Abstract
Dioscorea Rhizome is commonly used in traditional herbal medicines for the treatment of diabetes, hyperthyroidism, liver damage, neuropathy, and asthma. Here, we investigated the genotoxicity potential of D. Rhizome water extract (DRWE) using three standard battery systems in accordance with the test guidelines of the Organisation for Economic Cooperation and Development and Ministry of Food and Drug Safety as well as the principles of Good Laboratory Practice. A bacterial reverse mutation test (Ames test) was performed using the direct plate incorporation method in the presence or absence of a metabolic activation system (S9 mixture). The tester strains used included four histidine auxotrophic strains of Salmonella typhimurium, TA100, TA1535, TA98, and TA1537, along with a tryptophan auxotrophic strain of Escherichia coli, WP2 uvrA. An in vitro chromosome aberration test was performed using CHL/IU cells originally derived from the lung of a female Chinese hamster in the presence or absence of the S9 mixture. An in vivo mouse bone marrow micronucleus test was performed using male ICR mice. The micronucleus was confirmed after observation of the micro-nucleated polychromatic. The Ames test showed that DRWE did not induce gene mutations at any dose level in any of the tested strains. Additionally, DRWE did not result in any chromosomal aberrations specified in the in vitro chromosomal aberration and in vivo micronucleus tests. These results showed that DRWE exhibited neither mutagenic nor clastogenic potential in either the in vitro or in vivo test systems.
Keywords: Herbal medicine, Dioscorea rhizome, Genotoxicity, Bacterial reverse mutation test, Chromosome aberration test, Micronucleus test
Introduction
The use of herbal medicines has been of great interest in developed and developing countries during the last decade because of the common belief that herbal medicines are natural [1]. In the past, herbal medicines were considered safe and effective because it was believed that plant remedies have no side effects [2]. However, concerns about the side effects of herbal medicines, such as renal toxicity and genotoxicity, have recently emerged [3, 4]. Moreover, the lack of databases containing information on the pharmacology and toxicity of herbal medicines has raised medical concerns [5]. Therefore, research on the toxicity of herbal medicines is of great importance.
Dioscorea Rhizome, the root of D. batatas Decaisne, is widely used as a traditional herbal medicine for the treatment of diabetes, hyperthyroidism, liver damage, neuropathy, and asthma [6–8]. Moreover, it has anti-tumor, anti-oxidant, anti-inflammatory, and anti-osteoporotic immunomodulatory activities [9–12]. Allantoin is the main bioactive compound of D. Rhizome [13–15], and has anti-diabetic, anti-oxidant, anti-inflammatory, and anti-cancer activities [16, 17].
Although members of the family Dioscoreaceae are often used in traditional herbal medicines, data on their potential toxic effects on humans and experimental animals are insufficient. The median lethal dose (LD50) of D. bulbifera after single administration in mice was 25.49 mg/kg (intraperitoneal) or 79.98 mg/kg (oral) [18]. However, the potential genotoxic effects of D. Rhizome have not been rigorously evaluated in accordance with current regulatory guidelines. Thus, research on the genotoxicity of this herbal medicine is warranted in response to the increased public use of such compounds.
Therefore, this study was performed to evaluate the genotoxic potential of D. Rhizome water extract (DRWE). The genotoxicity of DRWE was evaluated using a bacterial reverse mutation test (Ames test), an in vitro chromosomal aberration test, and an in vivo mouse bone marrow micronucleus test. The study was conducted in accordance with the test guidelines recommended by the Organisation for Economic Cooperation and Development (OECD) and the Korean Ministry of Food and Drug Safety [19] for the testing of chemicals under the modern Good Laboratory Practice (GLP) regulations.
Materials and methods
Preparation of DRWE
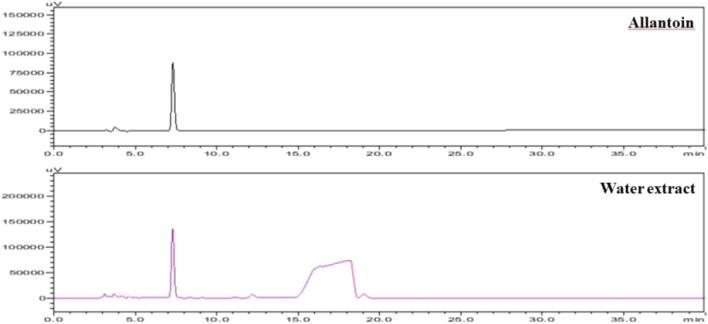
Dioscorea Rhizome was collected in Korea, and identification of this plant was managed by forming the herbal drug identification advisory committee. DRWE powder (Lot No. DBE-005R, DBE-006R, and DBE-007R) was provided by the Korea Bio Medical Science Institute (Seoul, Korea). Distilled water was added twice to the DRWE powder, and the mixture was refluxed for 12 h at 90 °C. The water extract was concentrated under vacuum and dried to obtain a final extract. The major component of the DRWE, allantoin, was measured using high-performance liquid chromatography (HPLC) with UV–visible detection. The results are shown in Fig. 1. The DRWE powder was prepared for injection via suspension in sterile distilled water (Daihan Pharm. Co., Seoul, Korea) at appropriate doses.
