Abstract
This study aimed to investigate the chemical composition, using GC–MS, and anti-biofilm potential of black cardamom essential oil (BCEO) against biofilms of Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748 through inhibition of bacterial quorum sensing. GC–MS quantification demonstrated that BCEO contains 1,8-cineole (44.24%), α-terpinyl acetate (12.25%), nerolidol (6.03%), and sabinene (5.96%) as the major bioactive compounds. Antioxidant assays for BCEO revealed the total phenolic and flavonoid mean values were 1325.03 ± 7.69 mg GAE 100/g and 168.25 ± 5.26 mg CE/g, respectively. In regards to antimicrobial potential, Candida albicans was the most sensitive species compared to Streptococcus mutans, Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus, and Salmonella Typhimurium with the following zones of inhibition; 14.4 ± 0.52, 13.2 ± 0.42, 11.2 ± 0.28, 11.0 ± 0.52, 8.2 ± 0.24 and 6.6 ± 0.18 mm in diameter, respectively. Biofilm inhibition by BCEO was concentration-dependent, when various concentrations of 0.03, 0.06, 0.12, 0.25 and 0.5% were applied, 33.67, 34.14, 38.66, 46.65 and 50.17% of Salmonella Typhimurium biofilm was inhibited, while 47.31, 54.15, 76.57, 83.36 and 84.63% of Escherichia coli biofilm formation was prevented. Chromobacterium violaceum ATCC 12,472 and its product violacein, was used as a microbial indicator for enhancement or inhibition of quorum sensing. Our data showed that 0.5% of BCEO inhibited violacein production without influencing the growth of Chromobacterium violaceum, while 1% of BCEO, caused 100% inhibtion of violacein production together with 30% inhibition of growth. This study shows that BCEO possesses promising antioxidant and antimicrobial potential, and found anti-biofilm activities linked to the quenching of the quorum sensing system of E. coli and S. Typhimurium.
Keywords: Black cardamom essential oil, GC–MS, Antioxidants, Anti-biofilm, Quorum sensing inhibition
Introduction
Essential oils and bioactive phytochemicals may contain natural antioxidants and antimicrobial compounds, which have been hypothesized to exhibit positive health effects and minimize the risk of chronic infections (Yousefi et al. 2019; Liu 2013). Several studies have reported on the desirable health effects of spices and aromatic herbs, including; gastro-intestinal protective (anti-ulcer), antiplatelet aggregation, anticancer, antihypercholesterolemic, and antihypertensive effects (Sharma et al. 2011; Chakraborty et al. 2019; Yousefi et al. 2019).
Cardamom (Amomum subulatum), commonly named as black cardamom, belongs to the family Zingiberaceae. India is the leading producer of black cardamom, manufacturing 4000 metric tons (MT) yearly, followed by Nepal and Bhutan at 2500 MT and 1000 MT, respectively (Thomas et al. 2009). Various techniques including hydrodistillation, conventional solvent extraction, and supercritical fluid extraction (SFE) have been used for the extraction of essential oils. Among these techniques, SFE has attracted the attention of researchers due to its potential advantages of less degradation, higher solvation/mass transfer, no clean-up steps, and eco-friendly nature. SFE is an efficient qualitative and quantitative technique when selective conditions, i.e. time, temperature, and pressure are controlled (Yousefi et al. 2019).
Essential oils and phytochemicals which contain phenolic acids, flavonoids, and stilbenes have been reported with antioxidant and antimicrobial potentials (Aneja and Radhika 2009; Liu 2013; Abdullah et al. 2017). Plant extracts containing flavonoids; kaempferol, quercetin, luteolin, and pelargonidin possessed antioxidant activities by increasing glutathione, reducing malondialdehyde levels, and preventing some microbial infections (Hossain et al. 2008; Neuman and Gragg 2016). During the last two decades, the impact of oxidative stress on the pathophysiology of various maladies, such as Alzheimer’s disease, atherosclerosis, diabetes, and hypertension has been discussed (Ramon 2009). Reactive oxygen species (ROS) are produced from the cellular metabolism of oxygen causing an imbalance of antioxidants and pro-oxidants, leading to oxidative stress. Excessive production of ROS has a substantial impact on cell aging, and can negatively impact critical cellular processes, through direct macromolecular damage and regulation of various cellular signaling pathways (Ray et al. 2012). Furthermore, chronic infections associated with bacterial biofilm formation and microbial resistance to conventional antibiotics reflect the urgent need for replacement of traditional antimicrobials (Livermore 2011; Neuman and Gragg 2016; Jamal et al. 2018). Some studies have reported the antimicrobial potential of essential oils and phytochemicals against Bacillus cereus, Candida albicans, Salmonella Typhimurium, Escherichia coli, Streptococcus mutans, Staphylococcus aureus and Aspergillus terreus (Aneja and Radhika 2009; Abdullah et al. 2017; Yousefi et al. 2019).
