 | 71 | 100 | 100 | |||||||
| Grass pea | Soaking (raw seed): Seed-water ratio,1:7 w/v; Time, 12 h | 16 | 8 | 9 | 13 | Grela et al. (2001); Srivastava et al. (2015) | |||||
| Dehulling: Using mini dal mill | 51 | 7 | 56 | ||||||||
| Extrusion cooking: Twin-screw extruder, Temp., zone I: 90–140 °C; zone II, 100–180 °C; zone III, 120–220 °C; and zone IV, 100–200 °C, Feed moisture, 14, 18, 22, 26 or 30% | 19–33 | 90–91 | 79–80 | 32–38 | |||||||
| Kidney bean | Soaking (raw seed): Seed-water (pH 6.9),1:3 w/v; Time, 12 h | 40–45 | 23–25 | 17–19 | 11 | 6–15 | Shimelis and Rakshit (2007, 2008) | ||||
| Germination (raw seed): Soaking of raw bean-water,1:5 w/v; Temp., 25 °C; Time, 12 h and kept in a filter paper lined petri-dish covered with perforated aluminium foil in dark at 25 °C for 4 days to germinate | 100 | 76 | 79–96 | 50–59 | 15–17 | ||||||
| Alkaline soaking (raw seed): Seeds-sodium bicarbonate solution (pH 8.2), 1:3 w/v; Time, 12 h | 43–48 | 25–27 | 15 | 14–23 | 9–18 | ||||||
| Cooking (raw seed): Seed-water, 1:3 (w/v); Temp., 97 °C; Time 35 min | 49–53 | 27–35 | 25–28 | 68 | 23–35 | ||||||
| Autoclaving (raw seed): Seed-water, 1:3 w/v; Temp.,121 °C; Time, 30 min | 65–71 | 50–72 | 65 | 100 | 100 | ||||||
| Soaking + Cooking | 60–66 | 58–70 | 61–65 | 90 | 45–60 | ||||||
| Alkaline soaking + Cooking | 67 | 55–68 | 61–64 | 100 | 48–65 | ||||||
| Soaking + Autoclaving | 74–77 | 56–75 | 62–66 | 100 | 100 | ||||||
| Alkaline soaking + Autoclaving | 77–80 | 55–75 | 62–65 | 100 | 100 | ||||||
| Germination + Autoclaving | 100 | 100 | 100 | 100 | 100 | ||||||
| Irradiation: 5, 7.5 and 10 kGy γ-rays | 11–25 | 7–27 | |||||||||
| Fermentation (natural): Time, 0–96 h | 100 | 24–47 | 100 | 60–77 | 52–82 | ||||||
| Fermentation (sterilized beans): Aseptically inoculated with a mixed culture of Lactobacillus acidophilus, Bifidobacterium, Streptococcus thermophillus and incubated; Temp., 42 °C; Time, 4 days | 18.6 | 27–48 | 100 | ||||||||
| Peanut | Dehulling | 100 | 25 | Ejigui et al. (2005) | |||||||
| Germination + dehulling | 100 | 23 | 56 | ||||||||
| Roasting + dehulling | 100 | 13 | 23 | 69 | |||||||
| Germination-roasting and dehulling | 100 | 42 | 71 | ||||||||
| Soybean | Soaking: Raw beans-water, 1:3 w/v; 12–14 h | 22 | 55 | Egounlety and Aworh (2003); de Toledo et al. (2007) | |||||||
| Dehulling: Manual | 100 | ||||||||||
| Cooking: washed dehulled beans-water, 1:6 w/v; 30 min | 100 | ||||||||||
| Soaking + dehulling + washing + cooking | 59 | ||||||||||
| Fermentation: Inoculated cooked beans with Rhizpus oligosporus and incubated at 27–30 °C for 48 h | 52 | ||||||||||
| Cooking (autoclaving): Soaked seeds-water, 1:2; Temp., 120 °C; Time, 10 min | 75–95 | 23–61 | |||||||||
| Irradiation (raw seeds): 2, 4 and 8 kGy γ-rays | 72–95 | 30–39 | |||||||||
*RFO raffinose family oligosaccharides, TC tannins content PAC phytic acid content, TIA trypsin inhibitor activity, AAIA α-amylase inhibitor activity, HA hemagglutinating activity, BOAA β-N-oxalylamino-L-alanine
Physical processing
Physical processing involves the use of simple equipment and facilities available in households or small industries. Physical processing basically includes dehulling, soaking, and heat treatments.
Dehulling
Dehulling involves removal of seed coat. In the household, mortar and pestle are used to dehull seeds, while in industry machines are used. The effect of dehulling on the content of antinutrients in seeds varies with legume species and cultivars. The reduction of antinutrients during dehulling in various legumes is shown in Table 5.
Soaking
Soaking is routinely carried out in households for the preparation of various legume-based foods. The solvents used for soaking are usually water, salt solution, and acidic and alkali solutions. After soaking, the soaked medium is discarded. The duration of soaking varies depending on the processing conditions. The property or type of legume seeds used, the soaking temperature, soaking medium used, and duration of soaking are important factors affecting the removal of antinutrients (Table 6) from raw legumes (Sharma et al. 2015, 2017, 2018).
