Abstract
Most forms of Alzheimer’s disease are sporadic. A model of sporadic Alzheimer’s disease induced with bilateral intraventricular injection of streptozotocin leads to insulin resistance in the brain accompanied by memory decline, synaptic dysfunction, amyloid plaque deposition, oxidative stress, and neuronal apoptosis, all of which mimic the pathologies associated with sporadic Alzheimer’s disease. Myelin injury is an essential component of Alzheimer’s disease, playing a key role in early cognitive impairment. Our previously research found that sporadic Alzheimer’s disease model showed myelin injury and that Shenzheling oral solution improved mild-to-moderate Alzheimer’s disease; therefore, the protective effect of Shenzheling oral solution on myelin injury in early cognitive impairment is worth attention. In this study, the Morris water maze test results showed impairments in the learning and memory functions of mice in the model group, whereas the learning and memory function significantly improved after drug intervention. Immunohistochemistry showed increased β-amyloid plaques in the model group and decreased amounts in the drug group. Moreover, results of electron microscopy, western blot, and polymerase chain reaction showed that Shenzhiling oral solution improved early cognitive impairment and repaired myelin sheath damage; the potential mechanism of these effects may relate to the PI3K/Akt-mTOR signaling pathway. These findings support the application and promotion of Shenzhiling oral solution to treat sporadic Alzheimer’s disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02900-x.
Keywords: Sporadic Alzheimer’s disease, Shenzhiling oral solution, Myelin sheath damage, Streptozotocin
Introduction
Alzheimer’s disease (AD) is a slowly progressive neurological disorder that is the most common form of dementia (Sorial and El Sayed 2017). During the past several years, neuroimaging studies have indicated that white-matter degeneration and demyelination may be important pathophysiological features in the risk and progression of AD (Nasrabady et al 2018; Butt et al 2019). There is emerging evidence that substantial white-matter microstructural impairment and demyelination exist in all stages of the AD process, including in high-risk population of AD, patients with AD clinical symptoms, patients with mild cognitive impairment, and patients with familial AD (FAD) and sporadic AD (SAD) (Shinohara et al 2014; Parra et al 2015). The PI3K/Akt-mTOR signaling pathway is an important signaling pathway that regulates nerve cell metabolism, and its conduction disorder can cause abnormal deposition of β-amyloid (Aβ) plaques, hyperphosphorylation of tau protein, insulin resistance, disordered energy metabolism, synaptic loss, and myelin damage (Tristão Pereira et al 2020). Furthermore, evidence demonstrates that the PI3K/Akt-mTOR signaling pathway is an important pathway for regulating myelin sheath-related proteins and maintaining myelin sheath integrity (Gaesser et al 2016).
Streptozotocin (STZ) is a glucosamine-nitrosourea compound derived from soil bacteria (Grieb 2016). Bilateral intraventricular injection of streptozotocin (ICV-STZ) causes brain glucose hypometabolism without systemic diabetes (Tristão Pereira et al 2020). The main advantage of an ICV-STZ model is that it closely resembles some pathology of SAD, including synaptic damage, Aβ plaque deposition, tau hyperphosphorylation, disordered glucose metabolism, and oxidative stress, characterized by central insulin resistance without increased peripheral blood glucose levels, which may also be referred to as type 3 diabetes (T3DM) (Kang and Cho 2014; Park 2011; Song et al 2014; Mansouri et al 2013; Neha et al 2014; Salkovic-Petrisic et al 2014). Much research has applied the ICV-STZ rodent model to SAD to evaluate a variety of marketed and novel compounds, including drugs and non-drug therapeutic strategies (Guardia de Souza, 2020; Yamini et al 2018; Hou et al 2014). Chinese herbal medicine may be a promising alternative therapy for AD due to good compliance, diminished toxicity, and reduced side effects (Howes et al 2017; Hou et al 2014). Shenzhiling oral solution (SZL) is mainly used for the clinical treatment of mild-to-moderate AD (Xing et al 2017). Previous evidence has demonstrated that SZL can improve the behavioral and psychological symptoms of dementia in patients with AD and that SZL affects multiple targets and pathways to offer great potential for treating AD (Wang Y et al 2019; Pan et al 2014). Our prior research examining SZL in STZ-induced SAD mice has demonstrated its propensity for improving synaptic plasticity and neuronal cell survival through glutamate receptors and GSK3β (Wang Y et al 2019). Additionally, our cell experiments have recently identified that SZL plays its therapeutic role in AD by promoting the PI3K/Akt-mTOR signaling pathway of oligodendrocytes (Zhenhong L et al 2020). On the basis of these findings, we hypothesized that the mechanism behind improvements in learning and memory with SZL in STZ-induced SAD mice was related to amelioration of myelin injury. To address this question, we conducted a study on whether SZL had an effect on myelin sheath integrity, ultrastructure, and myelin sheath-specific proteins, and we explored whether the mechanism of SZL was related to the PI3K/Akt-mTOR signaling pathway in a murine model of STZ-induced SAD.
