Abstract
The adverse effects of chemical compounds have limited their usage despite their relative success in improving meat tenderness. Thus, natural tenderizers have attracted attention. The present study aimed to evaluate the tenderization effects of asparagus (Asparagus officinalis L.) juice and balsamic vinegar on beefsteak; marination at 4 °C for 48 h significantly increased the water-holding capacity, total protein solubility, myofibrillar fragmentation index and hydroxyproline content but significantly decreased the pH value, Warner–Bratzler shear force, and energy to the peak rates (P < 0.05). Scanning electron microscopy images and electrophoresis findings revealed extensive degradation of connective tissues and changes in protein band patterns, respectively. The tenderness of the beefsteak samples was optimum by applying 25% asparagus juice, and 25% asparagus juice + 10% balsamic vinegar. Therefore, marinade solutions containing asparagus juice and balsamic vinegar can be considered as natural tenderizing agents in formulation of seasonings and sauces to promote tenderness in tough beefsteak and possibly improve other quality-related properties.
Keywords: Asparagus juice, Balsamic vinegar, Beefsteak, Natural tenderizer, Proteolytic activity
Introduction
Meat quality depends on a combination of sensory and technological properties, including tenderness, color, and water-holding capacity (Chen et al. 2018). Multiple parameters can affect the meat tenderness throughout the production processes, including biological, on-farm, processing, and consumer parameters. Meat tenderness is a result of the activity of endogenous proteases during aging; such activities cause postmortem protein degradation (Kemp et al. 2010). However, due to anatomical and biological differences, various muscles do not reach the same level of tenderization during aging, and consumers perceive toughness in meat even after a prolonged aging period. Increased activity of endogenous proteases stimulated by relatively high postmortem temperatures and high Ca2+ contents may result in increased meat tenderness. Nevertheless, other quality characteristics, such as flavor and color, can be negatively affected by such treatments. Toohey et al. (2011) focused on increasing the meat tenderness and monetary value/return of a carcass with the help of exogenous proteases. Among natural proteases, some plant-derived enzymes, including papain, bromelain, and ficin, were proposed as natural meat tenderizers with unique protein degradation activities in connective tissues and myofibrils (Maqsood et al. 2018). Aminlari et al. (2009) reported that kiwifruit juice has a controlled tenderizing action on the myofibrillar structure and is effective in dissolving connective tissues. It has also been shown that the ginger and pineapple juices have more activities on collagen and elastin in comparison to actomyosin (Żochowska-Kujawska et al. 2017). In softened meat, a higher water-holding capacity and better flavor can be achieved by the activity of bromelain compared with ficin and papain (Kadıoğlu et al. 2019). Although, the usage of plant-derived proteases with the aim of meat texture alteration and tenderization is a rapid, safe, cost-effective, non-thermal, and green technology, several studies have reported the indiscriminately degradation of muscle proteins due to their broad substrate specificity (Bhat et al. 2018). These natural compounds result in several unwanted attributes, such as mushy texture, bitterness, and off-flavor. Multiple reports are available on the new plant-derived proteases that are capable of displaying positive effects, such as mild tenderization, oxidative and meat color stability. Asparagus (Asparagus officinalis L.) contains a high level of antioxidants, such as flavonoids (mainly rutin), ascorbic acid, and other phenolic acids (Bhat et al. 2018). Yamaguchi et al. (1982) reported a cysteine protease derived from asparagus, with a high binding affinity and slow hydrolysis which was advantageous in preventing over-tenderization. Limited information is available regarding the potential of this protease for meat tenderization. The asparagus juice has proteins with a molecular weight of 5–75 kDa. The green asparagus juice contains cysteine protease with a structure similar to papain and a molecular weight of approximately 28 kDa. The optimal temperatures for the activity of green asparagus protease are 40–45 °C, whereas the optimal pH value for its activity is in neutral or slightly alkaline ranges (Yonezawa et al. 1998). The protease activity of Asparagus officinalis was 5 and 14.1 U/g in acidic and neutral pH values, respectively (Sun et al. 2016). Although the meat tenderization mechanism in acidic marinades has not been completely elucidated, marinating with different types of vinegars has been a traditional technique to enhance the flavor and tenderness of meat before cooking. For instance, Żochowska-Kujawska et al. (2017) reported that meat samples treated with low pH marinades, such as balsamic vinegar, were acceptable by panel members, probably due to the high tenderness and slight raw meat/vinegar aroma of samples, which vanished during marinating. Based on these reports, we hypothesized to apply the combination of plant-derived proteases and marination at acidic conditions by natural vinegar. So, the present study aimed to evaluate the influence of marinating with asparagus juice and balsamic vinegar on the quality characteristics and tenderization of beefsteak during storage.
Materials and methods
Materials
The beef M. semitendinosus steaks (breed: Holstein; sex: male; age: 1 year; weight: 210 ± 13 kg, after 24 h of slaughtering according to Islamic law), fresh asparagus (Asparagus officinalis L.) and balsamic vinegar (pH = 4.4; 6% aceto balsamico di Modena, Italy) were purchased from a local market. All chemicals used were of analytical reagent grade.
Extraction of asparagus crude juice
The extraction of asparagus juice was made according to Baker et al. (1980) with slight modifications. Fresh asparagus was ground with an Omni-mixer (Du Pont Instruments Corp., Wilmington, DE, USA), and then pressed through a triple-layer cheesecloth to extract crude juice, and centrifuged at 16,000×g for 5 min at 4 °C. The resulted supernatant (pH = 6.0; soluble solids content: 5.02%) was frozen at -20 °C in a dark container until testing.