Table 6.
Effect of optimized processing conditions using RSM on the content of antinutrients of some food legumes
| Legume | Food | Optimized processing condition | Antinutrient reduction (%)* | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| RFO | TC | PAC | TIA | HA | TBAC | ||||
| Bengal gram | Dhokla | Soaking: Raw dal-water ratio, 1:5 w/w; initial pH of water, 7.0; T, 23 °C; t, 20 h | 79 | 35 | 50 | 60 | 18 | Sharma et al. (2018) | |
| Fermentation (mixture of dal batter: rice slurry; 3:1 v/v): Salt, 8 g/kg unfermented mixed batter; T, 32 °C; t, 18 h | 57 | 64 | 50 | 70 | |||||
| Steaming of optimally prepared fermented batter: T, 20 min | 100 | 75 | 67 | 100 | 25 | ||||
| Black gram | Idli |
Soaking: Raw dal-water ratio, 1:5 w/w; initial pH of water, 4.0; T, 16 °C; t, 18 h Raw dal-water ratio, 1:10 w/w; initial pH of water, 6.0; T, 16 °C; t, 21 h |
95 | 49 | 26 | 35 | 26 | Rakshit et al. (2015); Sharma et al. (2017) | |
| Fermentation (mixture of dal batter: rice slurry; 1:2 v/v): Salt, 16 g/kg unfermented mixed batter; T, 35 °C; t, 19 h | 44 | 53 | 36 | 65 | |||||
| Steaming of optimally prepared fermented batter: T, 20 min | 100 | 63 | 19 | 100 | 7 | ||||
| Soybean | Kinema | Soaking: Raw beans-water of 1:10 w/w; initial pH of water, 8.0; T, 10 °C; t, 20 h | 55 | 26 | 7 | 18 | Sharma et al. (2015) | ||
| Cooking: Soaked beans-water of 1:5 w/w; pressure, 1.10 kg/cm2; t, 20 min | 96 | 9 | 68 | 100 | |||||
| Fermentation: Inoculum load of 103 total cells of Bacillus subtilis/g cooked beans; T, 37 °C; t, 48 h | 100 | 41 | 0 | 100 | |||||
*RFO raffinose family oligosaccharides, TC tannins content, PAC phytic acid content, TIA trypsin inhibitor activity, HA hemagglutinating activity, TBAC total biogenic amines content
Utilizing RSM, individual and interaction effects of soaking time, initial pH of soaking water, dal-water ratio, and soaking temperature were established. Optimized soaking conditions for maximum reduction of antinutrients from raw black gram dal, bengal gram dal, and soybeans were estimated by mathematic modelling (Table 6). After soaking followed by cooking of soybeans, there was 73–88% reduction in the contents of oligosaccharide (Sarkar et al. 1997). Solubility and leaching in soaking medium were considered the possible reason for the reduction of antinutrients (Luo and Xie 2013). Stimulation of endogenous enzyme activity, such as polyphenol-oxidase and phytase might also have a reducing effect on tannins and phytic acid during soaking (Kumar et al. 2010). Besides, increased permeability of cell membrane facilitates leaching of the water-soluble antinutrients during soaking (Luo and Xie 2013).
Cooking
Cooking is a process that facilitates leaching of antinutrients into the cooking medium. Heat treatment reduces antinutrients, such as phytic acid which mainly exists as a complex with mineral elements. Cooking effectively reduces heat-labile antinutrients, such as trypsin inhibitor and hemagglutinins, thereby enhancing protein quality of the cooked product (Shimelis and Rakshit 2007). Successive soaking and cooking are more effective in reducing antinutrients than cooking alone (Luo and Xie 2013). The effect of cooking in reducing antinutrients from legumes has been shown in Table 5. While considering the influence of cooking, it becomes essential to understand to what extent nutritional components get affected so as to improve the nutrition of legume-based product by reducing the level of antinutrients. Selection of shorter cooking time and use of steaming, rather than simple boiling, can be an alternative mode of cooking of legumes. One such example is autoclaving which is a moist heating technique where cooking is done under high pressure. Autoclaving shortens the duration of cooking. High-pressure cooking imparts hydrostatic pressure which sterilizes food, denatures proteins, and controls the enzymes and other chemical reactions without causing any physical damage to the raw material. However, at the household scale, pressure cookers instead of autoclaves are used. Sharma et al. (2015), using the RSM technique, studied the effect of cooking on antinutrients content, and the optimized condition of cooking of soybean for the reduction of antinutrients has been estimated. The effect of cooking by steaming on the contents of antinutrients of black gram and Bengal gram dal is shown in Table 6.