Materials and methods
Animals, model, and drug administration
The study used 3 month-old male C57BL/6 wild-type mice, weighing 22 ~ 25 g, purchased from Beijing Huafukang Biotechnology Co., Ltd [SCXK(Beijing)2019-0008, China]. All mice belonged to the SPF grade and were kept in the Animal Room of Chinese Medicine Pharmacology Laboratory of Dongzhimen Hospital, Affiliated Hospital of Beijing University of Traditional Chinese Medicine [SYXK(Beijing)2015-0001, China]. Protocols of the present investigation for all the animal experiments were approved by the guidelines of the Animal Research Ethics Board of Beijing university of Chinese medicine Dongzhimen Hospital. Animal suffering and discomfort were minimized (Approval No. 16-13).
One hundred and five C57BL/6 male mice were placed in the specific pathogen-free experimental environment for 7 days for acclimation. After 12 h of fasting, the animals were anesthetized with tribromoethanol (0.1 mL/10 g, Sigma-Aldrich, Batch No. A103417) dissolved in tert-amyl alcohol as intraperitoneal anesthesia. Then, anesthetized mice were fixed on a stereotactic locator.
The modeling method is consistent with our previous research (Wang Y et al 2019). SZL was purchased from Shandong Wohua Pharmaceutical Co., Ltd. (Batch No: Z20120010), which dissolved in 0.5% carboxymethyl cellulose (CMC). The doses of 1.3, 2.6, and 5.2 mg/kg/d were, respectively, for SZL high-dose treatment group (SZL-H), SZL medium-dose treatment group (SZL-M), and SZL low-dose treatment group (SZL-L). Hydrochloric acid donepezil tablets were purchased from Eisai Pharmaceutical Company Limited (Batch No.1706067), which was dissolved in 0.5% CMC at a dose of 0.92 mg/kg/d. The control group (C) and model group (M) mice were given an equal volume of 0.5% CMC at a dose of 10 ml/kg/day. Penicillin was given 1 week after modeling, and the mice were given an adaptive feeding regimen for 1 month. All mice were given the drug continuously for 3 months.
Morris water maze test
Morris water maze test (MWM) was carried out in a quiet room to evaluate spatial memory function. The water is injected into the pool, the temperature is kept at 22 ± 2 °C, and the depth is more than 1 cm above the platform. White paper is pasted on the inner wall of the pool to ensure that the black inner wall is completely covered. Dissolve 0.3 kg of skimmed milk powder in the water, stir well until completely dissolved, and then pour into the sink and mix well. Four quadrants on the inner wall of the pool are visual sign with different graphics and colors, which remain unchanged during the experiment. The circular pool was divided into four quadrants, among which there was a platform in the third quadrant, whose position remained unchanged throughout the experiment. The whole experiment lasted for 6 days, including 5 days of navigation testing and 1 day of the withdrawal experiment. The navigation test included 3 days of training and 2 days of testing. On the 3 days of training, the mice were first guided to stand on the platform for 10 s to help the mice recognize and remember the platform’s position, and the mice were then allowed to enter the water facing the pool wall in quadrants 1, 2, and 4. The time required for the mice to find the hidden platform was recorded within 120 s, which was the escape incubation period of the mice, and the distance and route the mice traveled were recorded. If the mouse could not find the platform hidden below the water’s surface, it was removed from the water and placed on the platform for 10 s to help it remember the platform’s position, and the escape latency period of the mouse was recorded as 120 s. The hidden platform test was carried out for 5 consecutive days, and on the fourth day, the mice were not guided to remember the platform position before swimming. In the platform withdrawal experiment, the platform was removed. The test time of each mouse was 120 s and the number of times before the mouse crossed the platform was recorded, and the time the mice remained in the target quadrant was recorded.