Marination of samples
The beefsteaks were sectioned into equal chunks in four batches. The meat chunks were injected with three marinade treatments, which were selected based on pre-treatment results; then transferred into low-density polyethylene bags, and stored at 4 °C. After 4, 24, and 48 h intervals, the samples were evaluated for designed tests. Repeated marinating experiments were conducted using beefsteaks and asparagus juice collected at different time points. The treatments included the following:
S0: control (beefsteak without marinade)
S1: 100 g beefsteak injected with 25 mL asparagus juice
S2: 100 g beefsteak injected with 25 mL asparagus juice diluted in 75 mL distilled water
S3: 100 g beefsteak injected with 25 mL asparagus juice diluted in 10 mL balsamic vinegar and 65 mL distilled water
pH measurement
Distilled water (50 mL) was added to 5 g prepared raw samples, placed in a homogenizer (Ultra-Turrax T25, Janke & Kunkel, Staufen, Germany) and centrifuged at 8000×g for 60 s to measure the pH values by a digital pH meter (Model 340, Mettler-Toledo GmbH, Schwerzenbach, Switzerland) (Kim et al. 2013).
Water-holding capacity (WHC)
The WHC of meat samples (0.3 g) was measured via filter paper press, using a 2 kg weight for 5 min, between eight pieces of filter paper, and two pieces of plastic board. The WHC was calculated as follows (Sultana et al. 2008):
Texture analysis
Each meat sample was wrapped with low-density polyethylene bags and cooked in a water bath at 75 °C for 15 min until its internal temperature reached 71 °C. The cooked samples were cooled at room temperature for 6 h and cut into 1 × 0.8 × 3 cm3 sections parallel to the orientation of muscle fibers. The Warner–Bratzler shear force (WBSF) values were calculated in accordance with Warner–Bratzler shear attachment (V-type blade set) (60° angle) by using a texture analyzer (TA-XT2i, Stable Micro Systems Ltd., UK). The test probe speeds were adjusted to 2 mm/s. The maximum force was in Newton (N), which was needed for shear through the samples (Kim et al. 2013).
Total protein solubility
The method proposed by Joo et al. (1999) with some modifications was used to measure total protein solubility. Raw samples of 2 g were mixed with 20 mL of ice-cold 1.1 M potassium iodide in 0.1 M potassium phosphate buffer (pH = 7.4) by using a homogenizer and then maintained at 4 °C overnight. The mixture was centrifuged at 6000×g for 15 min. The resulting supernatant was filtered with a Whatman No. 1 filter paper to measure the protein content in accordance with the Biuret method.
Hydroxyproline concentration
The method of Naveena et al. (2011) with slight modification was used to measure the hydroxyproline content. First, 2 g meat sample was hydrolyzed by 40 mL of 6 N HCl at 108 °C for 18 h. The filtered hydrolysate was diluted with distilled water to a final volume of 50 mL. The pH value of hydrolysate (25 mL) was adjusted to 7.0 by 40% NaOH, and diluted by mixing with distilled water to a final volume of 50 mL. An aliquot of 1 mL from the obtained solution was applied to measure the hydroxyproline content by reading the optical density (OD) with a UV–VIS spectrophotometer (UV-1700 PharmaSpec, SHIMADZU, Japan) at 540 nm in accordance with a standard curve.
Myofibrillar fragmentation index (MFI)
The MFI was measured using the method of Olson and Parrish (1977) with some modifications. MFI buffer containing 100 mM KCl, 20 mM K2HPO4/KH2PO4, 1 mM NaN3, and 1 mM EDTA with pH = 7.0 was used to extract the myofibrils. The protein content in the final suspension was determined using the Biuret method, followed by dilution with the MFI buffer to achieve a protein content of 0.5 ± 0.05 mg/mL. The OD was read by the UV–VIS spectrophotometer at 540 nm and multiplied by 200 to calculate the MFI values.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The method of Sikes et al. (2010) was used to separate the myofibrillar protein fractions to perform the SDS-PAGE, by using Bio-Rad’s vertical electrophoresis (PowerPac™ Basic model) and with the aid of 5% stacking gels and 12% running gels (20 μl loaded). Coomassie Brilliant Blue R250 (B7920, Sigma, USA) was used to stain the loaded gel, which was subsequently destained by a solution at a ratio of methanol (50): distilled water (40): acetic acid (10). Protein bands were identified in comparison with a standard protein marker (Precision Plus Protein Standards, Bio-Rad Lab., Hercules, CA, USA).
Analysis of microstructure
The specimens were cut (2–3 mm thickness) and then fixed with 2.5% glutaraldehyde in the presence of 0.1 M phosphate buffer (pH = 7.2) at 4 °C for 24 h. They were re-fixed by osmium tetroxide (2% in H2O, Sigma, USA), and then dehydrated by incremental ethanol serial dilutions (50, 70, 90, and 100%), followed by sectioning using a razor blade in liquid nitrogen (Naveena et al. 2011). A thin layer of gold was used to coat the samples by using an ion sputter (E-1010, Hitachi, Tokyo, Japan), and the samples were scanned via SEM (S-3000 N, Tokyo, Japan) at ×300 magnification.
Statistical analysis
The normal distribution of variables was checked using the Shapiro–Wilk test before analysis. A general linear model procedure was used to conduct the effect of marinade treatments (S0, S1, S2, and S3) and storage times (0, 4, 24, and 48 h) and their interaction on responses. All designed tests were conducted in triplicates and all experiments (treatments) were conducted twice (two different batches). Values are expressed as mean ± standard deviation. The data were analyzed by SPSS (version 25.0, SPSS Inc., Chicago, IL, USA) using analysis of variance (ANOVA) test and Duncan’s multiple range at a significance level of P < 0.05.