Extrusion cooking
This is a high-temperature, short-time process which can be applied to foods to modify or improve their quality. Extrusion cooking has been applied to various legume-based foods for the production of ready-to-eat food products. The extruder consists of a screw rotating inside a smooth or grooved cylindrical barrel. For the production of legume-based products, legume flour or prepared blended meal with water is fed into the extruder barrel. After feeding the barrel, it is subjected to rapid heating caused by the high pressure. The legume flour–water mixture then goes through a process called thermoplastic melting, also known as thermoplastic extrusion. The intense heat and pressure applied to the product result in the denaturation of legume proteins and puffing of the mixture. Extrusion processes destroy antinutrients, increase soluble dietary fiber, gelatinize starch, and decrease lipid oxidation (Nikmaran et al. 2017).
Irradiation
Application of ionizing radiation for the treatment of foods commenced after approval by the FAO/International Atomic Energy Agency (IAEA)/World Health Organization (WHO), who accepted 10 kGy overall average dose for foods (FAO/WHO 2013). Food irradiated at 50 kGy or less can be considered as safe for human consumption (FDA 1981). Ionizing radiation treatment could also be a possible method for inactivating antinutrients content in plant foods. Up to 10 kGy level of γ-irradiation is effective for inactivating or decomposing protease inhibitors, lectin, phytic acid, and oligosaccharides without affecting the nutritional value of foods. The effect of irradiation on the reduction of antinutrients of legumes is shown in Table 5.
Bio-processing
In a households processing soaked seeds are allowed to germinate or fermented before cooking depending on the type of food being prepared. During germination as well as fermentation, there is an involvement of enzyme(s) which are either present endogenously in the seeds or produced by microorganisms. Some of the bio-processing strategies used and their possible role in the reduction of antinutrients are discussed below.
Germination
Germination is a process starting with uptake of water by seeds followed by seed coat weakening, metabolism, and initiation of sprouting. In legumes, several enzymes take part in the hydrolysis of seed storage proteins. The effect of germination on the levels of antinutrients of legumes is shown in Table 5. Many proteinaceous antinutrients, such as hemagglutinin, amylase inhibitor, and trypsin inhibitors are reduced during germination (Uwaegbute et al. 2000). During germination, the activity of polyphenol oxidase is high- the condition which is beneficial for the reduction of tannins. Germination induces the synthesis of phytase too. Plant seeds utilize phytic acid as a source of inorganic phosphorus during germination and in turn increase the nutritive value of seeds (Kumar et al. 2010). When seeds germinate, reserve nutrients are released, and vitamin and mineral contents are increased. Germination mobilizes reserve nutrients, such as native proteins, required for the growth of plant seedlings and removal of antinutrients (Uwaegbute et al. 2000).
Fermentation
Fermentation is one of the oldest methods of food processing and preservation. It is defined as a biochemical modification of primary food products brought about by the activities of microorganisms and their enzymes.The substrate for fermentation can be whole grains, ground products, or processed products. Fermentation can be initiated either by following natural fermentation or by selected pure cultures. In traditional food preparation, fermentation is accelerated by addition of a starter culture of selected microorganisms or addition of a small amount of already fermented material (back-slopping) (Sarkar and Nout 2014). This accelerates the initial phase of fermentation and imparts a desirable change to the product. Legume fermentation is popular as it provides improved digestibility, micronutrient availability, vitamins, essential amino acids, and reduced antinutrients. Reduction of antinutrients in various legumes by fermentation is shown in Table 5. Tannins reduce the solubility of iron, zinc, and calcium by forming insoluble complexes between them; fermentation lowers the pH and provides an optimal condition for enzymic degradation of tannins. In addition, low pH favors phytase activity, resulting in the reduction of phytic acid content (PAC) (Kumar et al. 2010). Microbial phytase originates either from microbiota on the surface of legumes or from inoculum of the starter culture which helps to hydrolyze phytic acid to inositol phosphates (Kumar et al. 2010). Fermentation reduces trypsin inhibitor activity (TIA), increases availability of essential amino acids, and improves protein digestibility. TIA reduced with the increase in microbial growth during fermentation (Shimelis and Rakshit 2008). Hemagglutinin is heat-labile and can be destroyed upon heat treatment prior to fermentation. Solid state fermentation with Aspergillus oryzae and Rhizopus microsporus var. chinensis was successful in reducing β-ODAP in Lathyrus sativus seeds. Further, hydrolysis of cyanide takes place during fermentation of locust beans to produce dawadawa, a condiment in Africa. Saponin content in various legumes remained undetectable when the duration of fermentation was increased (Shimelis and Rakshit 2008). Human physiological effect upon consumption of legumes rich in oxalates can be minimized by fermentation. These data indicate the effectiveness of fermentation on legume antinutrient inactivation (Table 5). Recently, it has been shown that an increased fermentation time and temperature have reducing effect on the levels of antinutrients of soybean, black gram dal and bengal gram dal during kinema, idli, and dhokla preparation (Sharma et al. 2015, 2017, 2018). The optimized fermentation condition for the reduction of antinutrients are shown in Table 6.