Transmission electron microscope
After paraformaldehyde perfusion in the mice, the hippocampus was carefully stripped from each animal. The CA1 area of the hippocampus was cut with a razor blade and immediately placed into an EP tube containing 2.5% glutaraldehyde to fix the hippocampal tissue. After standing at 4℃ for 2 h, the sample was rinsed with 0.1 M PBS buffer every 15 min. This process was repeated three times, and then, the specimen was placed in an EP tube filled with 0.1 M PBS buffer. Upon completion of these operations, the specimens were immediately sent to the the Electron Microscope Room of Brain Hospital of Beijing Army General Hospital for additional preparation into ultrathin sections.
Immunohistochemical
The whole-brain tissue of each mouse was removed and soaked in a 4% paraformaldehyde fixation solution for 2 days after perfusion fixation, and the fixation liquid was changed every 24 h. Then, embedding was carried out, and the slices were dehydrated and made transparent; the embedding thickness was 4 μm. After the slices were baked in an incubator at 60℃ for 1 h, gradient alcohol dewaxing was performed; then, the antigen repair box was filled with a citrate repair solution, and the slices were inserted for microwave repair. After repair, each section was washed with PBS 3 times for 5 min each time. The endogenous peroxidase blocker of the ultrasensitive SP rabbit (KTI-9707, MBX) was added and left to stand for 10 min; then, the brain slices was cleaned as before. After incubation by dropwise addition of a nonspecific dye blocker for 10 min, the droplets were removed directly, and the primary antibody was added for incubation overnight. On the second day, the wet box was removed and placed at room temperature for 1 h, washed with PBS, and combined with the biotin-labeled sheep anti-rabbit IgG polymer.
After washing, streptomycin anti-biotin protein-peroxidase was added. After DAB color development, hematoxylin was reapplied. After gradient alcohol dewatering, the film was sealed. Antibodies and dilutions used in this study include: PI3K (1:500, ab151549, Abcam, USA), Akt (1:500, ab179463, Abcam, USA), mTOR (1:1000, ab2732, Abcam, USA), MBP (1:500, ab78896, Cell Signaling Technology, USA), Aβ42 (1:2000, ab201060, Abcam, USA), and six mice were taken from each group. The slices were observed under a × 20 objective lens of an optical microscope. The Aβ plaques and the distribution of myelin sheathing were observed under a × 5 objective lens of an optical microscope.
Western blot
Brain tissue was added to RIPA tissue/cell lysate (R0010, Solarbio) at a ratio of 1:9 and left on ice for half an hour. Then, the brain tissue was centrifuged at 4℃ for 15 min at a speed of 12,000 r/ min. The Bicinchoninic acid (BCA) method was used to measure the protein concentration and calculate the amount of protein loading. The glue concentration was determined according to the molecular weight of the protein; then, separation of protein by SDS–polyacrylamide gel electrophoresis (SDS-PAGE), electron transfer, and sealing was carried out to incubate the primary antibodies overnight. Antibodies and dilutions used in this study include: PI3K (1:1000, ab151549, Abcam, USA), Akt (1:1000, ab179463, Abcam, USA), p-Akt (1:1000, ab8805, Abcam, USA), mTOR (1:1000, ab2732, Abcam, USA), p-mTOR (1:1000,5536, Cell Signaling Technology, USA), MAG (1:1000, ab89780, Abcam, USA), MBP (1:1000, ab78896, Cell Signaling Technology, USA), MOG (1:1000, ab32760, Abcam, USA), PLP (1:1000, ab28486, Abcam, USA), Aβ42 (1:1000, ab201060, Abcam, USA), and β-actin (1:10000, ab6276, Abcam, USA). On the second day, the primary antibody was shaken for 1 h and then cleaned three times with TBST for 10 min each time. Then, the HRP-conjugated secondary antibodies were incubated for 1 h and cleaned. ECL was performed for visualization. Each target protein was scanned across three bands, and the gray values of the target band and β-actin were analyzed. β-actin was taken as an internal reference, and Image J was used to calculate the gray value of the target band/β-actin, after which additional statistical analysis was performed.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Sample preparation, total RNA extraction of a tissue, RNA inversion in vitro, and qPCR were performed following the protocol described in our previous research (Mana et al 2019). The mRNA levels of GAPDH were used as normalization controls. Finally, the relative quantitative method of 2−∆∆Ct was used to determine the relative expression levels of each target gene. The primer sequences to use quantitative RT-qPCR were as follows in Table1;
Table 1.