A biofilm is defined as a spatial structure of mono- or multi-microbial communities embedded in an excreted extracellular matrix which provides protection to microorganisms within the biofilm against harsh environmental factors (Miquel et al. 2016). The process of biofilm formation consists of five stages; microbial adhesion, exopolysaccharide production, spatial structure formation, biofilm maturation, and cellular detachment, after which a new process of biofilm formation can be initiated on another available surface, furthering the spread of the microbial community. During biofilm formation, bacterial flagella or pili play an important role in bacterial adhesion through the activation of van der Waal’s forces and/or electrostatic interactions. In addition, the hydrophobicity of surfaces may reduce the repulsive force between bacterial cells, further facilitating biofilm attachment (Jamal et al. 2018). It has been reported that the biofilm formation process is under the control of bacterial communication systems collectively known as quorum sensing (QS) (Li et al. 2018). The QS system of bacterial cells has an important role in the production, release, and detection of chemical signaling molecules, called "auto-inductors" (Whiteley et al. 2017). Therefore, quorum quenching, defined as all processes that are involved in the disturbance of the QS systen, is related to inhibition of biofilm formation, and several mechanisms have been reported for QS inhibition including; (i) the inhibition of signal molecule synthesis; (ii) inactivation or degradation of signal molecules; (iii) prevention of the signal molecules binding with their receptor analogues; and, (iv) blocking the signal transduction cascades (Lade et al. 2014; Zhao et al. 2015; Torres et al. 2016).
Quorum sensing plays a significant role in biofilm formation, which is directly linked to persistant chronic infections (Jamal et al. 2018; Algburi et al. 2017). Recently, researchers have focused on finding quorum quenching compounds in order to inhibit microbial communication and prevent subsequent biofilm formation (Rahman et al. 2017). In this study, the authors have investigated the chemical profile and anti-biofilm potentials of black cardamom essential oil (BCEO), as safe alternative substances against biofilms of the tested pathogenic bacterial strains.
Materials and methods
Materials
Black cardamom was purchased from the market (Faisalabad, Punjab, Pakistan) and crushed into fine powder after dust cleaning, essential oil was obtained and used throughout the study. HPLC grade reagents used in the analysis were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (Tokyo, Japan).
Supercritical fluid extraction of BCEO
Black cardamom essential oil was obtained by the supercritical fluid extraction technique (SFT-150) according to Abdullah et al. (2017), using 99.8% pure CO2 at a constant pressure (300 bar), time (60 min), and temperature (30 °C). The samples (100 g) were placed in an extraction vessel, then CO2 was liquefied through optimization of time, pressure, and temperature conditions which accelerated the mass transfer of essential oil. The essential oil was placed in vials and kept in a refrigerator at 4 °C until it was used.
GC–MS quantification and phytochemicals screening of BCEO
The essential oil (10 µL) was diluted in tertiary butyl methyl ether and analyzed by GC–MS technique following the protocol of Adams (2017) with some modifications. Briefly, samples (1 μL) of diluted essential oil were injected by an auto sampler (AOC 6000) into a Shimadzu SH-Rxi-5Sil MS column (30 m long, 0.25 mm ID, 0.25 µm film thickness), and the inlet temperature was 220 °C. The temperature program was initially held at 35 °C for 4 min, which increased from 35 °C to 250 °C with a rate of 20 °C/min. Helium was used as a carrier with a constant flow rate of 1 mL/min. An ionization energy of 70 eV was used to perform ionization in electron impact mode at 230 °C. Mass spectra was acquired in full scan mode in the mass (m/z) range of 35—450. The identification of each component was made by matching spectral data with mass spectral libraries, and individual identity was confirmed by comparison of its Kovat's index with the compounds from literature (Adams 2017), and library (Wiley275.L). The compounds present in the table were listed in order of elution from the SH-Rxi-5Sil MS column. The relative percentage of each component was obtained by peak integration method. Furthermore, the presence of antioxidant compounds in BCEO was determined in terms of total phenolic contents (TPC) and total flavonoids (TF), using Folin-Ciocalteu reagent and the aluminum chloride colorimetric method (Chanda and Dave 2009).
Evaluation of the antimicrobial potential of BCEO
Bacterial strains and growth conditions
In this study, the following microorganisms were used; Chromobacteriun violaceum ATCC 12,472, Staphylococcus aureus ATCC 29,213, Listeria monocytogenes Scott A, Streptococcus mutans ATCC 33,402, Bacillus cereus (lab isolate), Escherichia coli O157:H7, Pseudomonas aeruginosa ATCC 14,213, Salmonella Typhimurium JSG 1748, and Candida albicans ATCC 11,006 to determine antimicrobial potential of BCEO. The microorganisms were selected as representative Gram-positive and Gram-negative pathogens of medical importance and those found in foodborne outbreaks. Tryptic soy broth (TSB) was used to maintain the growth of selected microbial strains. The inoculated culture media was incubated with shaking for 18–24 h at 37 °C. The spot-plate method was performed for bacterial cells count using tryptic soy agar (TSA) (Remel™ ThermoFisher Scientific, Waltham, MA, USA). Luria Bertani (LB, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) broth was used as a medium to propagate C. violaceum, which was incubated for a period of 24 h at 27 °C. Violacein production inhibition was an indicator for quorum sensing inhibition in C. violaceum bacteria.