Enzymic processing
Enzymic removal of antinutrients depends on the activity of endogenous enzymes and addition of extracellular enzymes from other biological sources. During food processing, many endogenous enzymes become activated, and the addition of extracellular enzymes further helps in the reduction of antinutrients. Polyphenol-oxidase reduces tannins content, and about 60% reduction of phenolic compounds and improvement of iron availability were achieved by applying 1500 U/g polyphenol oxidase (Sandberg 2002). Phytic acid is hydrolyzed by the activity of phytase which is naturally present in plants and microorganisms. A complete removal of phytic acid and an increase of mineral availability was achieved by the addition of extracellular phytase. Nowadays, commercial phytase of microbial origin is available for use in food processing industries. A number of microorganisms associated with fermentations are able to degrade biogenic amines through the production of mono- and di-amino oxidases (Shukla et al. 2011).
Starter cultures
Microbial starters have the potential to reduce antinutrients present in legumes. Lactobacillus plantarum (Rui et al. 2017) and Bacillus subtilis (Sharma et al. 2015), used as starter culture, caused reduction of antinutrients during soybean fermentation. A similar observation was reported using Aspergillus niger as a starter culture during fermentation of Vigna racemosa seeds (Difo et al. 2015). The reduction of antinutrients, such as generated biogenic amines has been reported in various soybean-fermented food preparations, such as kochujang (Shukla et al. 2011), kinema (Sharma et al. 2015), soy sauce (Cho et al. 2006), sufu (Guan et al. 2013), tempe (Nout et al. 1993), cheonggukjang (Kim et al. 2017), chunjang (Bai et al. 2013), doubanjiang (Byun et al. 2013), and natto (Tsai et al. 2007). For controlling the growth of various bacteria capable of forming biogenic amines, mixed starter cultures were more effective than single starter cultures (Naila et al. 2010). Culture of Rhozopus oligosporus, Klebsiella pneumoniae, Trichosporon beigelii and L. plantarum in soybean tempe fermentation (Nout et al. 1993) for controlling biogenic amines formation evidenced the effectiveness of mixed starter cultures. Use of autochthonous strains (i.e. selected strains originating from each specific fermented product) was found suitable for reducing the formation of biogenic amines. The degradation of biogenic amines such as histamine and tyramine and the presence of genes encoding histidine and tyrosine decarboxylase and amine oxidases in three selected strains of Bacillus spp. were studied by Eom et al. (2015). They found that there was no expression of histidine and tyrosine decarboxylase genes in Bacillus spp., although substantial levels of amine oxidase gene expression were observed. So, the three selected strains of non-biogenic amines producing bacteria have potentiality to degrade biogenic amines, i.e. histamine and tyramine, indicating such bacterial strains could be used as a starter culture for controlling the accumulation and degradration of amines in foods.
Additives and preservatives
Sodium sorbate, sodium hexametaphosphate, D-glucono-δ-lactone, citric acid, succinic acid, D-sorbitol, and malic acid limit the formation of biogenic amines by inhibiting decarboxylase activity (Naila et al. 2010; Gardini et al. 2016). Histamine formation in Klebsiella pneumoniae was delayed by using 0.5% sorbate. Histamine, cadaverine, and putrescine formation by Bacillus licheniformis was reduced by 10% glycine. Naturally occurring substances in spices have an inhibitory effect on biogenic amine formation. Spices are often defined as aromatic, dried plant substances used for flavoring and coloring during food preparations. Such substances include curcumin (turmeric), capsaicin (red pepper), and piperine (black pepper). 6-gingerol and allicin, the active ingredients in ginger and garlic, respectively, have some inhibitory effect on biogenic amine formation (Naila et al., 2010). Sodium chloride also has an inhibitory role in biogenic amine formation (Sharma et al. 2017, 2018).
Safety issues
There are various antinutritive components in food systems that are hazardous to human health. These hazards are due to the property of food itself which includes naturally present antinutrients or components generated during processing, such as biogenic amines. Household preparation of food products often involves the reduction of processing time. These may have a serious impact on safety and nutritional quality of food products. Time saved by shortening of fermentation period may affect functioning of beneficial microorganisms or enzymic degradation of antinutrients (Motarjemi 2001). The constraints of processing time can be alleviated by the use of fermentation starter cultures in the preparation of fermented foods (Sarkar and Nout 2014). Optimization of household processing stage parameters might be helpful in minimizing toxic compounds, like biogenic amines and other antinutrients. Such maneuvering of processing parameters can be helpful for the production of more nutritious and safe foods.
Food safety in a broader sense can be achieved through production, storage, and handling in a safer way to avoid food intoxicants and other detrimental effects associated with consumption. Safety provides commercial reputation to prepare foods. Application of proper safety measures leads to the improvement of overall quality of a food (Motarjemi 2001). Food safety assurances mainly fall into two categories, namely good manufacturing practices (GMP) where safety action is taken during purchase of raw materials, processing, transport, and distribution, and good hygienic practice (GHP) which ensures that food products are safe for consumption (Mortarjemi 2001). Such manufacturing and hygienic practices could be used to improve quality and safety by employing the hazard analysis critical control point (HACCP) system. HACCP is defined as a system which identifies, evaluates, and controls hazards during food processing and ensures safety of foods (Mortarjemi 2001). While improving safety and nutritional value by reducing antinutritional components present in foods, a regular interaction among the scientists, producers/processors, and consumers is necessary so as to ensure acceptable and cost-effective changes of food products.