The primer sequence
| Name | Forward | Reverse | Size (bp) |
|---|---|---|---|
| PI3K | TCCAAATACCAGCAGGATCA | ATGCTTCGATAGCCGTTCTT | 137 |
| Akt | GAACGGCCTCAGGATGTGGA | GGTGCGCTCAATGACTGTGG | 144 |
| mTOR | GCTCCTGGGTGAGAGAGCTG | CAGGCTGCTGGAGCTTGTTG | 137 |
| MAG | ACCTTGCAGTTCGAGGGTTA | CTCCCTCAACACCATCCCAT | 174 |
| MBP | GCCGTAGAGAAGCTGTGGGT | GACACGGTTGCTCTGCGATG | 90 |
| MOG | GAGCAAGCACCTGAATACCG | ACTCCATTGCTGCCTCTTCT | 160 |
| PLP | GCTGTCAGGCAGATCTTTGG | GAGCTTGATGTTGGCCTCTG | 112 |
Statistical analysis
SPSS 20.0 software and GraphPad Prism 8 were used to carry out the statistical analyses and data mapping. Pursuant to the assumptions of a normal distribution and homogeneous variances, one-way ANOVA between groups was selected, and an LSD t test was used to compare pairs. Conversely, the Kruskal–Wallis H test was used to compare multiple samples. P < 0.05 determined statistical significance, with P < 0.01 used to flag a greater significance level.
Results
SZL modulate the pathology and protein expression of Aβ42
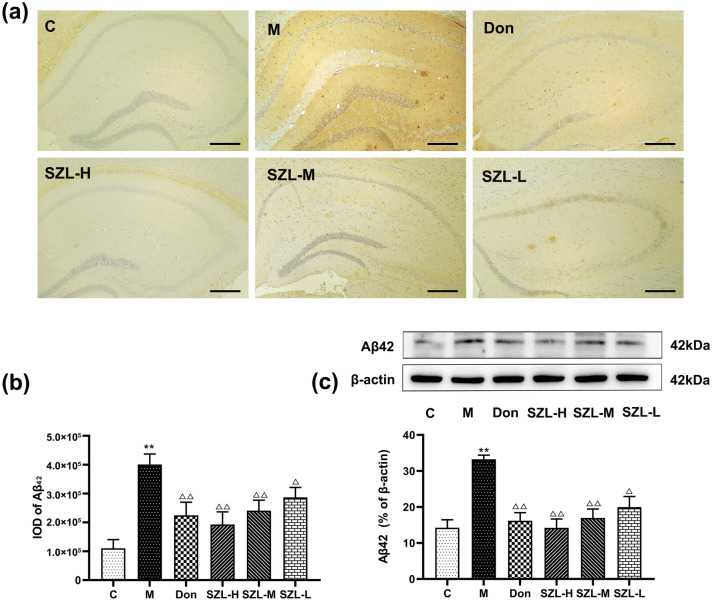
We tested the level of Aβ42 in different groups, and the immunohistochemical results showed that the brain slices of the M group showed significant deposition of Aβ plaques compared with the brain slices of the C group (P < 0.01). Mice in the Don group had Aβ plaques deposition, but levels were significantly lower in that group than in the M group (P < 0.01). For the mice in the different SZL dose groups, the amounts of Aβ plaques in the SZL-H and SZL-M groups were significantly lower than the amount in the M group (P < 0.01), whereas the SZL-L group had obvious Aβ plaques deposition (P < 0.05) (Fig. 1a, b).
Fig. 1.
Immunohistochemistry and Western blot results of Aβ42 in STZ-induced SAD mice. Results are shown as mean ± SD (n = 6, scale bar = 50 μm). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
Western blot showed that the level of Aβ42 in the M group was significantly higher than that in the C group (P < 0.01). The levels of Aβ42 in the Don, SZL-H, and SZL-M groups were significantly lower than that in the M group (P < 0.01), but the level in the SZL-L group was lower than that in the M group (P < 0.05) (Fig. 1c).