Disk diffusion assay
A disk diffusion assay was performed to assess antimicrobial activity of BCEO against the tested microbial strains, following CLSI guidelines (2012) with minor modifications. The microbial strains were incubated overnight at 37 °C and diluted (1:1000) with TSB to achieve the concentration ~ 106 CFU/mL. The sterile cotton swab applicator saturated with microbial suspension (106 CFU/mL) was used and streaked in three directions to cover the whole surface of TSA with microbial cells. Moreover, negative and positive controls were also used in this assay. Sterile filter paper disks (BD BBL™ Taxo™ Blank Discs, ThermoFisher Scientific, Waltham, MA, USA) were saturated with pre-determined concentrations of BCEO. Then, BCEO disks were placed on the TSA. Afterwards, all agar plates were incubated for 24 h at 37 °C aerobically. After incubation, the diameter of the zones of microbial inhibition were carefully noted using a digital caliper.
Biofilm inhibition assay
The biofilm formation assay was conducted following the method of Algburi et al. (2017) with minor modifications. Briefly, S. Typhimurium JSG 1748 and E. coli O157:H7 were grown aerobically in LB for 24 h at 37 °C. The overnight grown cells were diluted 1:40 (v/v) with fresh culture medium. Aliquots of 200 µL of bacterial growth dilutions were added into a 96-well tissue culture treated microplate and incubated for 48 h at 37 °C. After incubation, the planktonic cells were removed and the microplate was gently washed twice with fresh culture media. The plate was heat-fixed for 60 min at 60 °C, and 125 µL of 0.1% crystal violet was added into each well, and the plate was incubated for another 20 min at room temperature. Then, the plate was washed with sterile water 3—4 times, after which 200 µL ethanol (95%) was added into each well to solubilize the biofilm-stained crystal violet. Following solubilization of crystal violet, the plate was incubated for 30 min at 4 °C. Aliquots of 100 µL of the solubilized crystal violet were transferred separately from each well to a new 96-well microtiter plate and the absorbance was measured at 595 nm by a microplate reader (Model 550, Bio-Rad Laboratories, Hercules, CA). This experiment was performed at least three times and in duplicate.
Quorum sensing inhibition assay
The quorum sensing inhibition assay was conducted following the protocol of Zhu et al. (2011) with minor modifications. Briefly, C. violaceum ATCC 12,472 was cultured in LB broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and incubated for 24 h at 27 °C. The overnight growth of bacteria was diluted (1:1000 v/v) with LB broth to achieve (106 CFU/mL). The spot plate method was utilized to determine the total number of CFU/mL. Then, the BCEO was dissolved in 95% ethanol and further diluted into LB until achieving 10 mg/mL (1%). Then, the stock solution was twofold diluted with LB into a 48-well microtiter plate. A 500 µL suspension of diluted bacterial cells (106 CFU/mL) were added into each well, which was previously treated with 500 µL of various concentrations of BCEO. The microplate was incubated aerobically overnight at 27 °C. After incubation, 750 µL from each well was centrifuged for 5 min, 8000 × g, at room temperature. The supernatant was removed, and the precipitates were again re-suspended in 750 µL of DMSO and vigorously vortexed for 2 min. The sample was centrifuged again for 5 min at 8000 × g, at room temperature. Finally, 200 µL of the collected supernatants were poured in 96-wells plate and the optical density was measured at 595 nm by using a microplate reader (Model 550, Bio-Rad Laboratories, Hercules, CA). To confirm that BCEO inhibited quorum sensing but not the growth of C. violaceum, the microbial cells pellets were re-suspended in 750 µL sterile water, vortexed and microbial cells density was measured at 595 nm using a microplate reader.
Statistical analysis
The experiments were performed at least three times and in duplicate. The calculations and the statistical analysis were made using Microsoft Excel and shaped with SigmaPlot 11.0 (Systat Software Inc., Chicago, IL, USA).