Fig. 1.
HPLC chromatogram of allantoin standard and DRWE
Bacterial reverse mutation test (Ames test)
The mutagenic potential of DRWE was evaluated using a bacterial reverse mutation assay in accordance with OECD test guideline No. 471 for the testing of chemicals [20] according to previously described methods [21]. The tester strains used in this study were four histidine auxotroph strains of Salmonella typhimurium, TA100, TA1535, TA98, and TA1537, along with a tryptophan auxotroph strain of Escherichia coli, WP2 uvrA [22, 23]. The metabolic activation system (S9 mixture) comprised mixed cofactors in the S9 fraction, which is a rat liver homogenate pretreated with Aroclor-1254 [24]. The test strains were exposed to DRWE according to the direct plate incorporation method [25]. Based on the results of a dose-range finding test performed on DRWE tested at eight doses ranging from 5 to 5000 μg/plate (data not shown), the dose ranges were determined for the five test strains both in the presence or absence of the S9 mixture at three plates per dose. In the present study, the highest dose was set at 5000 μg/plate for all test strains, and five serially diluted concentrations (1500, 500, 150, 50, and 0 μg/plate) were selected. The positive control factors were 2-aminoanthracene (Sigma-Aldrich Co., St. Louis, MO, USA), benzo[a]pyrene (Sigma-Aldrich Co.), sodium azide (Sigma-Aldrich Co.), 2-nitrofluorene (Sigma-Aldrich Co.), 4-nitroquinoline-1-oxide (Sigma-Aldrich Co.), and acridine Mutagen ICR 191 (Sigma-Aldrich Co.). A small amount of bacterial growth from each master plate was taken and transferred to a flask containing 20 mL of liquid medium (2.5% Oxoid Nutrient Broth No. 2). The inoculated flasks were placed in a shaking incubator (37 ± 2 ºC, 120 rpm) for 10 h. The overnight cultures were removed from the incubator, and the viable cell counts were determined at an optical density of 600 nm; then, the cultures were refrigerated until use. For the plating assay, the following were added to each sterile culture tube containing 2 mL of top agar held at 45 ± 2 ºC in a dry bath: 0.5 mL of the S9 mixture (or sodium-phosphate buffer, pH 7.4 for the non-activating plates), 0.1 mL of bacterial culture, and 0.1 mL of DRWE solution. The contents were vortexed for 2–3 s and overlaid onto the surface of the bottom agar. The negative control plates were treated with 0.1 mL of vehicle instead of DRWE solution. The positive control plates were treated with positive control articles via the same method. The sterility of the highest dose DRWE solution was checked by plating a 0.1 mL aliquot (mixed with 2 mL of top agar) on minimal glucose agar. The S9 mixture was also checked for sterility by plating 0.5 mL via the same method. After the top agar solidified, the plates were inverted and incubated at 37 ± 2 ºC for 50 ± 2 h; subsequently, the revertant colonies were counted macroscopically.
In vitro chromosome aberration test
The chromosome aberration test was performed in CHL/IU cells originally derived from the lung of a female Chinese hamster in accordance with OECD test guideline No. 473 for the testing of chemicals [26]. The clastogenicity of DRWE was determined by evaluating its ability to induce chromosomal aberrations in CHL/IU cells. The CHL/IU cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were maintained in Eagle’s minimum essential medium supplemented with GlutaMax-I supplement, penicillin–streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). The cells were cultured in cell culture flasks (culture surface 75 cm2) at 37 ± 1 ºC in 5% CO2 in air in a humidified incubator (Forma 311 and 3111, Thermo Scientific, USA). Benzo[a]pyrene and 4-nitroquinoline-1-oxide were used as positive control articles in the presence or absence of the S9 mixture, respectively. The negative control used was sterile distilled water. Cell proliferation and cytotoxicity were evaluated using the relative increase in cell count (RICC) method. The dose ranges in this study were determined according to the cytotoxicity observed in a dose-range finding test (data not shown). In the dose-range finding test, the cells were treated with eight concentrations of DRWE, ranging from 5 to 5000 µg/mL, and turbidity was observed in the treatment mixtures at 5000 μg/plate. There were no signs of cytotoxicity at any of the doses in all the treatment series. The RICC values were greater than 77% at the highest dose in all the treatment series. Therefore, the DRWE concentrations were 1250, 2500, and 5000 μg/mL (short-term treatment and continuous treatment) in the absence of the S9 mixture. In the presence of the S9 mixture, the DRWE concentrations were 1250, 2500, and 5000 μg/mL (short-term treatment). Each 25-cm2 flask was seeded with cells (5 × 104 cells/mL) in complete culture medium and incubated for 3 days. After removing the complete culture medium, DRWE solution, S9 mixture, and complete culture medium were added to the flask. The cells were exposed to DRWE solution for 6 h (short-term treatment) or 22 h (continuous treatment). In the case of continuous treatment, DRWE solution was added for 22 h as excess precipitation. The cell cultures were treated with colchicine (1 μM), and the metaphasic cells were harvested by gentle shaking and centrifugation. The cell pellets were resuspended in a 75 mM potassium chloride solution for hypotonic treatment and then fixed with a fixative solution (methanol:glacial acetic acid, 3:1). A few drops of the cell pellet suspension were placed onto pre-cleaned glass microscope slides and air-dried. The slides were stained with 5% Giemsa stain solution, and two slides were prepared for each culture. The number of cells with chromosomal aberrations was recorded on 300 well-spread metaphases. Duplicate cultures were used for each dose. The classification of a chromosome aberration was conducted in accordance with the Japanese Environmental Mutagen Society-Mammalian Mutagenicity Group [27].
