Abstract
In this study, drying kinetics and quality of purple-fleshed sweet potato (PFSP) subjected to microwave-vacuum drying were investigated. The effects of hot water and steam blanching pretreatment on physicochemical characteristics of the dried products were also considered. The samples were dehydrated in a custom-made microwave-vacuum system at different power levels including 450, 600 and 850 W. Hot air drying at 70 °C was also conducted for comparison. The results showed that drying time of PFSP under microwave-vacuum conditions ranged from 6 to 12 min, significantly reduced as compared to that of hot air drying (600 min). The improvement of drying rate was also evidenced by increased effective moisture diffusivity (2.22 × 10−7–4.05 × 10−7 m2/s) of the samples. Drying kinetics of PFSP was best fitted by Page and logarithmic model with R2 ranging from 0.991 to 0.998, and RMSE from 0.016 to 0.030. PFSP dried under microwave-vacuum condition had lower water absorption index and swelling capacity than hot air drying. Color, antioxidant activity and total phenolic content of dried PFSP were also improved under microwave-vacuum drying. The effects of blanching pretreatment on quality of dried PFSP were more dominant in hot air than microwave-vacuum dried samples.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04789-5) contains supplementary material, which is available to authorized users.
Keywords: Blanching pretreatment, Drying kinetics, Microwave-vacuum drying, Power level, Quality
Introduction
Purple-fleshed sweet potato (Ipomoea batatas L.) (PFSP) contains high levels of anthocyanins (7.5 mg/g), polyphenols (74.6 mg/g) and dietary fiber (15.8%) (Lim et al. 2013). Anthocyanins can be used as food colorant and was found to exhibit strong radical scavenging activity, antimutagenic activity, to reduce high blood pressure, and to prevent colorectal cancer (Lim et al. 2013). Therefore, PFSP can be considered as a potential ingredient for functional foods with promising health benefits. PFSP is usually abundant during peak seasons, from late October through December. Due to its high water content (~ 64%), PFSP tuber is highly perishable. Therefore, processing technology is required to convert PFSP into stable ingredients, which can be used by food industries during off-season periods. Drying can improve the stability of foods by reducing their water and microbiological activity, and minimizing their physical and chemical changes during storage. Various drying methods can be used such as hot air drying, drum drying, spray and freeze-drying. Traditional hot air drying is usually preferred due to its simplicity and low cost (Ahmed et al. 2010). However, hot air dehydration is relatively slow and reduces the quality of food products significantly. Freeze-drying can produce products with superior quality. Yet, high production cost, high energy consumption, and low capacity is the downside of this process (Caparino et al. 2012). It is desirable to develop an alternative drying process, which can reduce drying time, better preserve physicochemical properties, and bioactive compounds of dried PFSP. Recently, microwave-vacuum drying has been explored for food dehydration. The process combines the advantages of both microwave heating and vacuum drying (Süfer and Palazoğlu 2019). Volumetric heating by microwave under vacuum helps increase drying rate and lower drying temperature. The process was reported to improve the retention of nutrients, color, and rehydration potential of foods (Ando et al. 2019). The oxidation of active compounds was also reduced due to the removal of air from the drying chamber (Zielinska and Michalska 2016). The technology was applied for drying various foods such as pomegranate arils (Dak et al. 2014), and blueberries (Zielinska and Michalska 2016).
In food dehydration, blanching prior to drying can further enhance the quality of the dried products. Blanching inactivates polyphenoloxidase and peroxidase enzymes causing discoloration (Liu et al. 2015), reduces microbial load, and enhances the retention of nutrients. Blanching was also shown to increase the drying rate and to reduce energy consumption of drying processes. Steam and hot water are commonly used blanching methods. Steam blanching is usually preferred due to reduced loss of soluble nutrients.
So far, there have been few studies investigating the roles of blanching pretreatment and microwave-vacuum drying in preserving quality of fried PFSP. The information would be useful for food processors to apply this technology in industrial settings. Therefore, the objective of this study was to evaluate the effects of blanching methods and microwave-vacuum drying on drying kinetics, physicochemical properties, and bioactive nutrients of PFSP.
Materials and methods
Materials and sample preparation
Purple-fleshed sweet potato (Ipomoea batatas L.), harvested about 100 days after planting, was purchased from the local market (Pathum Thani, Thailand). The tubers were sorted to minimize the size variations, and then stored at 18 °C in the dark to prevent germination. Prior to experiments, the tubers were cleaned with tap water and cut into slices of 4-mm thickness by a custom-made slicer. A stainless steel cork borer was used to punch the slices into cylindrical samples of 25 mm diameter (Fig. S1, Supplementary Information).
Folin–Ciocalteu reagents, sodium carbonate (Na2CO3), potassium chloride (KCl), sodium acetate trihydrate (NaCH3COO·3H2O) were supplied by Merk (Darmstadt, Germany). 1,1-diphenyl-2-picrylhydrazy (DPPH) was provided by Sigma Aldrich (St. Louis, MO, USA).
Blanching pretreatment
The effects of hot water and steam blanching on drying and quality of PFSP were investigated. Hot water blanching was conducted by immersing the samples in boiling water at 100 °C for 1 min. Steam blanching was also conducted for 1 min using saturated steam at 100 °C and atmospheric pressure. After blanching, the samples were cooled in cold water (5 °C) for 2 min to avoid further thermal degradation of the quality. Samples without pretreatment served as the control.
Microwave-vacuum drying
The design of the microwave-vacuum dryer was adopted from Monteiro et al. (2015) with some modifications (Fig. S2, Supplementary Information). The dryer was developed from a domestic microwave oven (MS28J5255UB, Samsung Electronics Co. Ltd., Suwon, South Korea) with maximum power rating of 1500 W. The vacuum drying chamber was made from polypropylene due to its mechanical strength, heat tolerance and microwave transparency (ε′ = 2.2; tan δ = 0.0003–0.0004) (Monteiro et al. 2015). The drying chamber was rotated by coupling with the turntable to improve heating uniformity during drying. The drying chamber was also connected to the vacuum system. Moisture in the exhaust air was absorbed by the silica gel before entering the vacuum pump (GEM 1.0 8890, WELCH, Illinois, USA). Microwave-vacuum drying was carried out at different power levels including 450, 600 and 850 W. The vacuum was maintained at 160 mm Hg during drying. Hot air drying was performed at 70 °C in a custom-made hot air tunnel dryer (Food Engineering Lab, Asian Institute of Technology, Thailand) for comparison. All samples were dried to final moisture content of 6.0-8.0% (wet basis). Dried samples were ground by a hammer mill (Polymix PX-MFC 90 D, Kinematica AG, Luzern, Switzerland) equipped with 60 mesh sieve. The powder was packed in polypropylene bag and stored at 4 °C for further analysis.