Results and discussion
Identification of the major bioactive components of BCEO using GC–MS
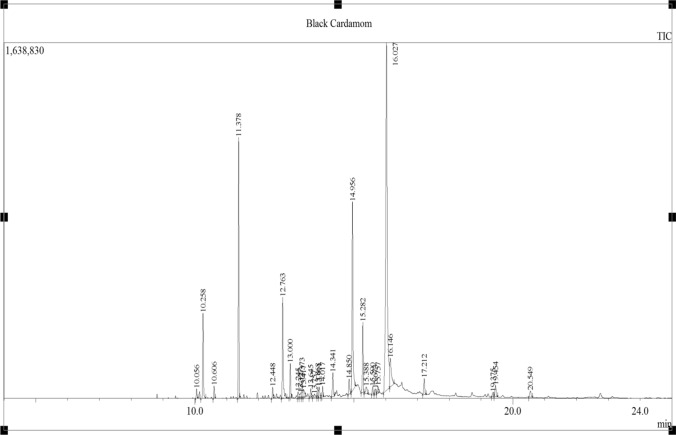
GC–MS was utilized for characterization and quantification of BCEO constituents; seventeen compounds (represented 98.47% of the essential oil) in total, were identified (Table 1). The major bioactive compounds were 1,8-cineole (44.24%), α-terpinyl acetate (12.25%), neridol (6.03%), and sabinene (5.96%), presented in Table 1. In addition, several natural compounds were also recognized including; limonene, α-pinene, α-terpineol, β-pinene, g-terpinene, linalool acetate, hexadecanol acetate, methyl linoleate, long chain hydrocarbons, and some fatty acids (Fig. 1).
Table 1.
GC–MS characterization and quantification of black cardamom essential oil (BCEO)
| Peak no. | Retention time | Bioactive compounds | Composition (%) |
|---|---|---|---|
| 1 | 10.056 | Limonene | 1.02 |
| 2 | 10.258 | α-Pinene | 3.41 |
| 3 | 10.606 | Linalool acetate | 1.23 |
| 4 | 11.378 | α-Terpinyl acetate | 12.25 |
| 5 | 12.448 | Hexadecanol acetate | 1.13 |
| 6 | 12.763 | Sabinene | 5.96 |
| 7 | 13.000 | Methyl linoleate | 3.11 |
| 8 | 13.373 | b-Pinene | 2.82 |
| 9 | 14.341 | α-Terpineol | 2.85 |
| 10 | 14.850 | n-Hexadecanoic acid | 2.74 |
| 11 | 14.956 | Nerolidol | 6.03 |
| 12 | 15.282 | g-Terpinene | 4.30 |
| 13 | 15.590 | Fatty acids (C18) | 2.81 |
| 14 | 16.027 | 1,8-Cineole | 44.24 |
| 15 | 16.146 | Fatty acids (C18) | 2.25 |
| 16 | 17.212 | Long chain hydrocarbons | 1.24 |
| 17 | 19.454 | Long chain hydrocarbons | 1.08 |
% = Relative percentage, individual component computed from the peak area of the chromatogram
Fig. 1.
Chromatogram indicating black cardamom essential oil components analyzed by Shimadzu GC 2010 Plus and GCMS-TQ8040 using Shimadzu SH-Rxi-5Sil MS column (30 m long, 0.25 mm ID, 0.25 μm film thickness). The area of the peak in the chromatogram reflect quantity (relative percentage, %) of each component present in the oil (for more details, see Table 1)
The data in Table 1 were in agreement with studies which described that 1,8-cineole and α-terpinyl acetate were the major bioactive compounds in BCEO. Rout et al. (2003) found that the cardamom essential oil mainly contains 1,8-cineole (38.7%), spathulenol (8.3%), 4-terpineol (4.5%) and α-pinene (2.8%) and some others compounds, which represented 98% of the total EO. Likewise Naik et al. (2004), reported that the major bioactive compounds of BCEO were 1,8-cineole (37.7%), α-terpineol (12.9%), β-pinene (13.8%), spathulenol (8.5%), germacrene D (3.3%), 4-terpineol (4.6%), and α-pinene (2.8%). Our results were also similar to the findings of Gilani et al. (2006) who reported 1,8-cineol (55.37%), α-terpinyl acetate (11.66%) and limonene (6.05%) as the main components of BCEO. Some studies showed that the levels of 1,8-cineole and α-terpinyl acetate in essential oil were associated with the plant's origin. The cardamom essential oil of Indian origin contained 1,8-cineole (36.30%) and α-terpinyl acetate (31.30%). Chempakam and Sindhu (2008) in their study elaborated that 1,8-cineole and α-terpinyl acetate varied between 26.50–63.30% and 23.20–52.50% of BCEO, respectively. The study of Parthasarathy et al. (2008) found that BCEO extracted by steam distillation using GC–MS, contained 26 components, including 1,8-cineole (61.50%) which was identified as a principle component. The difference between our results and the previous findings may be due to several factors, such as cardamom origin, extraction method (SFE and conventional), storage, and experimental conditions.