Results and discussion
Effect of marination on pH
The weakness of the protein structure and the improvement of proteolysis, particularly the myosin degradation, as well as the release of lysosomal enzymes due to the approach of environmental pH to the optimum pH activity, are the most important changes caused by marinade solutions (Bhat et al. 2018). As shown in Table 1, when tenderization time is progressed, the pH of sample S3 significantly (P < 0.05) decreased compared with other treatments. Also, a significant pH reduction was observed at 4 h and 24 h after marination compared with 48 h storage time in all treatments except S3 (P < 0.05). On the second day, the minimum pH was observed in the treatment S3 (P < 0.05). Also, no significant pH changes were seen between S1 and non-marinated (control) samples (P > 0.05). This observation can be due to low glycogen resources, high initial meat pH values, the intrinsic pH of the marinade solution ingredients, and their concentration (Kim et al. 2013). Therefore, raising the asparagus juice concentration in the marinade solution increased the pH of the samples, probably due to the release of basic amino acids during the decomposition of the marinated beefsteak proteins. Meanwhile, the presence of organic acids, such as acetic and benzoic acids, in the balsamic vinegar, can justify the reduced pH of marinated S3 sample. Balsamic vinegar has high levels of glucose and fructose (Verzelloni et al. 2010) which through their consumption by acid-based bacteria, pH reduction would occur over time. Besides, low glycogen content of the muscle at slaughter may decrease the glycolysis rate. Hence, a slow accumulation of lactic acid and a slow rate of pH reduction would take place after slaughter. Therefore, more time will be available for meat proteases to perform their proteolysis action on proteins and subsequently, postmortem tenderization. These findings were in line with the results of Kim et al. (2013) and Serdaroglu et al. (2007).
Table 1.
Effect of marinating with asparagus juice and balsamic vinegar on the physicochemical and textural properties of beefsteak chunks during storage at 4 °C
| Parameter | Treatment | Storage time (h) | |||
|---|---|---|---|---|---|
| 0 | 4 | 24 | 48 | ||
| pH | S0 | 5.76 ± 0.62Ab | 5.75 ± 0.37Ab | 5.60 ± 0.61Ac | 5.82 ± 0.37Aa |
| S1 | 5.76 ± 0.60Ab | 5.62 ± 0.88Ac | 5.64 ± 0.31Ac | 5.97 ± 0.36Aa | |
| S2 | 5.75 ± 0.59Aa | 5.54 ± 0.35ABb | 5.51 ± 0.42ABb | 5.57 ± 0.34Bb | |
| S3 | 5.74 ± 0.61Aa | 5.01 ± 0.28Cb | 5.04 ± 0.48Cb | 4.86 ± 0.51Cc | |
| WHC (%) | S0 | 46.17 ± 3.36Ac | 52.62 ± 3.86Bb | 57.24 ± 3.19Ba | 57.40 ± 3.77Ba |
| S1 | 46.19 ± 3.08Ac | 57.43 ± 5.54Aab | 59.22 ± 5.85Aa | 60.48 ± 5.36Aa | |
| S2 | 46.15 ± 3.26Ac | 50.98 ± 3.93Bb | 53.02 ± 4.04Ca | 55.55 ± 4.00Ca | |
| S3 | 46.16 ± 3.17Ac | 46.72 ± 3.27Cc | 53.35 ± 4.93Cab | 54.56 ± 3.40Ca | |
| WBSF (N) | S0 | 56.10 ± 5.70Aa | 49.95 ± 6.35Ab | 50.43 ± 6.88Aab | 38.96 ± 5.80Bc |
| S1 | 56.03 ± 5.43Aa | 35.51 ± 3.93Cc | 38.66 ± 3.17Db | 33.69 ± 3.17Dc | |
| S2 | 56.01 ± 5.74Aa | 51.41 ± 6.33Aab | 44.11 ± 4.52Bc | 43.23 ± 3.90Ac | |
| S3 | 56.02 ± 5.54Aa | 38.07 ± 5.98Bbc | 40.27 ± 2.13Cb | 36.38 ± 4.63Cc | |
| Energy to peak (N.mm) | S0 | 353.92 ± 8.30Aa | 347.84 ± 8.75Aa | 273.47 ± 9.70Ab | 210.51 ± 6.66Bc |
| S1 | 353.98 ± 8.38Aa | 217.97 ± 7.80Cc | 253.54 ± 10.08Bb | 183.28 ± 5.02Dd | |
| S2 | 353.94 ± 8.35Aa | 348.06 ± 9.90Aa | 275.98 ± 8.09Ab | 224.22 ± 6.93Ac | |
| S3 | 353.91 ± 8.33Aa | 227.48 ± 7.30Bc | 237.11 ± 8.53Cb | 195.03 ± 5.44Cd | |
Values are mean ± standard deviation in triplicate. A–D different letters within the marinade treatments and a–d different letters within the storage time indicate statistically significant differences at P < 0.05. Treatments: S0: control, S1: beefsteak + 25% asparagus juice, S2: beefsteak + 25% asparagus juice + 75% distilled water, S3: beefsteak + 25% asparagus juice + 10% balsamic vinegar + 65% distilled water
Effect of marination on WHC
As shown in Table 1, the changes in WHC during the storage followed a raising trend; thus after storage for 4 h, the WHC of S1 sample containing asparagus juice was significantly (P < 0.05) higher than those of control and other samples. The WHC of the S3 treatment increased after 24 h. Despite low pH values, a high WHC could be related to the free water, which easily disappears during cooking (simply being entrapped through capillary forces) (Zou et al. 2018). However, with extending the storage period up to 48 h, the highest WHC was observed only in the treatment S1. Other treatments showed lower WHC values compared with the control sample (P < 0.05). Also, as marination proceeded, in spite of slight WHC increments, no WHC difference was observed between 24 h and 48 h in all samples (P > 0.05). WHC is the capacity of meat to maintain natural water/added water into its spatial network. Physiological and anatomical factors and slaughtering/processing conditions could affect the WHC (Aminlari et al. 2009). Since, pH directly influences the level of negative charges on the protein particles that can bond to water molecules, pH values below or above the isoelectric point can increase the WHC level which can be attributed to the electrostatic repulsive forces in the muscle filaments, resulting in an expanded space; so, more water is entrapped in the space among them. Thus, the meat marinated at high or low pH values held a higher amount of water, and the cooked product showed increased levels of juiciness and tenderness. Considering the pH value of the S2 and S3 samples, sarcoplasmic proteins could precipitate on myofibrillar proteins, and an overall reduction happens in reactive groups of proteins available for water-holding. Subsequently, the WHC is reduced (Naveena et al. 2004). Some researchers have reported remarkable increases in WHC after enzymatic treatments for buffalo meat (Naveena et al. 2004), and turkey meat (Doneva et al. 2015), and some reported WHC decreases for beef, chicken, squid (Ketnawa and Rawdkuen 2011) and camel (Maqsood et al. 2018). Joo et al. (1999) described that WHC reduction in enzyme treated samples could be due to degradation and denaturation of both sarcoplasmic and myofibrillar proteins which might have caused the proteins to lose the capacity to hold water.