Maneuvering processing steps to prepare more nutritious and safe foods
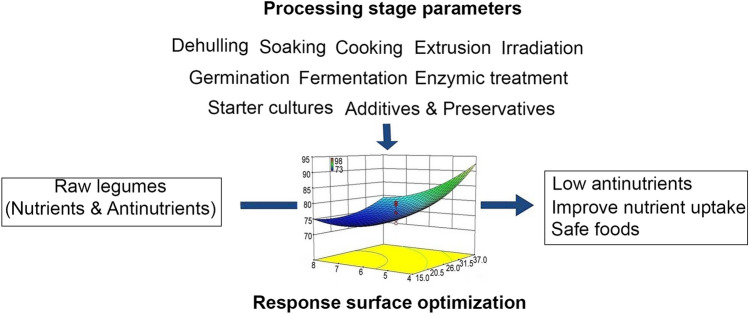
Household preparation of foods involves the use of simple traditional techniques without prior knowledge of science on food system. Such traditional processing consumes more time and might not be so effective in reducing the antinutritional components in food system. Optimization of processing variables will be valuable to tackle the problem by utilizing RSM where desired results can be achieved within a short period of time. It has been successfully used for optimization of various food processes (Nwabueze 2010). Some of the designs of RSM include full factorial design, fractional factorial design, saturated design, and central composite design. Designs depend on the type of food process being used. A conceptual approach of processing treatment strategies that can be employed for the reduction of antinutrients during legume-based food preparation is shown in Fig. 1.
Fig. 1.
Conceptual representation of processing treatment strategies which can be followed for safe food production having reduced levels of antinutrients
Finally, the shelf life of optimally processed products during packaging is also an important issue. The motif of packaging in food technology is to preserve food in order to inhibit the growth of undesirable microorganisms during storage of packaged products. It has been found that the inside atmosphere of the packages has qualitative and quantitative effects on the antinutrients, such as biogenic amines. Use of modified atmospheric packaging (MAP) and vacuum packaging on decarboxylating bacteria and the use of carbon-dioxide gas to prolong shelf life of foods by inhibiting biogenic amine formers ensures better safety of legume fermented foods (Naila et al. 2010; Gardini et al. 2016). Generally, MAP makes use of different scavengers of O2 and CO2 to control the environment within the pack. Vacuum packaging extends the shelf life of foods as compared to air packaging. In vaccum packaging, removal of O2 inhibits the growth of aerobic bacteria which in turn delay biogenic amine accumulation, thereby extending the shelf life of foods. A novel packaging method called “CO2-vacuum packed” has been developed which involves combination of organic acid and CO2 from the headspace dissolving into the product until a vaccum is formed (Schirmer et al. 2009). Compared to air packaging, biogenic amine formation can be checked or delayed or inhibited effectly by the use of active packaging, vacuum packaging, and MAP technique. However, the success of inhibition largely depends on the type of microbiota involved, environmental conditions especially temperature, and the use of gas mixture in case of MAP.
Conclusion
The increasing demand for nutritious and safe foods will likely continue to exert pressure on the supply of nutritionally superior and safe foods in the coming years. Legumes are an important source of nutrition. However, the presence of antinutrients limits consumption preference of legume-based foods. Therefore, monitoring and tailoring the (traditional) processing conditions and optimizing such parameters with the aim to reduce the levels of antinutrients could be a useful option to increase nutrition and safety of legume-based foods. Optimization techniques can be exploited to various processes for legume-based fermented as well as non-fermented foods. Furthermore, research should focus on the comparative study on nutritional and antinutritional components, dietary dose, threshold limits, shelf life, and antinutrient levels during legume-based food preparations.