Identification of antioxidant substances in BCEO
The average values of total phenolic contents (TPC) and total flavonoids (TF) in BCEO were 1325.03 ± 7.69 mg GAE 100/g and 168.25 ± 5.26 mg CE/g, respectively. Our results were in agreement with Bhatti et al. (2010) who found that the phenolic contents in 70% methanolic extracts of cardamom cultivar were 1660 ± 0.05 mg GAE 100/g. Moreover, our data were also close to the results reported by Tiveron et al. (2012) who stated that the phenolic content in Brazilian vegetable extract was 1279.53 mg GAE 100/g. Similarly, Ho et al. (2008) showed that flavonoids were present in cardamom at 226.8 mg CE/g.
Polyphenols, and flavonoids are the bioactive moieties which possessed a significant role in several biological functions, particularly antiradical activities. Oral absorption of quercetin has been reported in humans (Moon et al. 2008). Luteolin is another orally absorbed flavonoid which has been used as treatment for many chronic infections (Ying et al. 2008). Surprisingly, numerous polyphenols have been evaluated for reducing obesity and inhibiting inflammatory component in animal models. Previously, quercetin was orally administered to Zucker rats to improve the health status and control dyslipidemia, hyperinsulinemia, and hypertension (Rivera et al. 2008).
Evaluation of the antimicrobial potential of BCEO
BCEO inhibited growth of the targeted pathogens
The disk diffusion assay results reflected the inhibitory effects of BCEO against tested pathogenic microorganisms. Briefly, C. albicans ATCC 11,006 was the most sensitive pathogen to BCEO compared to S. mutans ATCC 33,402, S. aureus ATCC 29,213, L. monocytogenes Scott A, B. cereus, and S. Typhimurium JSG 1748 with the following zones of inhibition; 14.4 ± 0.52, 13.6 ± 0.42, 11.2 ± 0.28, 11 ± 0.52, 8.2 ± 0.24 and 6.6 ± 0.18 mm in diameter, respectively. However, BCEO did not inhibit the growth of P. aeruginosa ATCC 14,213 indicating that the bacterial strain was highly resistant to the antimicrobial agent.
BCEO inhibits biofilm formation by S. Typhimurium JSG 1748 and E. coli O157:H7
BCEO inhibited biofilm formation by S. Typhimurium JSG 1748 and E. coli O157:H7 in a concentration-dependent manner. When various concentrations of BCEO (0.03, 0.06, 0.12, 0.25 and 0.5%) were applied, 33.67, 34.14, 38.66, 46.65 and 50.17% of S. Typhimurium JSG 1748 biofilm were inhibited, respectively. The same concentrations of BCEO prevented 47.31, 54.15, 76.57, 83. 36 and 84.63%, respectively, of biofilm formation by E. coli O157:H7 (Table 2). However, BCEO did not inhibit the growth of the planktonic cells of S. Typhimurium JSG 1748 and E. coli O157:H7, and the results showed that greater biofilm inhibition was achieved by increasing the BCEO concentration, which reflect inhibition of virulence factors without interrupting bacterial growth (Figs. 2, 3).
Table 2.
BCEO effects on the biofilm formation inhibition and growth of the planktonic cells
| Bacterial strains | Concentration (mg/mL) | Biofilm integrity (%) | Inhibition (%) | Log10 CFU/mL |
|---|---|---|---|---|
| S. Typhimurium | 0.0 | 100 ± 0.00 | – | 9.65 ± 0.07 |
| 0.313 | 66.33 ± 2.62 | 33.67 | 9.68 ± 0.05 | |
| 0.625 | 65.86 ± 4.37 | 34.14 | 9.69 ± 0.06 | |
| 1.25 | 61.34 ± 4.17 | 38.66 | 9.74 ± 0.04 | |
| 2.5 | 53.35 ± 6.11 | 46.65 | 9.70 ± 0.02 | |
| 5.0 | 49.83 ± 6.26 | 50.17 | 9.69 ± 0.07 | |
| E. coli | 0.0 | 100 ± 0.00 | – | 9.71 ± 0.02 |
| 0.313 | 52.69 ± 1.43 | 47.31 | 9.71 ± 0.07 | |
| 0.625 | 45.85 ± 3.25 | 54.15 | 9.68 ± 0.01 | |
| 1.25 | 23.43 ± 4.32 | 76.57 | 9.65 ± 0.07 | |
| 2.5 | 16.64 ± 2.58 | 83.36 | 9.67 ± 0.07 | |
| 5.0 | 15.37 ± 6.02 | 84.63 | 9.72 ± 0.06 |
BCEO = Black cardamom essential oil
Fig. 2.