Textural evaluation of marinated meat samples
Table 1 shows the effects of marinade type and storage time on the textural property of beefsteak samples. Marination significantly (P < 0.05) affected WBSF and led to peaking of energy. Also, the changes in WBSF during the storage followed a decreasing trend; minimum WBSF was observed in S1 and S3 treatments after 4 h of marination, whereas shear force changes in S2 treatment was similar to that of the control group (Table 1). However, at the end of storage, the required force for shearing the cooked S2 sample was the highest value. Notably, the mean shear force value of S1 sample significantly (P < 0.05) decreased on the second day of storage (24–48 h). During marination, there was no significant WBSF differences (P > 0.05) between 4 h and 48 h storage time for S1 and S3 treatments. In addition, a trend similar to the WBSF values was observed for energy to peak. Kadıoğlu et al. (2019) claimed that the combined effect of enzymatic treatment with increasing duration of enzyme exposure to the substrate led to further hydrolysis followed by a significant decrease in WBSF and energy to peak values. Textural evaluation is used commonly to characterize meat tenderness. Proteolytic enzymes increase the tenderness of meat when they are uniformly distributed and properly applied. Swelling i.e., an expansion of connective tissues, including perimysium/endomysium, proteolytic weakening of muscle proteins structure, and solubilization of collagen while cooking are the tenderization effects of marination (Żochowska-Kujawska et al. 2017). These observations were evident in two S1 and S3 samples with a decrease in perimysium and endomysium thickness of beefsteak. As revealed by SEM images (will be discussed later), despite partially decreases in the connective tissues of S2 sample, the highest WBSF of this sample on the second day of storage might be due to low penetration of marinade solution and the remaining connective tissues or collagen in meat structure. Pre-slaughter and post-slaughter factors could also affect meat texture and tenderness (Aminlari et al. 2009). Accordingly, we evaluated the protein solubility and proteolysis to explore the effect of asparagus juice and balsamic vinegar on beefsteak tenderization. Significant decreases have been reported in WBSF after enzymatic treatments or marination with acidic/alkali marinades for home-style jerky (Żochowska-Kujawska et al. 2017), beef (Toohey et al. 2011; Kim et al. 2013), buffalo meat (Naveena et al. 2011), and duck breast muscles (He et al. 2015).
Total protein solubility (TPS) of marinated meat samples
Protein solubility has been used as an index for the meat quality changes during aging (Maqsood et al. 2018). Figure 1 shows the TPS of different samples during storage; S1 sample showed a consistent TPS increase during storage. Also, the S3 sample showed a slight decrease between 24-48 h whereas the reverse trend occurred for the S0 treatment; it showed a sudden increase on the second day of storage. Furthermore, S2 sample remained constant between 24 h and 48 h (P < 0.05). The enzymatic treatment of the meat samples could increase the permeability of myofibrils, easily degrade proteins, and justify their solubility (Gokoglu et al. 2016). The increase in TPS (salt + water soluble proteins) of S1 and S0 treatments could be attributed to the degradation of proteins and formation of small and low molecular weight peptides as well as free amino acids as a result of the proteolytic action of exogenous/endogenous enzymes (Zhao et al. 2019; Rawdkuen et al. 2013). Such increase in TPS reduced the toughness of the treated samples. Hence, liberating of amino acids, due to the activity of beef endogenous proteases caused pH elevation in non-marinated sample. Overall, the repulsive forces at pH values higher than isoelectric point, may increase the ability of proteins to bond water. Thereby, the WHC of proteins as well as their solubility enhanced which can justify the acquired results in S0 sample (24–48 h). Besides, as revealed by SEM images, up to 24 h, the fibrils in unmarinated sample (S0) were tightly organized to each other; which could prevent the penetration of buffer and caused resistance to extraction and solubilization (Naveena et al. 2004). Nonetheless, lower TPS of the S3 sample at the end of storage, might be associated with aggregation and precipitation of low molecular weight proteins produced from heavy chains of myosin or degradation of other high molecular weight proteins during marination. Such higher molecular weight units were not observed in soluble proteins (Lee et al. 2009). Enzymatic modification of proteins significantly improves their functional properties such as WHC, emulsifying capacity and stability through increasing the protein solubility. Improving the technological quality of meat and producing uniformly texturized meat products, as well as modifying the quality of folding and cutting in the meat products are affected by the degree of meat tenderization which is related to the soluble protein level (Aminlari et al. 2009). Other attributes of meat and meat products such as flavor, color and juiciness are also associated with WHC (Żochowska-Kujawska et al. 2017). Changes in TPS occur under a variety of extracting conditions. Hence, changes in solubility shows the changes in protein conformation degree, i.e., denaturation (Maqsood et al. 2018). The protein solubility is affected when pH level approaches the isoelectric point due to the reduction of repulsive forces and non-balance between the hydrophilicity and hydrophobicity ratios, which depends on the amino acid composition, especially on the protein surface. Denaturation may also affect the protein solubility due to changes in the aforementioned ratio. Higher protein solubility of enzyme-treated samples has been found in squid (Gokoglu et al. 2016), buffalo meat (Naveena et al. 2011), camel (Maqsood et al. 2018), pork, beef and chicken (Rawdkuen et al. 2013).