In vivo mouse bone marrow micronucleus test
The micronucleus test was performed in accordance with OECD test guideline No. 474 for the testing of chemicals [28]. Seven-week-old male ICR mice were obtained from a specific pathogen-free colony at Koatech Co. Ltd. (Pyeongtaek, Korea). The animals were housed in a room maintained at a temperature of 23 ± 3 °C and a relative humidity of 55 ± 15% under artificial lighting with a luminous intensity of 150–300 lx from 08:00 to 20:00 h and with 10–20 air changes per hour. The animals were permitted ad libitum access to an irradiation-sterilized pellet diet for laboratory animals (Teklad-certified irradiated global 18% protein rodent diet, 2918C, ENVIGO., UK) purchased from Dooyeol Biotech. Groundwater disinfected using an ultraviolet sterilizer and ultrafiltration was provided ad libitum via polycarbonate water bottles. The study protocol was approved by our Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. After 7-day periods of quarantine and acclimatization, the ICR mice were randomly assigned to one of five groups (N = 6 for each group). In the preliminary test, three males and three females per group were administered dose levels of 1250, 2500, and 5000 mg/kg/day of DRWE for 2 consecutive days. The animals were observed for 4 days (including the day of administration). There was no substantial difference in the toxicity profile between the sexes (data not shown). Based on the results of the preliminary test, DRWE was administered once daily for 2 days via gavage to the mice at dose levels of 1250, 2500, and 5000 mg/kg. The daily application volume (10 mL/kg) was calculated in advance based on the body weight of the mice on the day of treatment. The negative control group received an equivalent volume of sterile distilled water. Cyclophosphamide monohydrate (CPA) in normal saline was administered via intraperitoneal injection at 70 mg/kg/day as a positive control. During the study, all animals were observed at least once daily for any clinical signs of toxicity and mortality. The body weight of each mouse was measured on the days of receipt, administration, and necropsy. Bone marrow samples were prepared as previously described [29, 30]. The animals were euthanized using CO2 at 24 h after the last treatment. The femoral bone marrow cells were flushed out using a 23 G syringe with 2 mL of FBS. The selected cells were transferred to a centrifuge tube and centrifuged at 1000 rpm for 5 min. The suspended cells were smeared on a clean glass slide. The smeared slides were air-dried and fixed for 5 min in methanol thereafter. For the fluorescence microscopy observations, an acridine orange stock solution (0.05%) was prepared based on previously described methods [31]. The stock solution was diluted with Sorensen buffer (pH 6.8) to prepare a working solution for staining. The identification of the micronucleus was performed in accordance with the method described by Hayashi (1983). The polychromatic erythrocytes (PCEs) appeared red, whereas the normochromatic erythrocytes (NCEs) appeared dark gray. The micronucleus was then observed as a bright green spot on a red background. In total, 4000 PCEs per animal were counted to determine the number of micronucleated polychromatic erythrocytes (MNPCEs). In addition, the ratio of PCE to PCE + NCE (normochromatic erythrocytes) was calculated by counting 500 cells and detecting the possibility of cytotoxicity associated with the administration of DRWE.
Statistical analyses
In the Ames test, the result was considered positive if there was a dose-related increase over the range tested and/or a reproducible increase at one or more doses in the number of revertants per plate in at least one strain with or without the S9 mixture. A positive result indicated that the test substance induced point mutations in the test strain. The result was considered negative if the result did not meet the positivity criteria. A negative result indicated that the test substance was not mutagenic in the test strains. The biological relevance of the results was also considered in the evaluation of the results. For the in vitro chromosome aberration test, Fisher’s exact test was used to compare the frequency of the aberrant cells between the negative control group and the treated groups [32]. The dose–response relationship of the frequency of aberrant metaphase was tested via the linear-by-linear association of the chi-square test [33]. In the in vivo mouse bone marrow micronucleus test, the frequency of MNCPEs was analyzed using the non-parametric Kruskal–Wallis H-test [34]. If a statistically significant difference was observed between the groups, the Mann–Whitney U test was used to identify the groups that were significantly different from the negative control group [35]. The PCE:RBC ratio and body weights were assumed to be normally distributed and were analyzed by one-way analysis of variance. The assumption of homogeneity was tested using Levene’s test [36]. If the assumption of homogeneity of variance was met, Duncan’s multiple range test was used as the post hoc test [37]. If the assumption of homogeneity of variance was not met, Dunnett’s T3 test was used as the post hoc test [38]. Student’s t-test [39] and the Mann–Whitney U test were used to analyze the differences in means between two independent groups. SPSS 10.1 K was used for all statistical analyses (Chicago, IL, USA). A result was considered significant if p < 0.05.