Effects of SZL on spatial learning and memory in STZ-induced SAD mice
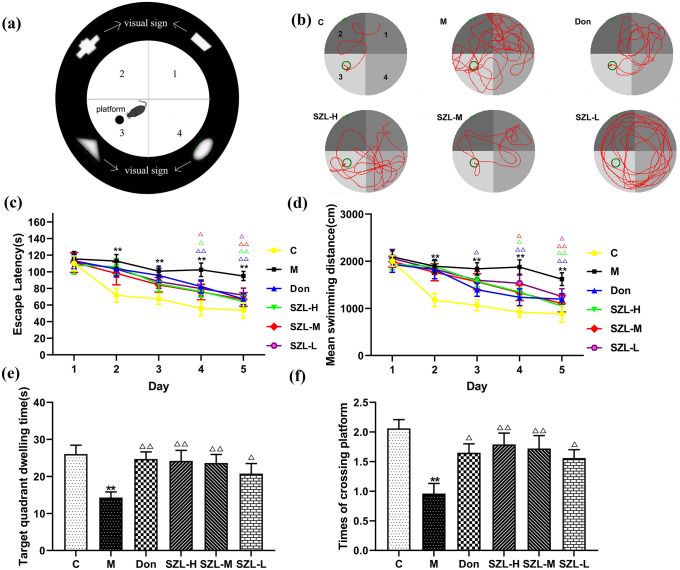
We used the MWM to assess the learning and memory of mice with STZ-induced SAD for 3 months and to investigate whether SZL could improve the memory deficits. We observed that mice in the C group moved the shortest distance before reaching the platform. The activity moving track of mice in the M group was the most complex in the moving track map, and the activity moving track of mice in the intervention group was less than that of the M group (Fig. 2b).
Fig. 2.
Behavioral tests of STZ-induced SAD mice. a Simple pattern of MWM: The figures represent the first, second, third, and fourth quadrants, respectively. Each quadrant of the inner wall of the MWM has visual signs to help the mice remember route. The platform is in the third quadrant. b effect of SZL treatment on the moving track. The first quadrant is in the upper left corner of the moving track map, and the remaining quadrants are 2, 3, and 4 in the counterclockwise direction. The moving track maps for the donepezil and SZL groups (SZL-H and SZL-L) were short. The effect of SZL treatment on Escape latency, d mean swimming distances, e target quadrant dwelling time, and f times of crossing platform in the MWM test. Results are shown as mean ± SD (n = 15). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
As the number of swimming days increased, the escape latency of the mice in each group was shortened. From the second to the fifth day, the escape latency of the M group was significantly longer than the C group (P < 0.01). On the first day of the test period, the escape latency of mice in the SZL-H and SZL-M groups was shorter than that of mice in the M group (P < 0.05), whereas the escape latency of mice in the Don group was significantly shorter than that of mice in the M group (P < 0.01). On the second day of the test period, the escape latency of the mice in each treatment group, except the SZL-L group (P < 0.05), was significantly shorter than that of mice in the M group (P < 0.01) (Fig. 2c).
Additional assessments showed that the mean swimming distances of the mice in each group decreased continuously with an increase in swimming days. Compared with results in the C group, the mean swimming distances of the mice in the M group increased significantly from the second to the fifth day of the experiment (P < 0.01). On the first day of the test period, the average swimming distance of the Don group decreased compared with that of the M group (P < 0.01). On the second day of the test period, the average swimming distances of all other groups except the SZL-L group were significantly reduced compared with the distance of the M group (P < 0.01) (Fig. 2d).
The time spent in the target quadrant (Fig. 2e) was significantly decreased, and the number of times crossing the platform (Fig. 2f) also was significantly decreased in the M group compared with the C group (P < 0.01). Compared with results in the M group, the numbers of times crossing the platform and the time spent in the target quadrant in the Don, SZL-H, and SZL-L groups were increased and prolonged, respectively (P < 0.01or P < 0.05).