Drying kinetics and effective moisture diffusivity
Drying curve of microwave-vacuum drying was developed by measuring moisture content of the samples at 1 min intervals. About 50.0 ± 0.2 g of sample was tested for each period of drying. The dryer was stopped to remove the samples of which the moisture content was determined by hot air oven method (AOAC 1999). For hot air drying, moisture content of the samples was determined by monitoring the weight loss during drying until the final moisture was reached. Drying kinetics of the samples was described by different models (Onwude et al. 2016) as below:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
where t is drying time. a, b, k, and n are constants. The moisture ratio (MR) was calculated by the following equation:
| 6 |
where Mt is moisture content at time t, Me is equilibrium moisture content, M0 is initial moisture content.
The constants of the models were obtained by nonlinear regression using R software (version 3.4.4, R Core Team). The goodness of fitting of the models was compared by root mean square error (RMSE) and coefficient of determination (R2). The effects of pretreatment and drying methods on drying rates were also evaluated based on effective moisture diffusivity (De) (m2/s) which was determined from the following correlation (Onwude et al. 2016):
| 7 |
where b1 is constant, r is radius (m) of the cylindrical sample, t is drying time (s).
Physicochemical properties
Color and microstructure
Color parameters (L*, a* and b*) of the sample were measured by a colorimeter (Hunter Lab, Inc., Reston, VA, USA) (Aurum and Nguyen 2019; Jalgaonkar et al. 2020).
Microstructures of PFSP samples were analyzed by a scanning electron microscope (SEM) (S 3400N, Hitachi, USA). The samples were fixed to SEM stubs by carbon tape and then coated with a thin gold layer by a JFC-1200 fine coater (JEOL, Tokyo, Japan). The pictures were taken at an accelerating potential of 20 kV.
Water solubility index (WSI), water absorption index (WAI), swelling capacity (SC)
WSI and WAI were measured by the method of Ahmed et al. (2010). Briefly, PFSP powder (0.83 g) was mixed with 10 mL distilled water in a 15 mL centrifuge tube using a vortex mixer (Thermolyne Maxi Mix II, Thermo Scientific, USA). The mixture was incubated at 30 °C in a water bath for 30 min. Then, the mixture was centrifuged (EBA 8S, Hettich, USA) at 4300 rpm for 15 min. The supernatant was poured on a pre-weighed petri dish and then dried in the hot air oven at 105 °C overnight. The weight of dried supernatant residue and the solid pellet remaining in the centrifuge tube was determined. WSI, WAI and SC were calculated by the following equations (Ahmed et al. 2010; Lai and Cheng 2004):
| 8 |
| 9 |
| 10 |
Antioxidant activity
Antioxidant activity of the samples was measured using protocols described by Blois (1958). The sample (0.1 g) was mixed with 10 mL of 50% ethanol for 1 min using a vortex mixer then sonicated (Ultrasonic bath, Branson 5210, USA) for 15 min. The extract obtained after filtration with Whatman filter paper No. 1 was mixed with 2 mL of 0.1 mM DPPH solution. After incubation in the dark for 60 min, the absorbance was measured at 517 nm using a UV–Vis spectrophotometer (UV-1800, Shimadzu Corporation, Kyoto, Japan). Percentage of DPPH inhibition was calculated as below (Blois 1958):
| 11 |
where A0 and A1 is the absorbance of the blank and sample, respectively.
Total phenolic content (TPC)
TPC in the samples was determined following the method of Ratanapoompinyo et al. (2017) with modifications. In brief, 0.3 g of sample was mixed with 10 mL of 80% ethanol using a vortex mixer for 1 min. The mixture was then sonicated for 5 min, centrifuged at 3500 rpm for 10 min and filtered by a Whatman filter paper No. 1. The extract (1 mL) was mixed with 2.5 mL of Folin–Ciocalteu reagent and then 2 mL of 7.5% sodium carbonate solution. The mixture was kept in the dark for 30 min and the absorbance was read at 765 nm using the UV–Vis spectrophotometer. TPC was calculated using standard curve of gallic acid and presented as mg gallic acid equivalent (GAE) per g sample.
Anthocyanin content
Anthocyanin content was determined by pH differential method (Giusti and Wrolstad 2001; Ratanapoompinyo et al. 2017). The extract was prepared as previously described. The absorbance at pH 1 (KCl buffer) and pH 4.5 (CH3COONa buffer) was then measured at 520 and 700 nm using the UV–Vis spectrophotometer. Anthocyanin content was calculated by:
| 12 |
where Mw is molecular weight (449.2 g/mol for cyanidin-3-glucoside (Cy3G)), DF is dilution factor, ε is molar extinction coefficient (26,900/cm/mg for Cy3G) and A was obtained from (A520nm − A700nm) at pH 1.0 − (A52 nm − A700nm) at pH 4.5.
Statistical analysis
All experiments were conducted in triplicates. One-way analysis of variance (ANOVA) was analyzed by SPSS statistical software (IBM, Armonk, NY, USA). Duncan’s multiple range test (DMRT) was used to compare mean values and statistical significance was expressed at 95% confident interval.
Results and discussion
Effects of drying methods on drying rate and effective moisture diffusivity
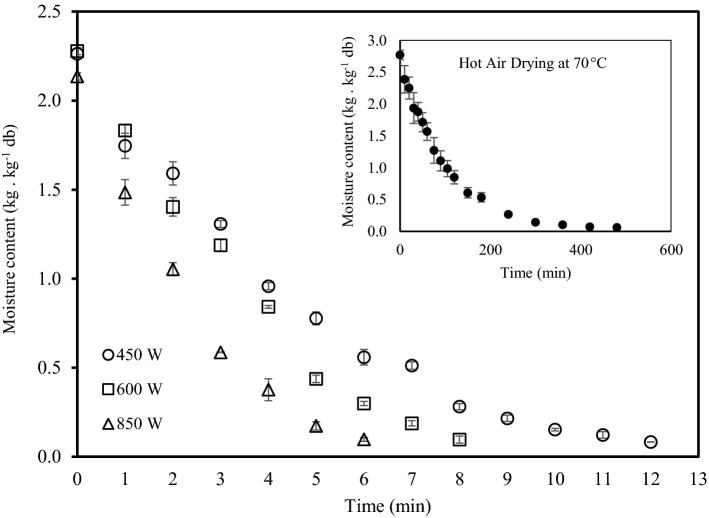
The effects of drying methods on drying rates of PFSP are illustrated in Fig. 1. The trend of drying curves is similar with decrease of drying rates over time. Microwave-vacuum drying accelerated the removal of moisture significantly. Moisture of the sample was reduced from 2.26 (kg/kg db) to 0.08, 0.10 and 0.10 (kg/kg db) at 450, 600 and 850 W, respectively in less than 15 min. The decrease of drying time (6–12 min) with increasing power level could be due to the increase of the applied power density (Mohapatra et al. 2014). The absorbed energy was converted into thermal energy to evaporate the free water (Monteiro et al. 2015). Hot air drying required considerably longer time (600 min) to achieve comparable final moisture content. The drying rate of microwave-vacuum method is usually higher than hot air drying due to rapid, volumetric heating, which facilitates mass, and heat transfer.