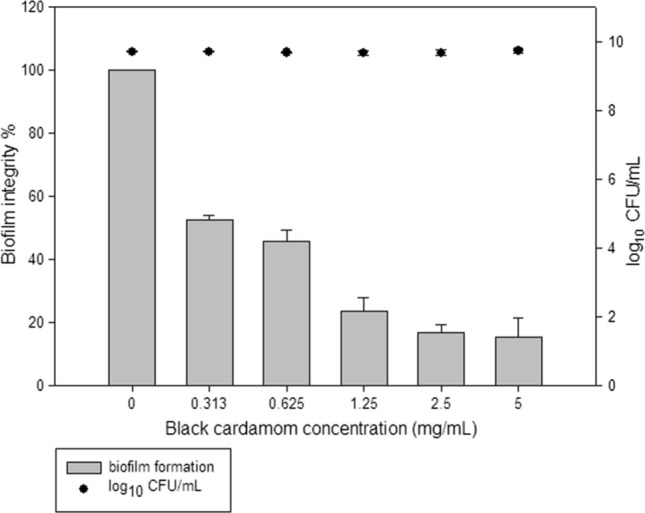
Antimicrobial activity of black cardamom essential oil against planktonic and biofilm associated cells of E. coli O157:H7
Fig. 3.

Antimicrobial activity of black cardamom essential oil against planktonic and biofilm associated cells of S. Typhimurium JSG 1748
BCEO inhibits quorum sensing in C. violaceum ATCC 12,472
BCEO was evaluated as a quorum sensing inhibitor utilizing C. violaceum ATCC 12,472 as a biological indicator. In order to identify the minimum biofilm inhibitory concentration (MIC-B), several concentrations of BCEO were prepared, and their quorum sensing inhibitory effects were evaluated. Inhibition of violacein production by C. violaceum was an indicator for the inhibition of quorum sensing in Gram-negative bacteria; E. coli and S. Typhimurium. Our data showed that 0.5% of BCEO prevented 35% of violacein production without influencing the growth of C. violaceum. When 1% of BCEO was utilized, a complete inhibition (100%) of violacein production was noticed, while only 30% of C. violaceum growth inhibition (< 1 log reduction) was reported (Fig. 4).
Fig. 4.
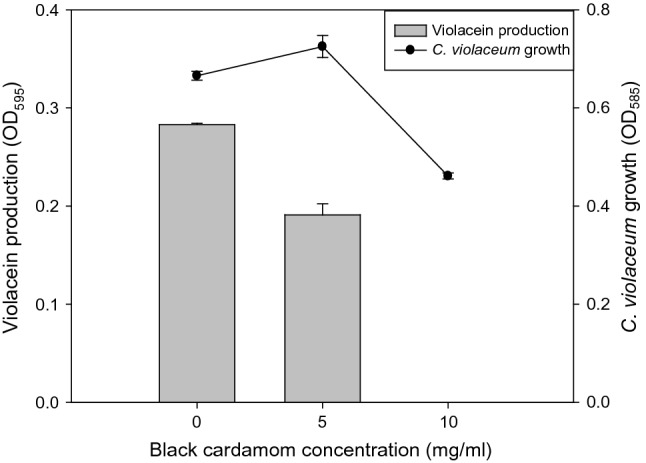
Quorum sensing inhibition (violacein production inhibition of C. violaceum ATCC 12,472) by black cardamom essential oil
Quorum sensing plays an important role in the regulation of biofilm formation (please see the recent review of Passos da Silva et al. (2017)). Therefore, quorum quenching is an important strategy, when it comes to prevention of biofilm formation. Recently, Rahman et al. (2017) reported that 4 mg/ mL of a cardamom extract prevented 51.96%, 47.06% and 45.28% of biofilm associated with S. Typhimurium ATCC 50,013, S. aureus ATCC 6538 and P. aeruginosa ATCC 9027, respectively. Kumar et al. (2014) reported that phenolic derivatives of ginger including; zingerone and gingerol exhibited anti-quorum sensing activities at 500 ppm by inhibiting 35% and 52.5%, respectively of biofilm-associated pathogens. Furthermore, a study of Soni et al. (2013) found that carvacrol, a component of oregano oil, effectively inhibited biofilm formation of Staphylococcus and Salmonella. Similarly, Niu and Gilbert (2004) stated that essential oil components (phytochemicals) inhibit biofilm formation by interrupting bacterial communication through inhibition of peptidoglycan synthesis.
It has been found that essential oils and phytochemicals contain natural bioactive substances which significantly act as anti-quorum sensing agents and inhibit biofilm formation (Abdullah et al. 2017; Kerekes et al. 2013). The halogenated furanones from an algae (Delispea pulchra) have proteolytic and anti-quorum sensing effects (Belapurkar et al. 2014). Moreover, Origanum majorana, Salvia sclarea, and Juniperus communis essential oils demonstrated anti-quorum sensing activities through inhibition of biofilm formation by the tested microbes (Kerekes et al. 2013). Likewise, the essential oil of Syzygium aromaticum showed a quorum sensing inhibitory effect (Khan et al. 2009). The reported mechanisms of quorum sensing and subsequent biofilm formation inhibition includes (i) degradation or inactivation of the quorum sensing signals by changing the medium pH (chemically), temperature (physically), or enzymatic content (Torres et al. 2016). In addition, (ii) inhibition of signaling substances production, and (iii) modification of the binding sites of signal substances through competitive inhibition (Zhao et al. 2015).