Effect of SZL on the ultrastructure of myelin in STZ-induced SAD mice
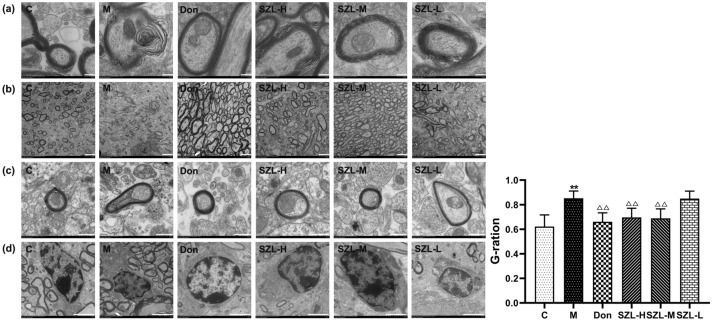
The lamellar structure of the myelin sheath in the C group was clear and complete, while the lamellar structure of the myelin sheath in the M group was severely degraded. In the picture, the edge of myelin sheath in the M group is blurred, and severe destruction of the myelin sheath structure is evident. Compared with the M group, the myelin sheath structure of the treated mice in each group was significantly repaired, among which the repair effect of D group was the best (Fig. 3a).
Fig. 3.
Treatment with SZL enhances remyelination in STZ-induced SAD mice. a The ultrastructures of the myelin sheaths in the hippocampal CA1 areas of mice in each group were observed under transmission electron microscope at × 1.2k. The effects of SZL on the G-ratio of mice with SAD were observed under a transmission electron microscope at b × 1.2k and c × 8k. d The ultrastructures of oligodendrocytes in the hippocampal CA1 areas of mice in each group were observed under a transmission electron microscope at × 2.5k. e The G-ratios of the myelin sheaths in hippocampal CA1 regions were calculated by randomly selecting five fields under the transmission electron microscope at 1200 times the field of vision. a Scale bar = 200 nm, b scale bar = 2.0 μm, c scale bar = 500 nm, and d scale bar = 2.0 μm (n = 3). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
The G-ratio is the ratio of axon diameter to the total fiber diameter, including the myelin sheath (Xie et al 2014). The G-ratio of the myelin sheaths in the CA1 region of the hippocampus was calculated by randomly selecting five fields under the transmission electron microscope under a visual field of × 1.2k. Previous G-ratio calculations reported a mean of 0.65, and the measure is a potential biomarker for white-matter microstructures (Jung et al 2018). Compared with the G-ratio in the M group, those of the Don group and C group were decreased in our study; the G-ratio of the Don group was the closest to 0.65 (Fig. 3b, d).
The single myelin sheaths in the hippocampal CA1 regions of mice in each group were observed under a visual field of × 8k. The diameter of single axons in the M group was small, and the myelin sheath was compact and thick. Compared with results in the C group, the axon diameter of the M group was significantly increased, and the myelin sheath became thinner. The layer structure remained unclear. Compared with results in the M group, the diameter of single axons decreased and the thickness of the myelin sheath increased in the Don, SZL-H, and SZL-M groups, whereas the diameter of single axons increased and the myelin sheath became thinner in the SZL-L group. These results indicate that SZL and donepezil can repair the pathological changes of myelin sheath ultrastructure injury to a certain extent and can increase the thickness of the myelin sheath in STZ-induced SAD mice (Fig. 3c).
Furthermore, the ultrastructure of oligodendrocyte in the hippocampal CA1 area of each group mice was observed under transmission electron microscope at × 2.5k. The oligodendrocytes in the C group had intact structures, continuous nuclear membranes, uniform distribution of nuclear chromatin, and intact myelin sheath structures. In the M group, oligodendrocytes were pyknotic, chromatin was concentrated, and the surrounding myelin lamellar structure was blurred or even disintegrated. Compared with those of the M group, the oligodendrocytes and the surrounding myelin structures in the Don group, SZL-H group, and SZL-M group were improved, whereas the oligodendrocytes in the SZL-L group were pyknotic, and the surrounding myelin lamellar structure was ambiguous (Fig. 3d).
Effect of SZL on the expression of PI3K in STZ-induced SAD mice
As shown in Fig. 4, the number of positive PI3K cells in the hippocampus of the M group was significantly reduced compared with that of the C group (P < 0.01). The numbers of immunohistochemical-positive cells in the other drug groups, except SZL-L (P < 0.05), were significantly increased compared with those in the M group (P < 0.01) (Fig. 4a, b).
Fig. 4.