Fig. 1.
Drying curve of purple-fleshed sweet potato by microwave-vacuum method at different power levels. Inset: Drying curve by hot air method at 70 °C
The effects of drying methods and power levels are also demonstrated by the difference in effective moisture diffusivity (Table 1). Deff value of 0.079 × 10−7 m2/s was obtained by hot air drying whereas those of microwave-vacuum drying were significantly higher, ranging from 2.22 × 10−7 to 4.05 × 10−7 m2/s. The increase in power level was found to improve the effective diffusivity of water in the sample. Dak et al. (2014) attributed the enhanced driving force of heat and mass transfer to increasing microwave power. The results of effective moisture diffusivity obtained this study are within the range of the values (10−11 to 10−6 m2/s) reported by Marinos-Kouris and Maroulis (2006).
Table 1.
Effective moisture diffusivity of purple sweet potato subjected to hot air and microwave-vacuum drying
| Drying method | Deff (× 10−7 m2/s) | R2 |
|---|---|---|
| HAD 70 °C | 0.079 | 0.995 |
| MVD 450 W | 2.22 | 0.985 |
| MVD 600 W | 3.36 | 0.966 |
| MVD 850 W | 4.05 | 0.984 |
HAD hot air drying, MVD microwave-vacuum drying
The drying curves were fitted by different empirical models and goodness of fitting is presented in Table 2. Most of the models have satisfactory performance with R2 from 0.970 to 0.998 and RMSE from 0.016 to 0.056. Page and logarithmic models had the best performance with the highest R2 (0.993–0.998) and the lowest RMSE (0.016–0.030). Page model was reported to be the best model for drying of prickly pear fruits (Lahsasni et al. 2004). Similarly, logarithmic model was found to show the best fitting to drying data of sweet potato when the influence of drying temperature and blanching methods was considered (Doymaz 2011).
Table 2.
Drying kinetics of purple-fleshed sweet potato described by different models
| Model | Drying method | Kinetics constants | Goodness of fit | ||||
|---|---|---|---|---|---|---|---|
| k (min−1) | n | a | b | R2 | RMSE | ||
| Lewis | HAD 70 °C | 0.0100 | 0.997 | 0.017 | |||
| MVD 450 W | 0.2213 | 0.982 | 0.041 | ||||
| MVD 600 W | 0.2782 | 0.970 | 0.056 | ||||
| MVD 850 W | 0.4138 | 0.989 | 0.034 | ||||
| Page | HAD 70 °C | 0.0113 | 0.9742 | 0.997 | 0.017 | ||
| MVD 450 W | 0.1583 | 1.2014 | 0.991 | 0.029 | |||
| MVD 600 W | 0.1733 | 1.3418 | 0.991 | 0.030 | |||
| MVD 850 W | 0.3311 | 1.2164 | 0.998 | 0.016 | |||
| Henderson and Pabis | HAD 70 °C | 0.0098 | 0.9871 | 0.997 | 0.017 | ||
| MVD 450 W | 0.2277 | 1.0296 | 0.983 | 0.040 | |||
| MVD 600 W | 0.2914 | 1.0480 | 0.974 | 0.052 | |||
| MVD 850 W | 0.4236 | 1.0250 | 0.990 | 0.033 | |||
| Logarithmic | HAD 70 °C | 0.0099 | 0.9850 | − 0.0032 | 0.997 | 0.017 | |
| MVD 450 W | 0.1721 | 1.1303 | − 0.1296 | 0.991 | 0.029 | ||
| MVD 600 W | 0.1680 | 1.3429 | − 0.3343 | 0.993 | 0.027 | ||
| MVD 850 W | 0.3187 | 1.1455 | − 0.1401 | 0.998 | 0.016 | ||
| Peleg | HAD 70 °C | 80.1430 | 0.8010 | 0.991 | 0.028 | ||
| MVD 450 W | 4.7047 | 0.6075 | 0.989 | 0.032 | |||
| MVD 600 W | 4.3610 | 0.4631 | 0.992 | 0.029 | |||
| MVD 850 W | 2.5547 | 0.5934 | 0.996 | 0.021 | |||
HAD hot air drying, MVD microwave-vacuum drying
Influence of blanching and drying methods on color parameters
Color parameters including L*, a*, b*, hue angle (H°) and chromaticity (C*) of PFSP subjected to different blanching pretreatment and drying methods are provided in Table 3.
Table 3.