Results
Bacterial reverse mutation test
The results of the Ames test are presented in Table 1. In TA100, TA1535, TA98, TA1537, and WP2 uvrA, there were no substantial increases in the numbers of colonies in comparison to that of the negative control group at 50, 150, 500, 1500, and 5000 μg/plate in the presence or absence of the S9 mixture. The mean number of revertants in the positive control for each test strain was clearly greater than that in the negative control for that strain.
Table 1.
Results of the bacterial reverse mutation test for Dioscorea Rhizome water extract in Salmonella typhimurium (TA100, TA1535, TA98, and TA1537) and Escherichia coli (WP2uvrA) with or without metabolic activation (S9 mixture)
| Tester Strain | Substance | Dose (μg/plate) | Colonies/plate [Factor]a | |||
|---|---|---|---|---|---|---|
| With S9 mixture | Without S9 mixture | |||||
| TA100 | DRWE | 0 | 117 ± 6 | 118 ± 4 | ||
| 50 | 109 ± 13 | [0.9] | 110 ± 12 | [0.9] | ||
| 150 | 109 ± 10 | [0.9] | 106 ± 5 | [0.9] | ||
| 500 | 113 ± 9 | [1.0] | 112 ± 9 | [0.9] | ||
| 1500 | 120 ± 9 | [1.0] | 111 ± 12 | [0.9] | ||
| 5000 | 144 ± 5 | [1.2] | 113 ± 6 | [1.0] | ||
| TA1535 | DRWE | 0 | 12 ± 1 | 13 ± 2 | ||
| 50 | 11 ± 3 | [0.9] | 13 ± 3 | [1.0] | ||
| 150 | 11 ± 2 | [1.0] | 13 ± 3 | [1.0] | ||
| 500 | 11 ± 3 | [0.9] | 12 ± 3 | [0.9] | ||
| 1500 | 10 ± 2 | [0.8] | 14 ± 2 | [1.1] | ||
| 5000 | 12 ± 2 | [1.1] | 12 ± 0 | [0.9] | ||
| TA98 | DRWE | 0 | 23 ± 3 | 23 ± 3 | ||
| 50 | 25 ± 3 | [1.1] | 22 ± 7 | [1.0] | ||
| 150 | 25 ± 1 | [1.1] | 21 ± 4 | [0.9] | ||
| 500 | 29 ± 2 | [1.2] | 21 ± 4 | [0.9] | ||
| 1500 | 29 ± 4 | [1.2] | 18 ± 2 | [0.8] | ||
| 5000 | 29 ± 7 | [1.2] | 20 ± 4 | [0.9] | ||
| TA1537 | DRWE | 0 | 14 ± 3 | 13 ± 2 | ||
| 50 | 17 ± 2 | [1.2] | 16 ± 2 | [1.2] | ||
| 150 | 15 ± 3 | [1.1] | 14 ± 2 | [1.1] | ||
| 500 | 12 ± 2 | [0.9] | 15 ± 4 | [1.1] | ||
| 1500 | 15 ± 4 | [1.1] | 14 ± 3 | [1.1] | ||
| 5000 | 17 ± 4 | [1.2] | 15 ± 3 | [1.2] | ||
| WP2uvrA | DRWE | 0 | 22 ± 4 | 20 ± 3 | ||
| 50 | 20 ± 6 | [0.9] | 23 ± 4 | [1.2] | ||
| 150 | 25 ± 4 | [1.1] | 22 ± 4 | [1.1] | ||
| 500 | 19 ± 3 | [0.8] | 17 ± 0 | [0.9] | ||
| 1500 | 23 ± 6 | [1.0] | 22 ± 6 | [1.1] | ||
| 5000 | 21 ± 3 | [0.9] | 20 ± 3 | [1.0] | ||
| Positive controls | ||||||
| TA100 | 2-AA | 1.0 | 1677 ± 58 | [14.4] | ||
| TA1535 | 2-AA | 2.0 | 109 ± 9 | [9.3] | ||
| TA98 | B[a]P | 1.0 | 210 ± 13 | [9.0] | ||
| TA1537 | 2-AA | 1.0 | 181 ± 10 | [12.9] | ||
| WP2uvrA | 2-AA | 6.0 | 152 ± 6 | [6.8] | ||
| TA100 | SA | 0.5 | 380 ± 9 | [3.2] | ||
| TA1535 | SA | 0.5 | 249 ± 9 | [18.7] | ||
| TA98 | 2-NF | 2.0 | 259 ± 9 | [11.4] | ||
| TA1537 | ICR-191 | 0.5 | 86 ± 15 | [6.5] | ||
| WP2uvrA | 4NQO | 0.5 | 108 ± 5 | [5.5] | ||
DRWE Dioscorea Rhizome water extract, 2-AA 2-aminoanthracene, SA sodium azide; B[a]P, benzo[a]pyrene; ICR-191, acridine mutagen ICR 191; 4NQO, 4-nitroquinoline N-oxide; and 2-NF, 2-nitrofluorene
Values are presented as the mean ± SD
aNo. of colonies of treated plate/No. of colonies of negative control plate
In vitro chromosome aberration test
As shown in Table 2, there was no statistically significant or dose-related increase in the frequencies of aberrant metaphases with structural and numerical chromosomal aberrations at any dose of the test article compared with the relevant negative control. The positive control articles induced clear positive responses in the mean frequency of aberrant metaphases with structural aberrations (p < 0.01).