Acknowledgments
This work was supported by grant, F.3-4/2013 (SAP-II), from the University Grants Commission, New Delhi, India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aidoo KE, Nout MJR, Sarkar PK. Occurrence and function of yeasts in Asian indigenous fermented foods. FEMS Yeast Res. 2006;6:30–39. doi: 10.1111/j.1567-1364.2005.00015.x. [DOI] [PubMed] [Google Scholar]
- Alajaji SA, El-Adawy TA. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J Food Compos Anal. 2006;19:806–812. [Google Scholar]
- Annor GA, Ma Z, Boye JI. Crop-legumes. In: Clark S, Jung S, Lamsal B, editors. Food processing: principles and applications. 2. Hoboken: John Wiley; 2014. pp. 305–337. [Google Scholar]
- Bai X, Byun BY, Mah JH. Formation and destruction of biogenic amines in chunjang (a black soybean paste) and jajang (a black soybean sauce) Food Chem. 2013;141:1026–1031. doi: 10.1016/j.foodchem.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Batista KA, Prudêncio SH, Fernandes KF. Changes in the functional properties and antinutritional factors of extruded hard-to-cook common beans (Phaseolus vulgaris L.) J Food Sci. 2010;75:C286–C290. doi: 10.1111/j.1750-3841.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- Bolade MK. Individualistic impact of unit operations of production, at household level, on some antinutritional factors in selected cowpea-based food products. Food Sci Nutr. 2016;4:441–455. doi: 10.1002/fsn3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora P. Anti-nutritional factors in foods and their effects. J Acad Ind Res. 2014;3:285–290. [Google Scholar]
- van Buul VJ, Brouns FJPH. Health effects of wheat lectins: a review. J Cereal Sci. 2014;59:112–117. [Google Scholar]
- Byun BY, Bai X, Mah JH. Occurrence of biogenic amines in doubanjiang and tofu. Food Sci Biotechnol. 2013;22:55–62. [Google Scholar]
- Campos-Vega R, Loarca-Pina G, Oomah BD. Minor components of pulses and their potential impact on human health. Food Res Int. 2010;43:461–482. [Google Scholar]
- Champ MM. Non-nutrient bioactive substances of pulses. Br J Nutr. 2002;88:S307–319. doi: 10.1079/BJN2002721. [DOI] [PubMed] [Google Scholar]
- Chettri R, Tamang JP. Microbiological evaluation of maseura, an ethnic fermented legume-based condiment of Sikkim. J Hill Res. 2008;21:1–7. [Google Scholar]
- Cho TY, Han GH, Bahn KN, Son YW, Jang MR, Lee CH, Kim SH, Kim DB, Kim SB. Evaluation of biogenic amines in Korean commercial fermented foods. Korean J Food Sci Technol. 2006;38:730–737. [Google Scholar]
- Dahiya PK, Nout MJR, van Boekel MA, Khetarpaul N, Grewal RB, Linnemann A. Nutritional characteristics of mung bean foods. Br Food J. 2013;116:1031–1046. [Google Scholar]
- Difo VH, Onyike E, Ameh DA, Njoku GC, Ndidi US. Changes in nutrient and antinutrient composition of Vigna racemosa flour in open and controlled fermentation. J Food Sci Technol. 2015;52:6043–6048. doi: 10.1007/s13197-014-1637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egounlety M, Aworh OC. Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and groundbean (Macrotyloma geocarpa Harms) J Food Eng. 2003;56:249–254. [Google Scholar]
- Ejigui J, Savoie L, Marin J, Desrosiers T. Influence of traditional processing methods on the nutritional composition and antinutritional factors of red peanuts (Arachis hypogea) and small red kidney beans (Phaseolus vulgaris) J Biol Sci. 2005;5:597–605. [Google Scholar]
- Eom JS, Seo BY, Choi HS. Biogenic amine degradation by Bacillus species isolated from traditional fermented soybean food and detection of decarboxylase-related genes. J Microbiol Biotechnol. 2015;25:1519–1527. doi: 10.4014/jmb.1506.06006. [DOI] [PubMed] [Google Scholar]
- Evans E, Musa A, Abubakar Y, Mainuna B. Nigerian indigenous fermented foods: processes and prospects. In: Makun HA, editor. Mycotoxin and Food Safety in Developing Countries. London: InTech; 2013. pp. 153–180. [Google Scholar]
- FAO/WHO (2013) Dietary Protein Quality Evaluation in Human Nutrition. FAO Food and Nutrition Paper 92, FAO, Rome, Italy [PubMed]
- FDA Irradiation in the production, processing, and handling of food, final rule. 21 CFR Part 179. Food Drug Adm Fed Reg. 1981;51:13376–13399. [Google Scholar]
- Gardini F, Özogul Y, Suzzi G, Tabanelli G, Özogul F. Technological factors affecting biogenic amine content in foods: a review. Front Microbiol. 2016;7:1218. doi: 10.3389/fmicb.2016.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grela ER, Studziñski T, Matras J. Antinutritional factors in seeds of Lathyrus sativus cultivated in Poland. Lathyrus Lathyrism Newsl. 2001;2:101–104. [Google Scholar]
- Guan RF, Liu ZF, Zhang JJ, Wei YX, Wahab S, Liu DH, Ye XQ. Investigation of biogenic amines in sufu (furu): a Chinese traditional fermented soybean food product. Food Control. 2013;31:345–352. [Google Scholar]