Color specifications and water absorption properties of purple-fleshed sweet potato under different pretreatment and drying methods
| Pretreatment | Drying methods | Color specification | Water absorption properties | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | a* | b* | H° | C* | WAI (g/g) | WSI (%) | SC (g/g) | ||
| UT | HAD 70 °C | 52.96 ± 1.29aA | 12.90 ± 0.19bC | 1.48 ± 0.49aA | 6.59 ± 2.27aA | 12.98 ± 0.13bC | 3.87 ± 0.18bA | 16.62 ± 1.79aA | 4.64 ± 0.31bA |
| MVD 450 W | 48.79 ± 1.47aB | 22.16 ± 0.34aB | − 10.69 ± 0.83aB | − 25.7 ± 1.41aB | 24.61 ± 0.67aB | 2.96 ± 0.39bB | 15.30 ± 0.58bA | 3.49 ± 0.44bB | |
| MVD 600 W | 47.90 ± 0.95aB | 22.81 ± 0.50aAB | − 12.03 ± 0.33aB | − 27.8 ± 1.71aB | 25.78 ± 0.29aAB | 2.96 ± 0.15cB | 16.63 ± 1.25cA | 3.55 ± 0.23cB | |
| MVD 850 W | 49.77 ± 0.95aAB | 23.42 ± 0.54aA | − 11.34 ± 0.11bB | − 25.8 ± 0.29abB | 26.02 ± 0.53aA | 2.58 ± 0.04bB | 17.50 ± 1.84aA | 3.13 ± 0.03cB | |
| SB | HAD 70 °C | 37.03 ± 1.81cB | 21.20 ± 0.67aA | − 7.21 ± 0.23bA | − 18.8 ± 0.01bA | 22.39 ± 0.71aB | 4.34 ± 0.41bA | 28.59 ± 7.63aA | 6.08 ± 0.07aA |
| MVD 450 W | 49.91 ± 0.16aA | 22.22 ± 0.01aA | − 11.10 ± 0.19aB | − 26.5 ± 0.41aC | 24.84 ± 0.07aA | 4.44 ± 0.09aA | 30.55 ± 3.08aA | 6.39 ± 0.15aA | |
| MVD 600 W | 47.52 ± 1.64aA | 22.95 ± 0.16aA | − 9.91 ± 0.87aB | − 23.3 ± 1.68aAB | 25.00 ± 0.49aA | 4.52 ± 0.09bA | 25.91 ± 0.92aA | 6.11 ± 0.20bA | |
| MVD 850 W | 47.49 ± 1.97aA | 23.71 ± 1.46aA | − 10.44 ± 0.04aB | − 23.8 ± 1.38aB | 25.90 ± 1.33aA | 4.79 ± 0.04aA | 21.19 ± 0.90aA | 6.08 ± 0.02bA | |
| HWB | HAD 70 °C | 46.22 ± 2.11bA | 20.68 ± 0.25aB | − 9.65 ± 0.06cA | − 25.00 ± 0.41cA | 22.81 ± 0.20aAB | 5.83 ± 0.12aA | 23.40 ± 0.47aA | 6.63 ± 0.11aB |
| MVD 450 W | 50.67 ± 3.08aA | 21.32 ± 1.77aAB | − 11.34 ± 0.83aA | − 28.00 ± 0.22aA | 24.15 ± 1.95aAB | 5.02 ± 0.06aB | 23.67 ± 2.38aA | 6.58 ± 0.13aB | |
| MVD 600 W | 45.70 ± 1.00aA | 20.01 ± 0.24bB | − 9.54 ± 1.48aA | − 25.40 ± 3.20aA | 22.19 ± 0.86bB | 5.38 ± 0.00aAB | 22.97 ± 0.37bA | 6.98 ± 0.03aA | |
| MVD 850 W | 47.69 ± 0.50aA | 22.91 ± 0.54aA | − 11.56 ± 0.37bA | − 26.80 ± 0.19bA | 25.66 ± 0.65aA | 4.94 ± 0.39aB | 23.91 ± 5.07aA | 6.49 ± 0.08aB | |
HAD hot air drying, MVD microwave-vacuum drying, WAI water absorption index, WSI water solubility index, SC swelling capacity Superscripts denoted by small letter indicate significant difference among pretreatment (p < 0.05); Superscript denoted by uppercase letters indicate significant difference among drying methods (p < 0.05)
For hot air drying, blanching significantly led to darkening of the samples, indicated by reduction in L value. The result can be ascribed to the release of color pigments when the samples were exposed to hot steam or water. In addition, drying process at high temperature and in the presence of oxygen may increase the activity of polyphenolic oxidase, resulting in browning of dehydrated foods (Wojdyło et al. 2014). L values of microwave-vacuum dried samples, either blanched or untreated one, were quite close. Microwave heating was known to induce volumetric heating and rupture of cellular structure (Dandekar and Gaikar 2002). Consequently, the process increased anthocyanin content on the sample surface and made the appearance of the sample darker. Power levels did not have significant effects on the parameter L of the samples. a* values of most of the samples were relatively similar. Untreated samples dried by hot air had the lowest a* value which could be caused by degradation of anthocyanin upon exposure to high temperature and oxygen. Blanching can protect anthocyanin due to the inactivation of oxidative enzymes such as peroxidase, lipoxygenase and the removal of air pockets in the sample. In addition, blanching and microwave volumetric heating partially gelatinized the starch in PFSP which enhanced the light transmission and color of the samples. Starch gelatinization can also protect the phenolic compounds in the amylose/amylose pectin chains against thermal treatment (Watson 2018). Similar to L value, other color parameters of the dried samples were affected by the migration of color pigments following the rupture of PFSP cellular structures after blanching or microwave heating. b* value of food materials correlates to the content of anthocyanin (Ruttarattanamongkol et al. 2016). Samples underwent blanching or microwave drying experienced an increase in negative b* value, which was consistent with the increase of anthocyanin on the sample surface. Only untreated PFSP samples dried by hot air had positive b* value of 1.48. Microwave-vacuum drying of untreated and steam blanched samples resulted in products with higher b* values as compared to hot air drying. The effects could be derived from additional release of anthocyanin during microwave heating. For water blanched PFSP, blanching process might have extensively damaged the structure of the samples. Hence, additional impact of microwave heating on the final b* value of the sample was not significant. It was noted that, hot water treatment had more pronounced effects on b* values of the hot air dried than microwave-vacuum dried samples. The trend is understandable as volumetric heating ruptured the cellular structures of PFSP, which reduced the difference in b* value of untreated, hot water and steam blanched samples. Similarly, the impact of drying methods on color of PFSP can be observed via Hue angle and chromaticity (C*) values. Hue angle of untreated, hot air dried sample (6.59) is in the brownish region, whereas others are in the purplish color range (− 18.8 to − 28.0). C* values of other samples were also higher than untreated, hot air dried sample. These visual changes in color and appearance of dried PFSP can be clearly seen in Table S1 (Supplementary Information).
Impacts of key process factors on WAI, WSI, and SC of dried PFSP powder
WAI represents the amount of water absorbed by starch and can be used as an index of gelatinization. The effects of blanching pretreatment and drying methods on WAI of dried PFSP are presented in Table 3. Pretreatment method had significant influence on WAI of all the samples. Hot water blanching induced higher increase in WAI than steam blanching. For hot air dried PFSP, steam blanching resulted in an increase of 51% WAI as compared to untreated and hot water blanched samples. On the other hand, WAI of microwave vacuum dried PFSP increased from 50 to 86% and from 70 to 91%, after being blanched with hot water and steam, respectively. Drying methods had significant impacts on WAI of dried products. Hot air drying produced samples with higher WAI as compared to microwave-vacuum drying. The levels of microwave power studied were not found to have any effects on WAI values. Water absorption of the starch is governed by its molecular structure, including the crystalline structure and chemical composition. Gelatinization during thermal pretreatment and drying possibly led to structural modifications that affected water binding capacity of the starch (Chen et al. 2017). Various changes such as swelling of granules, water absorption, loss of crystallinity and amylose leaching were observed during starch gelatinization (Eliasson 2004). Zhang et al. (2013) reported that cooking the pasta made from durum semolina flour above gelatinization temperature destroyed hydrogen bonds of starch granules. Consequently, these granules swelled and absorbed water in both the crystalline and non-crystalline phases, resulting in increased water absorption. Wiriyawattana et al. (2018) observed an increase in WAI in the pre-gelatinized riceberry flour samples subjected to drum drying. The trend was attributed to the destruction of starch granules which eventually produced porous structure of the flour. The lower WAI of microwave-vacuum dried as compared to hot air dried PFSP could be due to drying time and temperatures of the samples. Microwave-vacuum drying just took from 6 to 12 min to reach desired moisture content, whereas hot air drying required up to 600 min. In addition, vacuum inside microwave chamber was maintained at 160 mm Hg at which water evaporated at 62 °C which was significantly lower than 70 °C. Higher temperature of hot air drying therefore resulted in higher WAI (Ahmed et al. 2010). On the other hand, difference in water absorption capacity observed in samples blanched in hot water and steam was possibly caused by different degree of starch gelatinization. At the same blanching temperature and time, hot water with abundant availability of water might have induced more starch gelatinization than steam.