Table 2.
Results of the in vitro chromosome aberration test for Dioscorea Rhizome water extract in CHL/IU cells with or without metabolic activation (S9 mixture)
| Concentration (μg/mL) | S9 mixture | Time (h) | Platycodi radix water extract | |
|---|---|---|---|---|
| % Numerical aberration | % Structural aberration (Exclusive to gap) | |||
| 0 | – | 6 | 0.0 | 0.0 |
| 1250 | – | 6 | 0.0 | 0.0 |
| 2500 | – | 6 | 0.0 | 0.0 |
| 5000 | – | 6 | 0.0 | 0.3 |
| Positive controla | – | 6 | 0.0 | 9.7** |
| 0 | + | 6 | 0.0 | 0.7 |
| 1250 | + | 6 | 0.0 | 0.0 |
| 2500 | + | 6 | 0.0 | 0.3 |
| 5000 | + | 6 | 0.0 | 0.3 |
| Positive controlb | + | 6 | 0.0 | 18.0** |
| 0 | – | 22 | 0.0 | 0.0 |
| 1250 | – | 22 | 0.0 | 0.3 |
| 2500 | – | 22 | 0.0 | 0.0 |
| 5000 | – | 22 | 0.0 | 0.3 |
| Positive controla | – | 22 | 0.0 | 11.0** |
**P < 0.01 compared with the negative control group
a4-Nitroquinoline-1-oxide 0.4 μg/mL
bBenzo[a]pyrene 20 μg/mL
In vivo bone marrow micronucleus test
No treatment-related mortality was observed in the animals treated with DRWE during the experimental period (data not shown). Death was observed in one animal in the 5000 mg/kg/day group. However, this finding was not considered related to the DRWE treatment. There were no significant differences in body weight between the negative control group and the treatment groups (Table 3). There were no significant or dose-related increases in the frequencies of MNPCEs at any DRWE dose level compared with the negative control group. The PCE/(PCE + NCE) ratio was used as an index of bone marrow cytotoxicity. This ratio did not show any significant differences among the treatment groups or between the treatment groups and the negative control group (Table 4).
Table 3.
Body weight changes of ICR mice exposed to Dioscorea Rhizome water extract
| Substance | Dose (mg/kg) | Body weightsa | ||
|---|---|---|---|---|
| Administration | Sacrifice | |||
| 1st | 2nd | |||
| Vehicle | 0 | 34.69 ± 1.4 | 34.30 ± 1.7 | 34.69 ± 1.9 |
| DRWE | 1250 | 34.50 ± 1.3 | 34.42 ± 1.4 | 34.45 ± 1.1 |
| DRWE | 2500 | 34.26 ± 1.1 | 34.39 ± 1.2 | 34.48 ± 1.6 |
| DRWE | 5000 | 34.26 ± 1.4 | 34.05 ± 1.4 | 34.42 ± 2.0 |
| CPA | 70 | 34.60 ± 1.2 | 34.01 ± 1.1 | 34.64 ± 1.2 |
DRWE Dioscorea Rhizome water extract, CPA cyclophosphamide monohydrate
aValues are presented as the mean ± SD (g)
Table 4.
Results of the in vivo micronucleus test for Dioscorea Rhizome water extract in the bone marrow of ICR mice
| Substance | Dose (mg/kg) | No. of animals | MNPCE/4000 PCE (%) | PCE/(PCE + NCE) (%) |
|---|---|---|---|---|
| Vehicle | 0 | 6 | 0.83 ± 1.0a | 0.54 ± 0.0a |
| DRWE | 1250 | 6 | 0.67 ± 0.8 | 0.50 ± 0.0 |
| DRWE | 2500 | 6 | 1.00 ± 0.0 | 0.55 ± 0.0 |
| DRWE | 5000 | 5 | 1.40 ± 2.2 | 0.53 ± 0.0 |
| CPA | 70 | 6 | 66.33 ± 19.2** | 0.39 ± 0.0** |
DRWE Dioscorea Rhizome water extract, CPA cyclophosphamide monohydrate, MNPCE micronucleated polychromatic erythrocyte, NCE normochromatic erythrocyte, PCE polychromatic erythrocyte
**P < 0.01 compared with the negative control group
aValues are presented as the mean ± SD (%)
Discussion
Genotoxicity refers to the ability of chemical compounds and their metabolites to damage DNA or chromosomes in cells [40]. It is well known that genotoxic carcinogens initiate carcinogenesis via direct interaction with DNA [41]. Genotoxicity testing is usually conducted to identify potential genotoxic carcinogens, and the minimal batteries of genetic toxicology tests recommended by regulatory agencies include an Ames test, a mammalian cell chromosome damage test, and a bone marrow micronucleus test [42]. The Ames test using Salmonella and E. coli bacterial strains is a short-term bacterial reverse mutation assay specifically designed to identify test substances that can produce genetic damage that induces a gene mutation [43–45]. The National Toxicology Program (NTP) has validated this test using several hundred chemicals, and positive results in the bacterial reverse mutation test are well correlated with the results of rodent carcinogenicity tests [46]. A chromosomal aberration is a major biomarker of human exposure to genotoxic chemicals, and the chromosomal aberration test is well established as a complementary test to the Ames test [47, 48]. Micronucleus testing is a typical in vivo assay used to determine the mutagenicity of test substances, and micronucleus induction is well correlated with the induction of chromosomal aberrations [49].