- Guillon F, Champ MMJ. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 2002;88:293–306. doi: 10.1079/BJN2002720. [DOI] [PubMed] [Google Scholar]
- Hanbury CD, White CL, Mullan BP, Siddique KHM. A review of the potential of Lathyrus sativus L. and L. cicera L. grain for use as animal feed. Anim Feed Sci Technol. 2000;87:1–27. [Google Scholar]
- He FJ, Chen JQ. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: differences between Chinese women and women in Western countries and possible mechanisms. Food Sci Hum Wellness. 2013;2:146–161. [Google Scholar]
- Jezierny D, Mosenthin R, Bauer E. The use of grain legume as a protein source in pig nutrition: a review. Anim Feed Sci Technol. 2010;157:111–128. [Google Scholar]
- Judprasong K, Charoenkiatkul S, Sungpuag P, Vasanachitt K, Nakjamanong Y. Total and soluble oxalate contents in Thai vegetables, cereal grains and legume seeds and their changes after cooking. J Food Comp Anal. 2006;19:340–347. [Google Scholar]
- Kim B, Byun BY, Mah JH. Biogenic amine formation and bacterial contribution in natto products. Food Chem. 2012;135:2005–2011. doi: 10.1016/j.foodchem.2012.06.091. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim HE, Kim YS. The potentials of Bacillus licheniformis strains for inhibition of B. cereus growth and reduction of biogenic amines in cheonggukjang (Korean fermented unsalted soybean paste) Food Control. 2017;79:87–93. [Google Scholar]
- Kumar V, Sinha AK, Makkar HPS, Becker K. Dietary roles of phytate and phytase in human nutrition: a review. Food Chem. 2010;120:945–959. [Google Scholar]
- Luo YW, Xie WH. Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia faba L.) CyTA-J Food. 2013;11:43–49. [Google Scholar]
- Madodé YE, Nout MJR, Bakker EJ, Linnemann AR, Hounhouigan DJ, Boekel MAJS. Enhancing the digestibility of cowpea (Vigna unguiculata) by traditional processing and fermentation. LWT-Food Sci Technol. 2013;54:186–193. [Google Scholar]
- Massey LK, Palmer RG, Horner HT. Oxalate content of soybean seeds (Glycine max: Leguminosae), soyfoods, and other edible legumes. J Agric Food Chem. 2001;49:4262–4266. doi: 10.1021/jf010484y. [DOI] [PubMed] [Google Scholar]
- Megías C, Cortés-Giraldo I, Alaiz M, Girón-Calle J, Vioque J, Santana-Méridas O, Herraiz-Peñalver D, Sánchez-Vioque R. Determination of the Neurotoxin 3-N-Oxalyl-2,3- Diaminopropionic Acid and other free amino acids in Lathyrus cicera and L. sativus seeds by reversed-phase high-performance liquid chromatography. Food Anal Methods. 2015;8:1953–1961. [Google Scholar]
- Miles CA, Lumpkin TA, Zenz L (2000) Edamame. In: Farming west of the cascades series. A pacific northwest extension publication. Washington State University Cooperative Extension, Pullman, WA, Bulletin No. PNW0525, pp 1–8
- Motarjemi Y. An introduction to the hazard analysis and critical control point (HACCP) system and its application to fermented foods. In: Adams MR, Nout MJR, editors. Fermentation and Food Safety. Gaithersburg: Aspen Publishers; 2001. pp. 53–66. [Google Scholar]
- Naila A, Flint S, Fletcher G, Bremer P, Meerdink G. Control of biogenic amines in food-existing and emerging approaches. J Food Sc. 2010;75:R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LA. Health-promoting microbes in traditional vietnamese fermented foods: a review. Food Sci Hum Wellness. 2015;4:147–161. [Google Scholar]
- Nikmaran N, Leong SY, Koubaa M, Zhu Z, Barba FJ, Greiner R, Oey I, Roohinejad S. Effect of extrusion on the anti-nutritional factors of food products: an overview. Food Control. 2017;79:62–73. [Google Scholar]
- Nout MJR, Ruikes MMW, Bouwmeester HM, Beljaars PR. Effect of processing conditions on the formation of biogenic amines and ethyl carbamate in soybean tempe. J Food Safety. 1993;13:293–303. [Google Scholar]
- Nout MJR, Sarkar PK, Beuchat LR. Indigenous fermented foods. In: Doyle MP, Beuchat LR, editors. Food Microbiology: Fundamentals and Frontiers. 3. Washington: ASM Press; 2007. pp. 817–835. [Google Scholar]
- Nwabueze TU. Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. Int J Food Sci Technol. 2010;45:1768–1776. [Google Scholar]
- Ogunshe AAO, Ayodele AE, Okonko IO. Microbial studies on Aisa: a potential indigenous laboratory fermented food condiment from Albizia saman (Jacq.) F. Mull Pak J Nutr. 2006;5:51–58. [Google Scholar]
- Rakshit M, Sharma A, Saha J, Sarkar PK. Optimization of soaking condition of blackgram to minimize flatogenic sugar content in blackgram-based products. LWT-Food Sci Technol. 2015;63:814–820. [Google Scholar]
- Vaz Patto MC, Amarowicz R, Arye ANA, Boye JI, Chung HJ, Martín-Cabrejas MA, Domoney C. Achievements and challenges in improving the nutritional quality of food legumes. Crit Rev Plant Sci. 2015;34:105–143. [Google Scholar]
- Ray M, Ghosh K, Singh S, Mondal KC. Folk to functional: An explorative overview of rice-based fermented foods and beverages in India. J Ethn Foods. 2016;3:5–18. [Google Scholar]