WSI measures the extent of starch dissolving in water. The results obtained suggested that WSI was mainly influenced by pretreatment rather than drying methods. The increase of WSI during drying could stem from the disruption of starch granules and exposure of hydrophilic groups (Ahmed et al. 2010).
SC of dried PFSP was significantly affected by hot water and steam blanching. The highest increase in SC after pretreatment was 43 and 107% in hot air and microwave-vacuum dried samples, respectively. Drying methods did not affect SC of pretreated samples. Untreated samples subjected to hot air drying had higher SC than microwave-vacuum drying. SC of starch is controlled by hydrogen bonding and the crystallinity of the starch (Zhang et al. 2013). A low SC is usually associated with the presence of a large number of crystallites. The gelatinization of starch at certain temperature leads to disruption of the molecular organisation within the granules and increased starch–water interactions will result in increase in the swelling (Liu et al. 2003). Ndangui et al. (2014) found that SC of dried sweet potato flour produced from blanched samples was higher than that of untreated samples. The change was attributed to higher degradation of starch during thermal pretreatment.
Effects of microwave-vacuum drying on antioxidant activity, total phenolic content, and anthocyanin of PFSP
Antioxidant activity
Figure 2a demonstrates the effects of blanching and drying methods on antioxidant activity of dried PFSP products. It was noted that steam blanched, microwave-vacuum (600 W) dried sample had the highest antioxidant activity (84.97%), whereas untreated, hot air dried sample had the lowest value (74.91%). PFSP retained more antioxidant activity when the samples were blanched by steam than were left untreated or blanched in hot water. Thermal pretreatment was reported to affect antioxidant activity of foods (Severini et al. 2016). Specifically, blanching caused cellular breakage which facilitated the extraction of antioxidant compounds (Ruttarattanamongkol et al. 2016). Enhanced DPPH radical scavenging activity was also observed in microwave-vacuum dried samples at some power levels investigated. The trends are similar to those reported by Izli et al. (2018). Volumetric heating during microwave drying led to cell disruption and rupture. Consequently, more antioxidant constituents could be released during extraction process.
Fig. 2.
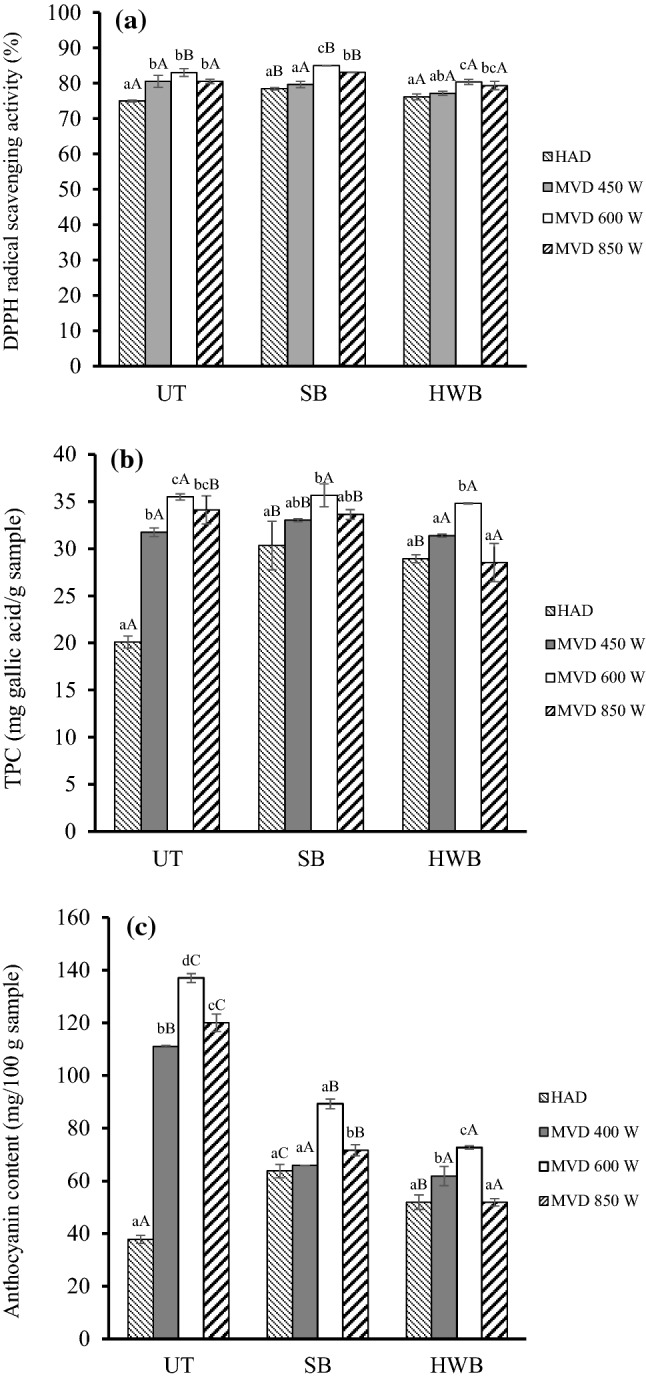
Physicochemical properties of purple-fleshed sweet potato. a DPPH free radical scavenging activity, b total phenolic content and, c anthocyanin content. HAD: Hot air drying at 70 °C; MVD 450 W: Microwave-vacuum drying at 450 W; MVD 600 W: Microwave-vacuum drying at 600 W; MVD 850 W: Microwave- vacuum drying at 450 W. UT Untreated, SB steam blanched, HWB hot water blanched. Small letters (a–c) indicate significant difference among drying methods (p < 0.05); Uppercase letters (A–B) indicate significant difference among blanching pretreatment (p < 0.05)
The mode of heat transfer during drying can play vital role in preserving antioxidant activity of dried products. In hot air drying, convective and conductive heat transfer was relatively slow. Therefore, the activation of oxidative enzymes during heat penetration period resulted in significant loss of antioxidant activity. Thermal pretreatment might help inactivate these enzymes and reduced oxidative reactions. On the other hand, rapid volumetric heating under microwave can effectively inactivate oxidative enzymes in a short time and improved antioxidant activity of microwave-vacuum dried PFSP (Fig. 2a). Microwave heating has been used as a thermal pretreatment for kiwifruit puree and retention of antioxidant capacity was observed by Benlloch-Tinoco et al. (2013). Similarly, enhanced preservation of antioxidant activity was reported when fruit peelings were blanched by microwave heating (Dibanda et al. 2020).