Fig. 1.
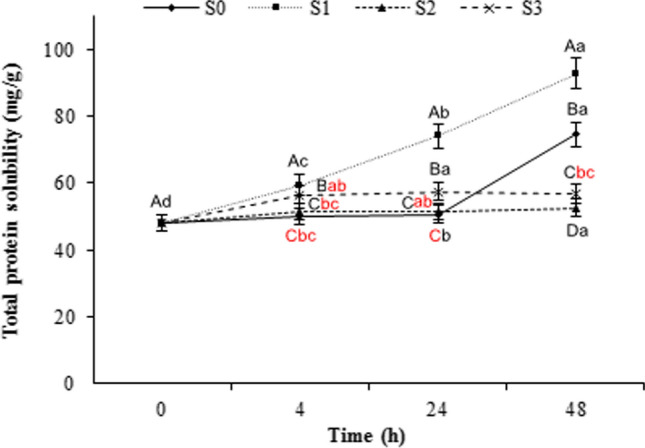
Total protein solubility of beefsteak chunks marinated with asparagus juice and balsamic vinegar during storage at 4 °C
Hydroxyproline (HP) content of marinated meat samples
The muscle protein solubility influences the textural attributes and improvement in the solubility of proteins can be achieved by chemical and enzymatic modifications (Maqsood et al. 2018). The maximum HP content after 4 h marination as shown in Fig. 2 was observed in the S1, S3 and S2 samples compared with S0 (control) sample; which was increasing until the end of storage period. The changes in the S1 sample were more remarkable than other samples (P < 0.05). HP is one of the main constituents of collagen protein and an indicator for measuring collagen values. Collagen has swelling characteristics in acidic or alkali conditions at high temperatures. Also, swollen collagen can be converted to gelatin, such as those encountered during cooking. High collagen solubility due to acidic or alkali conditions improves meat tenderness (Naveena et al. 2011). Proteases appear to be effective on proteoglycans, which bind collagen type II fibers, as intramuscular bridges. Meanwhile, the muscle membrane may begin to rupture as the pH drops; collagen, which begins to dissolve in acidic conditions, slowly exits from the muscle and subsequently elevates the meat tenderness (Chang et al. 2010). Based on the present findings, the higher HP content of marinated samples may be associated with protease activities (including natural meat proteases and asparagus proteases) under low pH conditions. Furthermore, heat-soluble collagen and perimysium denaturation could lead to reduced denaturation temperature and so, increased collagen extraction, thereby resulting in a high concentration of HP detected in the treated samples. Maqsood et al. (2018) reported that increased total collagen content of treated samples was well expressed in the higher content of HP in camel meat treated with plant proteases.
Fig. 2.
Hydroxyproline content of beefsteak chunks marinated with asparagus juice and balsamic vinegar during storage at 4 °C
Myofibrillar fragmentation index (MFI) of marinated meat samples
The meat tenderness is highly dependent on the degree of weakness and breakdown of myofibrillary structure. Break-up of thin filaments of band I and the loss of myofibril joints due to the activity of the enzymes are the most important events during aging (Olson and Parrish 1977). The MFI of marinated samples was affected by the storage time and marination type (P < 0.05, Fig. 3). In general, MFI increased with aging time, reaching the highest values at 48 h storage in all treatments (however, with different rates). Also, the MFI values in the S1 sample was higher than other samples. As revealed by the electrophoretic data (next section), the results on desmin degradation together with the major increase of MFI observed in marinated beefsteak samples during aging confirm a highly intense proteolysis of specimens. In the present study, an increase in the solubility of proteins and lower WBSF values of S1 treatment was well reflected in higher WHC and myofibrillar proteins degradation over time. MFI can be used to determine the degree of meat tenderness during marination, since there is a positive correlation between the rate of myofibrillar fragmentation and tenderness of the meat during aging time. A high MFI value means a further tender meat (Kim et al. 2013). However, the elevated MFI under acidic conditions is associated with intrinsic factors and also increased enzymatic proteolysis of μ-calpain and extrinsic factors, such as added ingredients i.e., asparagus juice. Moreover, the differences in the enzyme level or ratio to the inhibitor affects the MFI (Soltanizadeh et al. 2008). He et al. (2015) reported that disruption of Z-disk of meat structure can shorten the length of myofibrils and reduce the number of sarcomere units, to the cost of increasing myofibrillar short segments during aging time. Moreover, endogenous proteases (including calpains and cathepsins) in muscle cells and cytoplasmic matrix as well as natural meat tenderizers contribute to MFI increase and meat tenderness. Li et al. (2017) reported that CaCl2 marination could contribute to myofibrillar fractions directly through the conversion of F-ation to G-actin. The calpains are more effective in disintegration of myofibrillar proteins into short segments and the increase in MFI compared with cathepsins (Li et al. 2017). Similar findings have been reported in MFI increases after enzymatic treatments and marinating with low pH marinades as well as calcium ion solution for duck breast muscles (He et al. 2015), beef (Kim et al. 2013), and goose meat (Li et al. 2017), respectively.
Fig. 3.