The use of herbs for primary treatment continues to increase worldwide [50]; however, herbal medicines should be considered to have the same potential side effects as a drug. Unfortunately, an evaluation of herbal medicines revealed that only 15% of studies provided information on safety or side effects [51]. In addition, some traditional herbal medicines and their constituents are suspected of being carcinogens [52]. Therefore, considerable efforts are needed to prevent the side effects caused by the misuse of herbs that have not been accurately evaluated for toxicity. Although D. Rhizome extracts display wide-ranging health benefits on inflammation, ulcers, and diabetic disorders [53], genotoxicity information on these extracts is insufficiently documented. Therefore, the present study investigated the genotoxicity potential of DRWE using the in vitro and in vivo genotoxicity tests suggested in the OECD and MFDS test guidelines.
The major constituents of Dioscorea, including allantoin, dioscorine, sapogenin, prosapogenin, gracillin, choline, l-arginine, polysaccharides, and proteins, exhibit immunomodulatory and antitumor effects [54]. Allantoin, as the principle active compound, is abundant in D. Rhizome [55]. This compound is a well-known wound healing agent and prevents asthma and hypertension [55, 56]. The HPLC analysis conducted in the present study confirmed the presence of allantoin in DRWE.
According to a report by Yoon [57], D. Rhizome shows genotoxic effects when extracted with solvents such as 70% ethanol in the Ames test. The number of revertant colonies was higher in the presence of the S9 mixture than in the negative control in this study. However, in the present study, there was no significant increase in the number of revertants per plate in all the test strains in the presence or absence of the S9 mixture upon extraction with water. Thus, the results indicated that DRWE was not mutagenic in this bacterial assay system. In general, herbal medicines are extracted with water, but it is common to extract them using many organic solvents in order to find many useful ingredients that are not extracted from water [57]. In addition, it is known that mutagenicity results can be observed differently depending on the extraction solvent [57–59].
In the in vitro chromosome aberration test, no significant or dose-related increase in the frequency of aberrant metaphases with structural or numerical aberrations occurred compared with the negative control, regardless of the treatment regimen at any dose level of DRWE. Based on the results of the test, DRWE did not induce chromosomal aberrations in the CHL/IU cells in this assay system, and the result was a clear negative.
There are no detailed data on the genotoxicity potential of D. Rhizome extracts except for the Ames test using a 70% ethanol extract [57]. In our micronucleus testing, DRWE did not induce any significant increase in the MNPCEs, and there was no significant decrease in the PCE/(PCE + NCE) ratio up to 5000 mg/kg in the DRWE treatment groups compared with that in the negative control group. As shown in our results, DRWE did not induce micronuclei in the mammalian bone marrow cells used in this study under the present experimental conditions. The present study is therefore the first to evaluate the genotoxicity potential of DRWE based on current regulatory guidelines and GLP. The results provide valuable information on the genotoxicity of DRWE through in vitro and in vivo genotoxicity studies, which may be useful in toxicity evaluations.
In summary, DRWE neither exhibits mutagenic nor clastogenic potential under the present experimental conditions. The results of this study suggest that there is no risk of genotoxicity when using D. Rhizome as an herbal medicine.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A4A1019395).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Haq I. Safety of medicinal plants. Pakistan J Med Res. 2004;43:203–210. [Google Scholar]
- 2.Ardalan MR, Rafieian-Kopaei M. Is the safety of herbal medicines for kidneys under question? J Nephropharmacol. 2013;2:11–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Asif M. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012;1:44. doi: 10.4103/2277-9175.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Ouedraogo M, Qu F, Duez P. Potential genotoxicity of traditional Chinese medicinal plants and phytochemicals: an overview. Phytother Res. 2013;27:1745–1755. doi: 10.1002/ptr.4942. [DOI] [PubMed] [Google Scholar]
- 5.Shin JW, Kim HG, Park HJ, Sung NW, Son CG. Safety of the traditional Korean herbal medicine CGX: a 6-month repeated-dose study in rats. J Ethnopharmacol. 2010;128:221–229. [Google Scholar]