Total phenolic content
The effects of pretreatment and drying methods on TPC of dried PFSP are presented in Fig. 2b. Pretreatment was found to improve the retention of TPC in hot air dried samples significantly. Samples blanched in hot water and steam had TPC of 28.93 and 30.35 mg GAE/g, respectively as compared to the untreated one (20.1 mg GAE/g). The inactivation of oxidative enzymes by thermal pretreatment might lessen the degradation of TPC in hot air drying. Early inhibition of oxidative enzymes is critical as significant loss of TPC could occur during heat penetration period of hot air drying. On the other hand, the effect of pretreatment on TPC was less obvious in microwave-vacuum dried samples. Rapid volumetric heating by microwave can inhibit the peroxidase enzyme within 80s (Severini et al. 2016). Additional blanching treatment might not be significant to further inactivate oxidative enzymes in the samples. Instead, blanching in this case could lead to partial loss of TPC due to thermal degradation and leaching of water-soluble compounds (Tang et al. 2015). Therefore, TPC of blanched, microwave-vacuum dried samples slightly decreased as compared to that of untreated one (Fig. 2b). The results also showed that steam blanching resulted in lower loss of TPC than hot water for the same drying method. Similar results were reported by Severini et al. (2016).
The influence of drying methods on TPC can be compared within the same pretreatment. As expected, microwave-vacuum drying preserved TPC of PFSP better than hot air drying, especially in untreated samples. Enhanced TPC in microwave-vacuum dried samples could originate from the combination of various factors. Oxidative reactions detrimental to TPC might have been minimized by vacuum condition and blanching effects of microwave volumetric heating (Wojdyło et al. 2014). In addition, the simultaneous volumetric heating and vacuum applied might have ruptured the cellular structure and release bound phytochemicals from the matrix which helped improve the extraction of phenolic compounds from the sample during analysis (Izli et al. 2018). In pretreated samples, the impact of drying methods on TPC was less significant. Blanching probably inactivated oxidative enzymes and prompted cellular breakage prior to drying. Therefore, the benefits of microwave-vacuum heating were no longer noticeable. Nevertheless, microwave-vacuum dried samples still had better TPC retention, possibly due to shorter drying time and lower drying temperature. TPC content at 600 W was higher than that at 450 and 850 W. The discrepancy could be caused by the difference of drying time and temperature at these power levels.
Anthocyanin
Both blanching pretreatment and drying methods had significant effects on anthocyanins contents of dried PFSP (Fig. 2c). Stability of anthocyanins is dependent on their structures, composition of the matrices, and processing parameters such as temperature, time, light and oxygen exposure, pH, and water activity (Paul and Das 2018). Dehydration processes often result in loss of anthocyanins and other phytochemicals in fruits, vegetables, and herbs (Wojdyło et al. 2014). Hot air drying reportedly degraded anthocyanins in fruit and vegetable samples (Mejia-Meza et al. 2010). Similar to phenolic compounds, anthocyanins are destroyed by peroxidase and lipoxygenase enzymes during drying processes (Deepika and Sutar 2018). Therefore, blanching helped inhibit these oxidative enzymes and reduce the loss of PFSP anthocyanins. However, since anthocyanins are highly soluble in water, hot water or steam blanching could cause significant leaching of anthocyanins to heating medium. Considering all of these factors, the influence of blanching on PFSP anthocyanin content would depend on the treatment conditions and characteristics of the samples. In this study, blanching had diverse impacts on anthocyanin content of hot air and microwave-vacuum dried samples. In hot air drying, samples subjected to blanching had had higher anthocyanin content than that of the untreated one. The amount of anthocyanins lost due to oxidative degradation during drying might be higher than the amount leached to blanching water. Therefore, blanching resulted in improved retention of anthocyanins. The opposite trend was observed for microwave-vacuum dried PFSP. In this drying method, untreated PFSP had higher anthocyanin content than the blanched samples (Fig. 2c). It is widely known that, microwave volumetric heating can be used to blanch food samples. Since no water is required, the leaching of anthocyanins to heating medium is avoided. Microwave-vacuum drying can blanch and dehydrate PFSP samples simultaneously. Additional blanching pretreatment was not necessary and even led to more loss of anthocyanins. The results agree with those reported by Liu et al. (2015) which found that microwave blanching improved anthocyanin content of spouted bed dried PFSP in comparison to hot water and steam blanching.
In all treatments, drying under microwave-vacuum condition had better retention of anthocyanins than hot air. Drying time, temperature and pressure could have profound influence on anthocyanin content of dried samples. Wojdyło et al. (2014) observed that the duration and intensity of heat treatment was directly related to the extent of anthocyanin loss. Reduced drying time and temperature under microwave-vacuum drying might help minimize the loss of anthocyanins in the dried products. Vacuum condition also reduced the oxidation of anthocyanins during drying. Microwave-vacuum drying could be a viable choice to produce dried PFSP with high retention of anthocyanins.
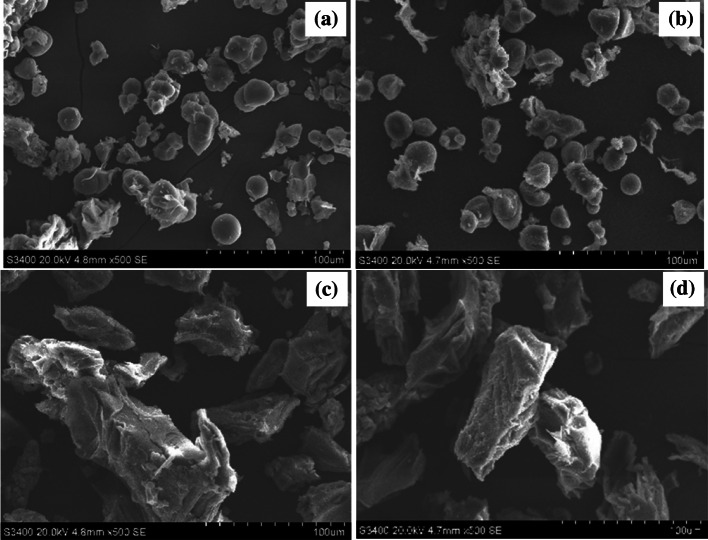
Microstructure of PFSP under microwave-vacuum drying
Figure 3 shows the influence of drying and pretreatment on microstructures of PFSP samples.