MFI of beefsteak chunks marinated with asparagus juice and balsamic vinegar during storage at 4 °C
Electrophoretic data of marinated samples
The SDS-PAGE findings in our study were markedly affected by the factor of time. The recognized protein bands in samples were probably nebulin and/or titin (> 220 kDa), myosin heavy chain (MHC) (220 kDa), alpha-actinin (105 kDa), desmin (50 kDa), actin (45 kDa), and alpha and beta-tropomyosin (35–33 kDa) (Ha et al. 2013). A significant difference was observed in electrophoresis patterns between the marinated beefsteak samples and the control groups (Fig. 4), thereby confirming the MFI findings. Accordingly, during 48 h storage at 4 °C, reduction in intensity and the number of high molecular weight (MW) protein bands (> 65 kDa) was observed in the two S1 and S3 treatments. Meanwhile, all treatments containing asparagus juice showed a higher content of low MW protein bands (< 25 kDa) compared with the control. The 50 kDa band associated to desmin was clearly detected in unmarinated samples, whereas it became less intense in asparagus juice treated samples in prolonged storage period, suggesting that extensive degradation could occur during cold storage. A correlation between desmin destruction and WHC was reported in postmortem tenderization, which possibly improved the meat tenderness. The major contractile proteins, actin and myosin underwent different degrees of degradation in all marinated samples during meat storage. The strength rigor bonds of the myosin and actin proteins generated during rigor development, might be affected or modified by MHC degradation, and could contribute to meat tenderness (Tsai et al. 2012). Meanwhile, less intensity of alpha-actinin and complete disappearance of alpha and beta-tropomyosin bands were observed in S1 treatment after 48 h. Alpha-actinin as a major Z-line protein is responsible for cross-linking with thin filaments inside the Z-lines. Consequently, its destruction might lead to the decomposition of the Z-lines and the attachment sites of the thin filaments to the Z-lines, thus resulting in extensive fragmentation of myofibrils in the marinated samples and improved meat tenderness (Tsai et al. 2012). Moreover, the S3 treatment showed higher proteolysis of myofibrillar proteins by asparagus cysteine protease, as well as the synergistic effect of asparagus juice and pH reduction induced by balsamic vinegar over time, which correlated well with WBSF and MFI results. However, it showed more intense bands similar to untreated samples, particularly in 50 kDa band, suggesting that accumulation or aggregation of low MW proteins bands came from MHC or other high MW proteins degradation (Lee et al. 2009). The MFI results as depicted in SDS-PAGE data are increased in marinated beefsteak samples due to low MW protein bands. The proteolysis of postmortem meat muscle can be attributed to calpains, lysosomal cathepsins, and multicatalytic proteinase complex. The proteolytic activity of the μ-calpain enzyme has a pivotal function in postmortem proteolysis and meat tenderization. Titin and nebulin, which are central agents in the beef tenderization, are significantly degraded by L-cathepsin instead of calpain (Li et al. 2017). Nonetheless, the main reason for the proteolytic degradation and the increased breakdown of myofibrillar proteins (MHC, alpha-actinin, desmin, actin, alpha and beta-tropomyosin) might be the cysteine protease activities of asparagus and activation of D-cathepsin enzyme in acidic pH during extended cold storage. The main target of asparagus juice is the heavy chain of myosin. However, 24 h incubation of meat myofibrillar proteins in the presence of asparagus juice could degrade all myofibrillar proteins, except for troponin-C (Ha et al. 2013). Therefore, the myosin degradation due to the asparagus juice and balsamic vinegar may contribute to the tender beefsteak. In addition, degradation of troponin-T which was supposed to produce the 30 kDa band was not virtually recognized. In beef, the 30 kDa band, which is a proteolytic product of troponin-T, was absent up to 3 days of storage and then started to appear, thereby showing a high degree of proteolysis (Soltanizadeh et al. 2008). Tsai et al. (2012) reported that titin, myosin heavy chain, desmin and alpha-actinin broke down more quickly in the samples marinated with ginger extract and 32 and 30 kDa MW components were produced by troponin-T degradation during 14 day storage at 5 °C. To conclude, bands between 11 and 25 kDa were closely contributed to the tender beefsteak. These bands came from a notable degradation of dense proteins in all asparagus juice treated samples. Han et al. (2009) and Maqsood et al. (2018) reported a 20 kDa peptide band after enzymatic treatment of lamb and camel meat during storage, respectively.
Fig. 4.
SDS-PAGE photograph of isolated myofibrillar protein fraction of beefsteak chunks marinated with asparagus juice and balsamic vinegar during 48 h storage at 4 °C
Microstructural changes of marinated meat samples
Microstructural changes in the beefsteak marinated with asparagus juice and balsamic vinegar are shown in Fig. 5. The unmarinated beefsteak filaments were tightly organized to each other indicating a high meat hardness (Fig. 5a–c, e, i, m), whereas the SEM images of beefsteak marinated with asparagus juice and balsamic vinegar showed remarkable gaps between muscle fibers (Fig. 5d, f–h, j, n, p) and larger cavities (Fig. 5k, l, o) due to degradation and solubilization of connective tissues when compared with the control sample. The above-mentioned conditions also caused myofibrillar disintegration with huge exudates increased with prolonging the storage period, indicating texture softening. In a study by Chang et al. (2010), the acidic conditions created swollen connective tissues, including perimysium or endomysium, which coated the single muscle fibers and related bundles. The perimysium contains greater than 90% collagen. The loss of connective tissues can be attributed to the higher collagen solubility (Naveena et al. 2004). Enzymatic hydrolysis of meat proteins by cysteine protease of asparagus juice could result in higher solubilization of free amino groups and HP. This phenomenon may result in loss of muscle integrity and reduced WBSF or increased tenderness. Yogesh et al. (2012) confirmed that appearance of gaps between muscle fibers can control the amount of maintained water by the muscles. Thereby, a higher WHC observed in the S1 treatment may be due to the disruption of muscle ultrastructure and larger cavities in meat as revealed by SEM images in this study. Notably, some dot-shaped components in the SEM images, might be salts, sugars, or other materials on the surface of the marinated samples, which were used as ingredients of marinade solution. Similar findings have been observed in previous studies (Kim et al. 2013; Naveena et al. 2011).