- 6.Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 2011;13(4):289–301. doi: 10.1111/j.1463-1326.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Sung HK, Hong KE. Effects of Dioscoreae Rhizoma (SanYak) on peripheral neuropathy and its safety. J Pharmacopunct. 2013;16:7–10. doi: 10.3831/KPI.2013.16.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Kang SY, Meng X, Kang AN, Park JH, Park YK, Jung HW. Effects of rhizome extract of Dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in C2C12 myotubes. Molecules. 2018;23:2023. doi: 10.3390/molecules23082023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu K, Yao X. The cytotoxicity of methyl protoneogracillin (NSC-698793) and gracillin (NSC-698787), two steroidal saponins from the Rhizomes of Dioscorea Collettii Var. hypoglauca, against human cancer cells in vitro. Phytother Res. 2003;17:620–626. doi: 10.1002/ptr.1211. [DOI] [PubMed] [Google Scholar]
- 10.Choi EM, Koo SJ, Hwang JK. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea Batatas) J Ethnopharmacol. 2004;91:1–6. doi: 10.1016/j.jep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Kim HN, Kang KS, Baek NI, Kim DK, Kim YS, Jeon BH, Kim SH. Methanol extract of Dioscoreae Rhizoma inhibits pro-inflammatory cytokines and mediators in the synoviocytes of rheumatoid arthritis. Int Immunopharmacol. 2004;4:1489–1497. doi: 10.1016/j.intimp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Jeon JR, Lee JS, Lee CH, Kim JY, Kim SD, Nam DH. Effect of ethanol extract of dried chinese yam (Dioscorea Batatas) flour containing dioscin on gastrointestinal function in rat model. Arch Pharm Res. 2006;29:348–353. doi: 10.1007/BF02968583. [DOI] [PubMed] [Google Scholar]
- 13.Sagara K, Ojima M, Suto K, Yoshida T. Quantitative determination of allantoin in Dioscorea Rhizome and an oriental pharmaceutical preparation, hachimi-gan, by high-performance liquid chromatography. Planta Med. 1989;55:93. doi: 10.1055/s-2006-961841. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Liu YH, Chen GN. Simultaneous determination of allantoin, choline and L-arginine in Rhizoma Dioscoreae by capillary electrophoresis. J Chromatogr A. 2004;1043:317–321. doi: 10.1016/j.chroma.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Yoon KD, Yang MH, Chin YW, Park JH, Kim JW. Determination of allantoin in Dioscorea Rhizoma by high performance iquid chromatography using cyano columns. Nat Prod Sci. 2008;14:254–259. [Google Scholar]
- 16.Fu YC, Ferng ALH, Huang PY. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin and bulbil of the Dioscorea species. Food Chem. 2006;94:541–549. [Google Scholar]
- 17.Lebot V, Faloye B, Okon E, Gueye B. Simultaneous quantification of allantoin and steroidal saponins in yam (Dioscorea spp.) powders. J Appl Res Med Aromat Plants. 2019;13:100200. [Google Scholar]
- 18.Guan XR, Zhu L, Xiao ZG, Zhang YL, Chen HB, Yi T. Bioactivity, toxicity and detoxification assessment of Dioscorea bulbifera L.: a comprehensive review. Phytochem Rev. 2017;16:573–601. [Google Scholar]
- 19.MFDS (2014) Nonclinical study guideline for herbal (crude) medicinal preparations (Notification No. B1–2014–3–014). Ministry of Food and Drug Safety, Korea
- 20.OECD (1997) OECD guideline for testing of chemicals. TG 471: bacterial reverse mutation test
- 21.Yun JW, Kim YS, Kwon E, Kim SH, You JR, Kim HH, Che JH, Kang BC. Evaluation of in vitro and in vivo genotoxicity of Angelica acutiloba in a standard battery of assays. Lab Anim Res. 2017;33:231–236. doi: 10.5625/lar.2017.33.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta T, Tokishita S, Tsunoi R, Ohmae S, Yamagata H. Characterization of Trp+ reversions in Escherichia coli strain WP2uvrA. Mutagenesis. 2002;17:313–316. doi: 10.1093/mutage/17.4.313. [DOI] [PubMed] [Google Scholar]
- 23.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;133:173–175. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 24.Hyde R, Smith JN, Ioannides C. Induction of the hepatic mixed-function oxidases by Aroclor 1254 in the hamster: comparison of Aroclor-induced rat and hamster preparations in the activation of pre-carcinogens in the Ames test. Mutagenesis. 1987;2:477–482. doi: 10.1093/mutage/2.6.477. [DOI] [PubMed] [Google Scholar]
- 25.Tejs S. The Ames test: a methodological short review. Environ Biotechnol. 2008;4:7–14. [Google Scholar]
- 26.OECD (2014) OECD Guideline for testing of chemicals. TG 473: in vitro mammalian chromosome aberration test
- 27.JEMS-MMS (1988) Japanese Environmental Mutagen Society-Mammalian Mutagenicity Study Group. In: Atlas of chromosome aberration by chemicals. JEMS-MMS, Tokyo
- 28.OECD (2014) OECD Guideline for testing of chemicals. TG 474: mammalian erythrocyte micronucleus test
- 29.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 30.Kim HY, Kim MH, Kim HK, Park YC. Genotoxicity study of glycopeptide (G-7%NANA) Toxicol Res. 2018;34:259–266. doi: 10.5487/TR.2018.34.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi M, Sofuni T, Ishidate M. An application of acridine orange fluorescent staining to the micronucleus test. Mutat Res. 1983;120:241–247. doi: 10.1016/0165-7992(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 32.Fisher RA. Statistical methods for research workers. 14. Edinburgh: Oliver and Boyd; 1970. [Google Scholar]