Fig. 3.
Microstructure of different purple-fleshed sweet potato samples subjected to different pretreatment and drying methods. a Untreated, hot air dying, b untreated, microwave vacuum drying, c steam blanched, microwave vacuum drying, d hot water blanched, microwave-vacuum drying
Drying methods did not affect the microstructure of the untreated PFSP samples (Fig. 3a, b). Both images show oval starch granules of different sizes. The morphology of the granules agree with that of sweet potato starch observed by Noda et al. (2009) which have rather smooth and non-cracked surface. Steam and hot water blanching (Fig. 3c, d) tended to produce swollen, irregular granules with rough surface. These changes could be derived from the extensive gelatinization of starch subjected to steam and hot water blanching. Gelatinization temperature of sweet potato starches is in the range of 58–84 °C (Garcia and Walter 1998). The gelatinization resulting from thermal treatment can affect the integrity and the original shape of the structure and starch granule. Chen et al. (2017) studied the effects of blanching and drying temperatures on microstructure of yam flour and noted that gelatinization produced dried flour with characteristic blocks and irregular structure with cranny and rough surfaces. SEM images further confirmed the effects of starch gelatinization during blanching on the WAI, WSI and SC of dried PFSP powder.
Conclusion
The study demonstrated that microwave-vacuum condition accelerated the drying rate of PFSP significantly. Increase in the drying rate was also achieved with increasing microwave power level. Drying kinetics of the sample was best described by Page and logarithmic model. Microwave-vacuum drying produced PFSP with lower WAI and SC than hot air drying and microwave power level did not have significant impact on the WAI of dried samples. However, microwave-vacuum drying enhanced retention of color, antioxidant activity and TPC of dried PFSP. Steam and hot air blanching had more pronounced effects on the quality of PFSP dried by hot air than microwave-vacuum method. Microwave volumetric heating might have had similar effects as blanching. Hence, additional blanching did not help further improve the quality of the dried products. The obtained results are helpful for applying microwave vacuum drying in production of dried PFSP with superior quality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Author Sigit Uji Marzuki is grateful to Indonesian Agency for Agricultural Research and Development (IAARD) (Indonesia) which provided the full scholarship for his Master’s study at Asian Institute of Technology.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed M, Sorifa AM, Eun JB. Effect of pretreatments and drying temperatures on sweet potato flour. Int J Food Sci Technol. 2010;45:726–732. doi: 10.1111/j.1365-2621.2010.02191.x. [DOI] [Google Scholar]
- Ando Y, Hagiwara S, Nabetani H, Sotome I, Okunishi T, Okadome H, Orikasa T, Tagawa A. Improvements of drying rate and structural quality of microwave-vacuum dried carrot by freeze-thaw pretreatment. LWT. 2019;100:294–299. doi: 10.1016/j.lwt.2018.10.064. [DOI] [Google Scholar]
- AOAC (1999) Official methods of analysis, 16th edn. Method 930.15. Association of Official Analytical Chemists, Gaithersburg, Maryland
- Aurum FS, Nguyen LT. Efficacy of photoactivated curcumin to decontaminate food surfaces under blue light emitting diode. J Food Process Eng. 2019;42:e12988. doi: 10.1111/jfpe.12988. [DOI] [Google Scholar]
- Benlloch-Tinoco M, Igual M, Rodrigo D, Martínez-Navarrete N. Comparison of microwaves and conventional thermal treatment on enzymes activity and antioxidant capacity of kiwifruit puree. Innov Food Sci Emerg Technol. 2013;19:166–172. doi: 10.1016/j.ifset.2013.05.007. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Caparino OA, Tang J, Nindo CI, Sablani SS, Powers JR, Fellman JK. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’var.) powder. J Food Eng. 2012;111:135–148. doi: 10.1016/j.jfoodeng.2012.01.010. [DOI] [Google Scholar]
- Chen X, Lu J, Li X, Wang Y, Miao J, Mao X, Zhao C, Gao W. Effect of blanching and drying temperatures on starch-related physicochemical properties, bioactive components and antioxidant activities of yam flours. LWT Food Sci Technol. 2017;82:303–310. doi: 10.1016/j.lwt.2017.04.058. [DOI] [Google Scholar]
- Dak M, Jain MK, Jat SL. Optimization of microwave-vacuum drying of pomegranate arils. J Food Meas Charact. 2014;8:398–411. doi: 10.1007/s11694-014-9205-4. [DOI] [Google Scholar]
- Dandekar DV, Gaikar VG. Microwave assisted extraction of curcuminoids from Curcuma longa. Sep Sci Technol. 2002;37:2669–2690. doi: 10.1081/SS-120004458. [DOI] [Google Scholar]
- Deepika S, Sutar PP. Combining osmotic–steam blanching with infrared–microwave–hot air drying: production of dried lemon (Citrus limon L.) slices and enzyme inactivation. Dry Technol. 2018;36:1719–1737. doi: 10.1080/07373937.2017.1422744. [DOI] [Google Scholar]
- Dibanda RF, Akdowa PE, Rani PA, Tongwa QM, Mbofung FCM. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020;302:125308. doi: 10.1016/j.foodchem.2019.125308. [DOI] [PubMed] [Google Scholar]
- Doymaz I. Thin-layer drying characteristics of sweet potato slices and mathematical modelling. Heat Mass Transf. 2011;47:277–285. doi: 10.1007/s00231-010-0722-3. [DOI] [Google Scholar]
- Eliasson AC. Starch in food: structure, function and applications. Boca Raton: CRC Press; 2004. [Google Scholar]
- Garcia AM, Walter WM. Physicochemical characterization of starch from Peruvian sweet potato selections. Starch/Staerke. 1998;50:331–337. doi: 10.1002/(SICI)1521-379X(199808)50:8<331::AID-STAR331>3.0.CO;2-J. [DOI] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV–visible spectroscopy. Curr Protoc Food Anal Chem. 2001;1:F1–F2. doi: 10.1002/0471142913.faf0102s00. [DOI] [Google Scholar]