Fig. 5.
SEM images (longitudinal section) of raw beefsteak chunks marinated with asparagus juice and balsamic vinegar during 48 h storage at 4 °C (×300)
Conclusion
Marinating of beefsteak chunks with asparagus juice and balsamic vinegar at 4 °C for 48 h significantly increased the WHC, TPS, MFI and HP content but significantly decreased pH, WBSF and energy to the peak values. The SDS-PAGE findings and SEM images, respectively, exhibited alteration in the protein band patterns and high degradation of connective tissues. The tenderness results of the beefsteaks are very good in S1 and S3 samples. Accordingly, the asparagus juice and balsamic vinegar caused beefsteak tenderization by affecting myofibrillar protein and collagen tissues. In summary, the tenderization effect of asparagus juice and balsamic vinegar might be due to different mechanisms, including higher collagen solubility and proteolysis depending on the asparagus juice and balsamic vinegar level in the marinade. Therefore, marinade treatments containing 25% asparagus juice and 25% asparagus juice + 10% balsamic vinegar can be used as natural tenderizer agents for the formulation of seasonings and sauces to increase the tenderness and other quality properties of tough beefsteaks.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aminlari M, Shekarforoush SS, Gheisari HR, Golestan L. Effect of actinidin on the protein solubility, water-holding capacity, texture, electrophoretic pattern of beef, and on the quality attributes of a sausage product. J Food Sci. 2009;74(3):221–226. doi: 10.1111/j.1750-3841.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- Baker EN, Boland MJ, Calder PC, Hardman MJ. The specificity of actinidin and its relationship to the structure of the enzyme. Biochim Biophys Acta. 1980;616(1):30–34. doi: 10.1016/0005-2744(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Morton JD, Mason SL, Bekhit AED. Applied and emerging methods for meat tenderization: a comparative perspective. Compr Rev Food Sci Food Saf. 2018;17(4):841–859. doi: 10.1111/1541-4337.12356. [DOI] [PubMed] [Google Scholar]
- Chang H-J, Wang Q, Zhou G-H, Xu X-L, Li C-B. Influence of weak organic acids and sodium chloride marination on characteristics of connective tissue collagen and textural properties of beef semitendinosus muscle. J Texture Stud. 2010;41(3):279–301. doi: 10.1111/j.1745-4603.2010.00226.x. [DOI] [Google Scholar]
- Chen L, Li Z, Li X, Chen J, Everaert N, Zhang D. The effect of sarcoplasmic protein phosphorylation on glycolysis in postmortem ovine muscle. Int J Food Sci Technol. 2018;53(12):2714–2722. doi: 10.1111/ijfs.13882. [DOI] [Google Scholar]
- Doneva M, Miteva D, Dyankova S, Nacheva I, Metodieva P, Dimov K. Effıciency of plant proteases bromelain and papain on turkey meat tenderness. Biotechnol Anim Husb. 2015;31(3):407–413. doi: 10.2298/BAH1503407D. [DOI] [Google Scholar]
- Gokoglu N, Yerlikaya P, Ucak I, Yatmaz HA. Effect of bromelain and papain enzymes addition on physicochemical and textural properties of squid (Loligo vulgaris) J Food Meas Charact. 2016;11(1):347–353. doi: 10.1007/s11694-016-9403-3. [DOI] [Google Scholar]
- Ha M, Bekhit AED, Carne A, Hopkins DL. Characterization of kiwifruit and asparagus enzyme extracts, and their activities toward meat proteins. Food Chem. 2013;136(2):989–998. doi: 10.1016/j.foodchem.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Han J, Morton JD, Bekhit AED, Sedcole JR. Pre-rigor infusion with kiwifruit juice improves lamb tenderness. Meat Sci. 2009;82(3):324–330. doi: 10.1016/j.meatsci.2009.02.003. [DOI] [PubMed] [Google Scholar]
- He FY, Kim HW, Hwang KE, Song DH, Kim YJ, Ham YK, Kim SY, Yeo IJ, Jung TJ, Kim CJ. Effect of ginger extract and citric acid on the tenderness of duck breast Muscles. Korean J Food Sci Anim Resour. 2015;35(6):721–730. doi: 10.5851/kosfa.2015.35.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo ST, Kauffman RG, Kim BC, Park GB. The relationship of sarcoplasmic and myofibrillar protein solubility to color and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999;52(3):291–297. doi: 10.1016/s0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Kadıoğlu P, Karakaya M, Unal K, Babaoğlu AS. Technological and textural properties of spent chicken breast, drumstick and thigh meats as affected by marinating with pineapple fruit juice. Br Poult Sci. 2019;60(4):381–387. doi: 10.1080/00071668.2019.1621990. [DOI] [PubMed] [Google Scholar]
- Kemp CM, Sensky PL, Bardsley RG, Buttery PJ, Parr T. Tenderness—an enzymatic view. Meat Sci. 2010;84(2):248–256. doi: 10.1016/j.meatsci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ketnawa S, Rawdkuen S. Application of bromelain extract for muscle foods tenderization. Food Nutr Sci. 2011;2:393–401. doi: 10.4236/fns.2011.25055. [DOI] [Google Scholar]
- Kim HW, Choi YS, Choi JH, Kim HY, Lee MA, Hwang KE, Song DH, Lim YB, Kim CJ. Tenderization effect of soy sauce on beef M. Biceps femoris. Food Chem. 2013;139(1–4):597–603. doi: 10.1016/j.foodchem.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Lee E-J, Oh S-W, Lee N-H, Kim Y-H, Lee D-U, Yamamoto K, Kim Y-J. Application of a kiwifruit (Actinidia chinensis) to improve the textural quality on beef Bulgogi treated with hydrostatic pressure. Korean J Food Sci Anim Resour. 2009;29(3):317–324. doi: 10.5851/kosfa.2009.29.3.317. [DOI] [Google Scholar]