Conclusion
The GC–MS analysis of BCEO identified seventeen compounds (represented 98.47% of BCEO) in total, while 1,8-cineole (44.24%), α-terpinyl acetate (12.25%), neridol (6.03%), and sabinene (5.96%), were the major components along with several others, which may have a promising antioxidant potential. C. albicans ATCC 11,006 was the most sensitive among the tested species, followed by S. mutans ATCC 33,402, S. aureus ATCC 29,213, L. monocytogenes Scott A, B. cereus and S. Typhimurium JSG 1748. The biofilm formation of E. coli and S. Typhimurium was prevented in a concentration-dependent manner when various concentrations of BCEO were applied. Moreover, BCEO inhibited violacein production without influencing the growth of C. violaceum, indicating that BCEO inhibited biofilm formation but not the growth of planktonic cells (non-biofilm cells) through a quorum quenching mechanism. Thus, BCEO bioactive compounds could possibly be used to develop novel organic antimicrobials and alternatives to traditional antibiotics to reduce their risky side effects, including antibiotic resistance and infection recurrence. In addition, it could also be used to improve the antimicrobial status, enhance shelf life as an alternative to chemical preservatives, and may impart a pleasant aroma to commercial edibles viz. food, meat, and dairy products.
Acknowledgements
The authors gratefully recognized the support of Higher Education Commission (HEC), Pakistan and Rutgers, The State University of New Jersey, USA to accomplish present research work. Abdullah was financially supported by the postdoc office of the Zhejiang University, Hangzhou, China. MLC was supported by the Ministry of Science and Higher Education of the Russian Federation (Project Number 075-15-2019-1880).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdullah, Email: abdullah_ch2002@yahoo.com.
Warda Mustfa, Email: wardamustfa18@gmail.com.
References
- Abdullah AA, Butt MS, Shahid M, Huang Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J Food Sci Technol. 2017;54:2306–2315. doi: 10.1007/s13197-017-2668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. TX: Texensis Publishing Gruver; 2017. [Google Scholar]
- Algburi A, Zehm S, Netrebov V, Bren AB, Chistyakov V, Chikindas ML. Subtilosin prevents biofilm formation by inhibiting bacterial quorum sensing. Probiotics Antimicrob Protiens. 2017;9:81–90. doi: 10.1007/s12602-016-9242-x. [DOI] [PubMed] [Google Scholar]
- Aneja K, Radhika J. Antimicrobial activity of Amomum subulatum and Elettaria cardamomum against dental caries causing microorganisms. Ethnobotan Leafl. 2009;13:840–849. [Google Scholar]
- Belapurkar R, Tale VS, Madkaikar R. Exploiting quorum sensing to inhibit the bacterial pathogens. Int J Curr Microbiol App Sci. 2014;3:453–458. [Google Scholar]
- Bhatti HN, Zafar F, Jamal MA. Evaluation of phenolic contents and antioxidant potential of methanolic extracts of green cardamom (Elettaria cardamomum) Asian J Chem. 2010;22:4787–4794. [Google Scholar]
- Chakraborty S, Paul K, Mallick P, Pradhan S, Das K, Chakrabarti S, Bhattacharjee P. Consortia of bioactives in supercritical carbon dioxide extracts of mustard and small cardamom seeds lower serum cholesterol levels in rats: new leads for hypocholesterolaemic supplements from spices. J Nutr Sci. 2019;8:1–15. doi: 10.1017/jns.2019.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties An overview. Afr J Microbiol Res. 2009;3:981–996. [Google Scholar]
- Chempakam B, Sindhu S. Small and large cardamom; Chemistry of Spices. Cambridge, MA: CABI; 2008. pp. 41–69. [Google Scholar]
- CLSI (2012) Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Eleventh Edition. M02-A11
- Gilani SR, Shahid I, Javed M, Mehmud S, Ahmed R. Antimicrobial activities and physico-chemical properties of the essential oil from Amomum subulatum. Int J Appl Chem. 2006;2:81–86. [Google Scholar]
- Ho S-C, Tzung-Hsun T, Po-Jung T, Chih-Cheng L. Protective capacities of certain spices against peroxynitrite-mediated biomolecular damage. Food Chem Toxicol. 2008;46:920–928. doi: 10.1016/j.fct.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ahmad W, Andleeb S, Jali F, Imran M, Nazaw MA, Hussain T, Ali M, Rafiq M, Kamil MA. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Kerekes EB, Deak E, Tak M, Tserennadmid R, Petkovits T, Vagvolgyi C, Krisch J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related microorganisms. J Appl Microbiol. 2013;115:933–942. doi: 10.1111/jam.12289. [DOI] [PubMed] [Google Scholar]