- 33.Cochran WG. The Chi-square test of goodness of fit. Ann Math Stat. 1952;23:315–345. [Google Scholar]
- 34.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:614–617. [Google Scholar]
- 35.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 36.Levene H. Contributions to probability and statistics: essays in honor of Harold Hotelling. Palo ALto: Stanford University Press; 1960. pp. 278–292. [Google Scholar]
- 37.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 38.Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–491. [Google Scholar]
- 39.Fisher RA. Applications of “Student’s” distribution. Metron. 1925;5:90–104. [Google Scholar]
- 40.Ren N, Atyah M, Chen WY, Zhou CH. The various aspects of genetic and epigenetic toxicology: testing methods and clinical applications. J Transl Med. 2017;15:110. doi: 10.1186/s12967-017-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poivre M, Nachtergael A, Bunel V, Philippe ON, Duez P. Genotoxicity and carcinogenicity of herbal products. Berlin: Springer; 2017. pp. 179–215. [Google Scholar]
- 42.Thybaud V, Aardema M, Clements J, Dearfield K, Galloway S, Hayashi M, Jacobson-Kram D, Kirkland D, MacGregor JT, Marzin D, Ohyama W, Schuler M, Suzuki H, Zeiger E. Strategy for genotoxicity testing: hazard identification and risk assessment in relation to in vitro testing. Mutat Res. 2007;627:41–58. doi: 10.1016/j.mrgentox.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 44.Verheyen GR, Deun KV, Miert SV. Testing the mutagenicity potential of chemicals. J Genet Med. 2017;4:029. [Google Scholar]
- 45.Levya D, Zeiger E, Escobar PA, Hakura AM, van der Leedee BJ, Kato M, Moore MM, Sugiyama KI. Recommended criteria for the evaluation of bacterial mutagenicity data(Ames test) Mutat Res Gen Toxicol Eng. 2019;848:403074. doi: 10.1016/j.mrgentox.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Aubrecht J, Osowski JJ, Persaud P, Cheung JR, Ackerman J, Lopes SH, Ku WW. Bioluminescent Salmonella reverse mutation assay: a screen for detecting mutagenicity with high throughput attributes. Mutagenesis. 2007;22:335–342. doi: 10.1093/mutage/gem022. [DOI] [PubMed] [Google Scholar]
- 47.Ishidate M, Miura KF, Sofuni T. Chromosome aberration assays in genetic toxicology testing in vitro. Mutat Res. 1998;404:167–172. doi: 10.1016/s0027-5107(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 48.Natarajan AT. Chromosome aberrations: past, present and future. Mutat Res. 2002;504:3–16. doi: 10.1016/s0027-5107(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 49.Kasamoto S, Masumori S, Hayashi M. In vivo micronucleus assay in mouse bone marrow and peripheral blood. Methods Mol Biol. 2013;1044:179–189. doi: 10.1007/978-1-62703-529-3_9. [DOI] [PubMed] [Google Scholar]
- 50.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;10:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boullata JI, Nace AM. Safety issues with herbal medicine. Pharmacotherapy. 2000;20:257–269. doi: 10.1592/phco.20.4.257.34886. [DOI] [PubMed] [Google Scholar]
- 52.Moreira LDD, Teixeira SS, Monteiro DMH, De-Oliveira AXAC, Paumgartten JRF. Traditional use and safety of herbal medicines. Rev Bras Farmacogn. 2014;24:248–257. [Google Scholar]
- 53.Tsai CC, Chen LJ, Niu HS, Chung KM, Cheng JT, Lin KC. Allantoin activates imidazoline I-3 receptors to enhance insulin secretion in pancreatic β-cells. Nutr Metab. 2014;11:41. [Google Scholar]
- 54.Go HK, Rahman MM, Kim GB, Na CS, Song CH, Kim JS, Kim SJ, Kang HS. Antidiabetic effects of Yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients. 2015;7:8532–8544. doi: 10.3390/nu7105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen MF, Tsai JT, Chen LJ, Wu TP, Yang JJ, Yin LT, Yang YL, Chiang TA, Lu HL, Wu MC. Antihypertensive action of allantoin in animals. Biomed Res Int. 2014;2014:690135. doi: 10.1155/2014/690135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee MY, Lee NH, Jung D, Lee JA, Seo CS, Lee H, Kim JH, Shin HK. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int Immunopharmacol. 2010;10:474–480. doi: 10.1016/j.intimp.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Yoon WH. Genotoxicological safety evaluation of the solvent extracts for medicinal herbs that are of highly domestic spendings. Korean J Food & Nutr. 2013;26:814–823. [Google Scholar]
- 58.Florinsiah LF, Zuriana MY, Rajab NF, Nurshahirah N, Norfazlina N, Suziaina Zaila CF. Mutagenicity effect of Centella asiatica in aqueous extract by using Ames test. Open Conf Proc J. 2013;4:83–87. [Google Scholar]
- 59.Jadczyk P, Kołwzan B. Genotoxicity of soil pollutants extracted with different solvents. Pol J Environ Stud. 2013;22:141–147. [Google Scholar]