- Rehman Z, Shah WH. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005;91:327–331. [Google Scholar]
- Roy A, Moktan B, Sarkar PK. Microbiological quality of legume-based traditional fermented foods marketed in West Bengal, India. Food Control. 2007;18:1405–1411. [Google Scholar]
- Rui X, Wang M, Zhang Y, Chen X, Li L, Liu Y, Dong M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J Food Process Preserv. 2017;41:e13290. [Google Scholar]
- Ruiz-López MA, García-López PM, Castaneda-Vazquez H, Zamora NJF, Garzón-De la Mora P, Banuelos Pineda J, Burbano C, Pedrosa MM, Cuadrado C, Muzquiz M. Chemical composition and antinutrient content of three Lupinus species from Jalisco, Mexico. J Food Comp Anal. 2000;13:193–199. [Google Scholar]
- Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002;88:281–285. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–1117. [Google Scholar]
- Sarkar PK, Nout MJR. Kinema and similar products. In: Sarkar PK, Nout MJR, editors. Handbook of Indigenous Foods Involving Alkaline Fermentation. Boca Raton: CRC Press; 2014. pp. 33–53. [Google Scholar]
- Sarkar PK, Jone LJ, Craven GS, Somerset SM. Oligosaccharide profiles of soybeans during kinema production. Lett Appl Microbiol. 1997;24:337–339. [Google Scholar]
- Saura-Calixto F, Serrano J, Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007;101:492–501. [Google Scholar]
- Savitri BTC. Traditional foods and beverages of Himalchal Pradesh. Indian J Trad Know. 2007;6:17–24. [Google Scholar]
- Schirmer BC, Heiberg R, Eie T, Møretrø T, Maugesten T, Carlehøg M, Langsrud S. A novel packaging method with a dissolving CO2 headspace combined with organic acids prolongs the shelf life of fresh salmon. Int J Food Microbiol. 2009;133:154–160. doi: 10.1016/j.ijfoodmicro.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Schofield P, Mbugua DM, Pell AN. Analysis of condensed tannins: a review. Anim Feed Sci Technol. 2001;91:21–40. [Google Scholar]
- Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:S310–329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sarkar PK. Microbial diversity in ethno-fermented foods of Indian Himalayan region. ENVIS Bull Hima Ecol. 2015;23:85–91. [Google Scholar]
- Sharma A, Kumari S, Wongputtisin P, Nout MJR, Sarkar PK. Optimization of soybean processing into kinema, a Bacillus-fermented alkaline food, with respect to a minimum level of antinutrients. J Appl Microbiol. 2015;119:162–176. doi: 10.1111/jam.12826. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumari S, Nout MJR, Sarkar PK. Minimization of antinutrients in idli by using response surface process optimization. J Food Process Preserv. 2017;41:e13099. [Google Scholar]
- Sharma A, Kumari S, Nout MJR, Sarkar PK. Preparation of antinutrients-reduced dhokla using response surface process optimization. J Food Sci Technol. 2018;55:2048–2058. doi: 10.1007/s13197-018-3119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimelis EA, Rakshit SK. Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem. 2007;103:161–172. [Google Scholar]
- Shimelis EA, Rakshit SK. Influence of natural and controlled fermentations on α-galactosides, antinutrients and protein digestibility of beans (Phaseolus vulgaris L.) Int J Food Sci Technol. 2008;43:658–665. [Google Scholar]
- Shin D, Jeong D. Korean traditional fermented soybean products: Jang. J Ethnic Foods. 2015;2:2–7. [Google Scholar]
- Shukla S, Kim JK, Kim M. Occurrence of biogenic amines in soybean food products. In: Hany ES, editor. Soybean and Health. London: InTech; 2011. pp. 181–206. [Google Scholar]
- Srivastava RP, Singh J, Singh NP, Singh D. Neurotoxin and other anti-nutrients of khesari (Lathyrus sativus) genotypes and their reduction by water soaking and dehusking. Indian J Agric Biochem. 2015;28:172–177. [Google Scholar]
- Thakur N, Savitri BTC. Characterization of some traditional fermented foods and beverages of Himachal Pradesh. Indian J Trad Knowl. 2004;3:325–335. [Google Scholar]
- Tsai YH, Chang SC, Kung HF. Histamine contents and histamine-forming bacteria in natto products in Taiwan. Food Control. 2007;18:1026–1030. [Google Scholar]
- de Toledo TCF, Arthur V, Brazaca SGC, Piedade, SMS (2007) Effects of gamma radiation on antinutritional factors of soybean. International Nuclear Atlantic Conference, Sept. 30-Oct. 5, 2007, Santos, Brazil
- Uwaegbute AC, Iroegbu CU, Eke O. Chemical and sensory evaluation of germinated cowpeas (Vigna unguiculata) and their products. Food Chem. 2000;68:141–146. [Google Scholar]
- Venter CS, van Eyssen E. More legumes for better overall health. South Afr J Clin Nutr. 2001;14:32–38. [Google Scholar]
- Vetter J. Plant cyanogenic glycosides. Toxicon. 2000;38:11–36. doi: 10.1016/s0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]