- Kumar NV, Murthy PS, Manjunatha JR, Bettadaiah BK. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014;15:451–457. doi: 10.1016/j.foodchem.2014.03.039. [DOI] [PubMed] [Google Scholar]
- Lade H, Paul D, Kweon JH. Quorum quenching mediated approaches for control of membrane biofouling. Int J Biol Sci. 2014;10:550–565. doi: 10.7150/ijbs.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang D, Liu N, Ma Y, Ding T, Mei Y, Li J. Inhibition of quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas fluorescens by cinnamaldehyde. Int J Food Microbiol. 2018;269:98–106. doi: 10.1016/j.ijfoodmicro.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384–392. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM. Discovery research: the scientific challenge of finding new antibiotics. J antimicrob chemother. 2011;66:1941–1954. doi: 10.1093/jac/dkr262. [DOI] [PubMed] [Google Scholar]
- Miquel S, Lagrafeuille R, Souweine B, Forestier C. Anti-biofilm activity as a health issue. Front Microbiol. 2016;7:592–605. doi: 10.3389/fmicb.2016.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YJ, Wang L, DiCenzo R, Morris ME. Quercetin pharmacokinetics in humans. Biopharm Drug Dispos. 2008;29:205–217. doi: 10.1002/bdd.605. [DOI] [PubMed] [Google Scholar]
- Naik JP, Rao LJM, Kumar M, Sampathu S. Chemical composition of the volatile oil from the pericarp (husk) of large cardamom (Amomum subulatum Roxb.) Flav Fragr J. 2004;19:441–444. doi: 10.1002/ffj.1336. [DOI] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as source of new drugs over the period 1981–2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Niu C, Gilbert ES. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol. 2004;70:6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy VA, Chempakam B, Zachariah TJ. Chemistry of Spices. Cambridge, MA: CABI; 2008. [Google Scholar]
- Passos da Silva D, Schofield MC, Parsek MR, Tseng BS. An update on the sociomicrobiology of quorum sensing in gram-negative biofilm development. Pathogens. 2017;6:51. doi: 10.3390/pathogens6040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MRT, Lou Z, Yu F, Wang P, Wang H. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J Biol Sci. 2017;24:324–330. doi: 10.1016/j.sjbs.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon R. Oxidative stress and antioxidants: Their role in human disease. NY, USA: Nova publishers; 2009. [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Moron R, Sanchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- Rout PD, Sahoo KJ, Rao Y. Analysis of the oil of large cardamom (Amomum subulatum Roxb.) growing in sikkim. J Essent Oil Res. 2003;15:265–266. doi: 10.1080/10412905.2003.9712138. [DOI] [Google Scholar]
- Sharma S, Sharma J, Kaur G. Therapeutic uses of Elettaria cardamomum. Int J Drug Formul Res. 2011;2:102–108. [Google Scholar]
- Thomas VP, Sabu M, Gupta U. Taxonomic studies on cultivars of Amomum subulatum (Zingiberaceae) Rheedea. 2009;19:25–36. [Google Scholar]
- Tiveron AP, Melo PS, Bergamaschi KB, Vieira TM, Regitano-d MA, Alencar SM. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int J Mol Sci. 2012;13:8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Rubio-Portillo E, Anton J, Ramos-Espla AA, Quesada E, Llamas I. Selection of the N-acylhomoserine lactone-degrading bacterium Alteromonas stellipolaris PQQ-42 and of its potential for biocontrol in aquaculture. Front Microbiol. 2016;7:1–13. doi: 10.3389/fmicb.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Diggle SP, Greenberg EP. Bacterial quorum sensing: the progress and promise of an emerging research area. Nature. 2017;551:313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying JY, Ma JL, Xia ZL, Yao TW. Pharmacokinetics of luteolin from Elsholtzia blanda extracts in rats. Acta Pharm Sin. 2008;43:523–527. [PubMed] [Google Scholar]
- Yousefi M, Rahimi-Nasrabadi M, Pourmortazavi SM, Wysokowski M, Jesionowski T, Ehrlich H, Mirsadeghi S. Supercritical fluid extraction of essential oils. Trends Analyt Chem. 2019;118:182–193. doi: 10.1016/j.trac.2019.05.038. [DOI] [Google Scholar]
- Zhao J, Chen M, Quan CS, Fan SD. Mechanisms of quorum sensing and strategies for quorum sensing disruption in aquaculture pathogens. J Fish Dis. 2015;38:771–786. doi: 10.1111/jfd.12299. [DOI] [PubMed] [Google Scholar]
- Zhu H, He CC, Chu QH. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett Appl Microbiol. 2011;52:269–274. doi: 10.1111/j.1472-765X.2010.02993.x. [DOI] [PubMed] [Google Scholar]