- Li X, Sun Y, Pan D, Wang Y, Cao J. The effect of CaCl2 marination on the tenderizing pathway of goose meat during conditioning. Food Res Int. 2017;102:487–492. doi: 10.1016/j.foodres.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Maqsood S, Manheem K, Gani A, Abushelaibi A. Degradation of myofibrillar, sarcoplasmic and connective tissue proteins by plant proteolytic enzymes and their impact on camel meat tenderness. J Food Sci Technol. 2018;55(9):3427–3438. doi: 10.1007/s13197-018-3251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveena BM, Mendiratta SK, Anjaneyulu ASR. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome) Meat Sci. 2004;68(3):363–369. doi: 10.1016/j.meatsci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Sen AR, Muthukumar M, Babji Y, Kondaiah N. Effects of salt and ammonium hydroxide on the quality of ground buffalo meat. Meat Sci. 2011;87(4):315–320. doi: 10.1016/j.meatsci.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Olson DG, Parrish JRFC. Relationship of myofibril fragmentation index to measures of beefsteak tenderness. J Food Sci. 1977;42(2):506–509. doi: 10.1111/j.1365-2621.1977.tb01533.x. [DOI] [Google Scholar]
- Rawdkuen S, Jaimakreu M, Benjakul S. Physicochemical properties and tenderness of meat samples using proteolytic extract from Calotropis procera latex. Food Chem. 2013;136(2):909–916. doi: 10.1016/j.foodchem.2012.08.077. [DOI] [PubMed] [Google Scholar]
- Serdaroglu M, Abdraimov K, Onenc A. The effects of marinating with citric acid solutions and grapefruit juice on cooking and eating quality of turkey breast. J Muscle Foods. 2007;18(2):162–172. doi: 10.1111/j.1745-4573.2007.00074.x. [DOI] [Google Scholar]
- Sikes A, Tornberg E, Tume R. A proposed mechanism of tenderizing post-rigor beef using high pressure-heat treatment. Meat Sci. 2010;84(3):390–399. doi: 10.1016/j.meatsci.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Soltanizadeh N, Kadivar M, Keramat J, Fazilati M. Comparison of fresh beef and camel meat proteolysis during cold storage. Meat Sci. 2008;80(3):892–895. doi: 10.1016/j.meatsci.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Sultana A, Nakanishi A, Roy BC, Mizunoya W, Tatsumi R, Ito T, Tabata S, Rashid H, Katayama S, Ikeuchi Y. Quality improvement of frozen and chilled beef biceps femoris with the application of salt-bicarbonate solution. Asian-Australas J Anim Sci. 2008;21(6):903–911. doi: 10.5713/ajas.2008.70544. [DOI] [Google Scholar]
- Sun Q, Zhang B, Yan QJ, Jiang ZQ. Comparative analysis on the distribution of protease activities among fruits and vegetable resources. Food Chem. 2016;213:708–713. doi: 10.1016/j.foodchem.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Toohey ES, Kerr MJ, van de Ven R, Hopkins DL. The effect of a kiwi fruit based solution on meat traits in beef M. Semimembranosus (topside) Meat Sci. 2011;88(3):468–471. doi: 10.1016/j.meatsci.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Tsai L-L, Yen N-J, Chou R-GR. Changes in muscovy duck breast muscle marinated with ginger extract. Food Chem. 2012;130(2):316–320. doi: 10.1016/j.foodchem.2011.07.044. [DOI] [Google Scholar]
- Verzelloni E, Tagliazucchi D, Conte A. From balsamic to healthy: traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem Toxicol. 2010;48:2097–2102. doi: 10.1016/j.fct.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Yamashita Y, Takeda I, Kiso H. Proteolytic enzymes in green asparagus, kiwi fruit and miut: occurrence and partial characterization. Agric Biol Chem. 1982;46(8):1983–1986. doi: 10.1080/00021369.1982.10865376. [DOI] [Google Scholar]
- Yogesh K, Jha SN, Yadav DN. Antioxidant activities of Murraya koenigii (L.) spreng berry extract: application in refrigerated (4 ± 1°C) stored meat homogenates. Agric Res. 2012;1(2):183–189. doi: 10.1007/s40003-012-0018-6. [DOI] [Google Scholar]
- Yonezawa H, Kaneda M, Uchikoba T. A cysteine protease from young stems of asparagus: isolation, properties, and substrate specificity. Biosci Biotechnol Biochem. 1998;62(1):28–33. doi: 10.1271/bbb.62.28. [DOI] [PubMed] [Google Scholar]
- Zhao C-C, Benjakulb S, Eun J-B. Changes in protein compositions and textural properties of the muscle of skate fermented at 10°C. Int J Food Prop. 2019;22(1):173–185. doi: 10.1080/10942912.2019.1575396. [DOI] [Google Scholar]
- Żochowska-Kujawska J, Kotowicz M, Lachowicz K, Sobczak M. Influence of marinades on shear force, structure, and sensory properties of home-style jerky. Acta Sci Pol Technol Aliment. 2017;16(4):413–420. doi: 10.17306/J.AFS.2017.0508. [DOI] [PubMed] [Google Scholar]
- Zou Y, Zhang W, Kang D, Zhou G. Improvement of tenderness and water-holding capacity of spiced beef by the application of ultrasound during cooking. Int J Food Sci Technol. 2018;53(3):828–836. doi: 10.1111/ijfs.13659. [DOI] [Google Scholar]