- Izli N, Izli G, Taskin O. Impact of different drying methods on the drying kinetics, color, total phenolic content and antioxidant capacity of pineapple. CyTA J Food. 2018;16:213–221. doi: 10.1080/19476337.2017.1381174. [DOI] [Google Scholar]
- Jalgaonkar K, Mahawar MK, Vishwakarma RK, Shivhare US, Nambi VE. Optimization of process condition for preparation of sapota bar using refractance window drying method. Dry Technol. 2020;38:269–278. doi: 10.1080/07373937.2018.1482314. [DOI] [Google Scholar]
- Lahsasni S, Kouhila M, Mahrouz M, Jaouhari JT. Drying kinetics of prickly pear fruit (Opuntia ficus indica) J Food Eng. 2004;61:173–179. doi: 10.1016/S0260-8774(03)00084-0. [DOI] [Google Scholar]
- Lai HM, Cheng HH. Properties of pregelatinized rice flour made by hot air or gum puffing. Int J Food Sci Technol. 2004;39:201–212. doi: 10.1046/j.0950-5423.2003.00761.x. [DOI] [Google Scholar]
- Lim S, Xu J, Kim J, Chen TY, Su X, Standard J, Carey E, Griffin J, Herndon B, Katz B, Tomich J, Wang W. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol Nutr Food Res. 2013;57:1908–1917. doi: 10.1002/mnfr.201300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weber E, Currie V, Yada R. Physicochemical properties of starches during potato growth. Carbohydr Polym. 2003;51:213–221. doi: 10.1016/S0144-8617(02)00138-8. [DOI] [Google Scholar]
- Liu P, Mujumdar AS, Zhang M, Jiang H. Comparison of three blanching treatments on the color and anthocyanin level of the microwave-assisted spouted bed drying of purple flesh sweet potato. Dry Technol. 2015;33:66–71. doi: 10.1080/07373937.2014.936558. [DOI] [Google Scholar]
- Marinos-Kouris D, Maroulis ZB. Transport properties in the drying of solids. In: Mujumdar AS, editor. Handbook of industrial drying. Boca Raton: CRC Press; 2006. pp. 106–145. [Google Scholar]
- Mejia-Meza EI, Yanez JA, Remsberg CM, Takemoto JK, Davies NM, Rasco B, Clary C. Effect of dehydration on raspberries: polyphenol and anthocyanin retention, antioxidant capacity, and antiadipogenic activity. J Food Sci. 2010;75:H5–H12. doi: 10.1111/j.1750-3841.2009.01383.x. [DOI] [PubMed] [Google Scholar]
- Mohapatra D, Giri SK, Prasad S, Kar A, Nema PK. Vacuum-microwave drying characteristics of spearmint leaves. J Food Res Technol. 2014;2:87–92. [Google Scholar]
- Monteiro RL, Carciofi BAM, Marsaioli A, Laurindo JB. How to make a microwave vacuum dryer with turntable. J Food Eng. 2015;166:276–284. doi: 10.1016/j.jfoodeng.2015.06.029. [DOI] [Google Scholar]
- Ndangui CB, Petit J, Gaiani C, Nzikou JM, Scher J. Impact of thermal and chemical pretreatments on physicochemical, rheological, and functional properties of sweet potato (Ipomea batatas Lam) Flour. Food Bioprocess Technol. 2014;7:3618–3628. doi: 10.1007/s11947-014-1361-3. [DOI] [Google Scholar]
- Noda T, Isono N, Krivandin AV, Shatalova OV, Błaszczak W, Yuryev VP. Origin of defects in assembled supramolecular structures of sweet potato starches with different amylopectin chain-length distribution. Carbohydr Polym. 2009;76:400–409. doi: 10.1016/j.carbpol.2008.10.029. [DOI] [Google Scholar]
- Onwude DI, Hashim N, Janius RB, Nawi NM, Abdan K. Modeling the thin-layer drying of fruits and vegetables: a review. Compr Rev Food Sci Food Saf. 2016;15:599–618. doi: 10.1111/1541-4337.12196. [DOI] [PubMed] [Google Scholar]
- Paul ID, Das M. Effect of freeze, microwave-convective hot air, vacuum and dehumidified air drying on total phenolics content, anthocyanin content and antioxidant activity of jamun (Syzygium cumini L.) pulp. J Food Sci Technol. 2018;55:2410–2419. doi: 10.1007/s13197-018-3158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanapoompinyo J, Nguyen LT, Devkota L, Shrestha P. The effects of selected metal ions on the stability of red cabbage anthocyanins and total phenolic compounds subjected to encapsulation process. J Food Process Preserv. 2017;41:e13234. doi: 10.1111/jfpp.13234. [DOI] [Google Scholar]
- Ruttarattanamongkol K, Chittrakorn S, Weerawatanakorn M, Dangpium N. Effect of drying conditions on properties, pigments and antioxidant activity retentions of pretreated orange and purple-fleshed sweet potato flours. J Food Sci Technol. 2016;53:1811–1822. doi: 10.1007/s13197-015-2086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severini C, Giuliani R, De Filippis A, Derossi A, De Pilli T. Influence of different blanching methods on colour, ascorbic acid and phenolics content of broccoli. J Food Sci Technol. 2016;53:501–510. doi: 10.1007/s13197-015-1878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süfer Ö, Palazoğlu TK. Microwave-vacuum drying of pomegranate arils (Punica granatum L. cv. Hicaznar): effect on quality and nutrient content. J Food Process Preserv. 2019;43:14085. doi: 10.1111/jfpp.14085. [DOI] [Google Scholar]
- Tang Y, Cai W, Xu B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Sci Hum Wellness. 2015;4:123–132. doi: 10.1016/j.fshw.2015.07.003. [DOI] [Google Scholar]
- Watson RR. Polyphenols in plants: isolation, purification and extract preparation. London: Academic Press; 2018. [Google Scholar]
- Wiriyawattana P, Suwonsichon S, Suwonsichon T. Effects of drum drying on physical and antioxidant properties of riceberry flour. Agric Nat Resour. 2018;52:445–450. doi: 10.1016/j.anres.2018.11.008. [DOI] [Google Scholar]
- Wojdyło A, Figiel A, Lech K, Nowicka P, Oszmiański J. Effect of convective and vacuum-microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014;7:829–841. doi: 10.1007/s11947-013-1130-8. [DOI] [Google Scholar]
- Zhang L, Nishizu T, Hayakawa S, Nakashima R, Goto K. Effects of different drying conditions on water absorption and gelatinization properties of pasta. Food Bioprocess Technol. 2013;6:2000–2009. doi: 10.1007/s11947-012-0976-5. [DOI] [Google Scholar]
- Zielinska M, Michalska A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016;212:671–680. doi: 10.1016/j.foodchem.2016.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.