Immunohistochemistry, western blot, and RT-qPCR results of PI3K in STZ-induced SAD mice. Results are shown as mean ± SD (n = 6, scale bar = 50 μm). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
RT-qPCR results showed that, compared with the C group, the expression of PI3K mRNA in the M group showed a tendency to decrease, but there was no statistical difference (P > 0.05). Compared with the M group, the expression of PI3K mRNA in Don and SZL groups showed an increased trend, but there was no statistical significance (P > 0.05) (Fig. 4c). Western blot results showed that PI3K protein expression was significantly reduced in the M group compared with the C group (P < 0.01). Compared with the M group, all drug groups except SZL-L had significantly increased expression (P < 0.01) (Fig. 4d).
Effect of SZL on the expression of Akt in STZ-induced SAD mice
The immunohistochemical results showed that the number of positive Akt cells in the hippocampus of the M group was significantly reduced compared with the C group (P < 0.01). The number of immunohistochemical-positive cells in the other drug groups was significantly increased compared with the number in the M group (P < 0.01) (Fig. 5a, b).
Fig. 5.
Immunohistochemistry, western blot, and RT-qPCR results of Akt in STZ-induced SAD mice. Results are shown as mean ± SD (n = 6, scale bar = 50 μm). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
Furthermore, the expression of Akt mRNA in the M group was decreased compared with expression in the C group (P < 0.05). Compared with expression in the M group, the Akt mRNA expression in the Don and SZL-H groups increased (P < 0.05) (Fig. 5c).
Western blot results showed that Akt and p-Akt protein expressions were significantly reduced in the M group compared with the C group (P < 0.01). Compared with the M group, Akt and p-Akt protein expressions in all drug groups were significantly increased except SZL-L group (P < 0.01) (Fig. 5d, e).
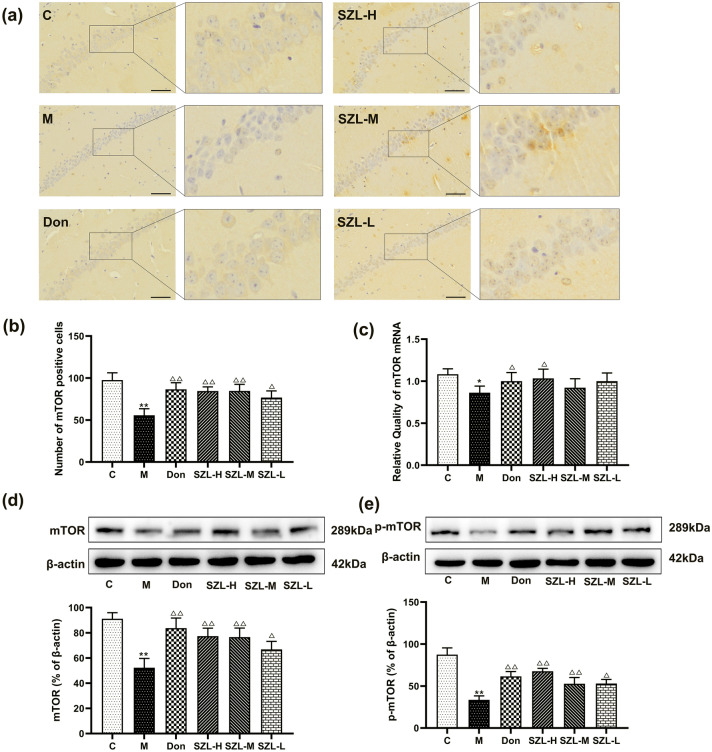
Effect of SZL on the expression of mTOR in STZ-induced SAD mice
Immunohistochemical results showed that, compared with the C group, the M group had a significantly reduced number of mTOR-positive cells (P < 0.05). Compared with the number in the M group, the numbers of mTOR-positive cells in the Don, SZL-H, and SZL-M groups were significantly increased (P < 0.05) (Fig. 6a, b). The expression of mTOR mRNA in the M group was decreased compared with the expression in the C group (P < 0.05). Compared with expression in the M group, the mTOR mRNA expression in the Don and SZL-H groups was increased (P < 0.05) (Fig. 6c).
Fig. 6.
Immunohistochemistry, western blot, and RT-qPCR results of mTOR in STZ-induced SAD mice. Results are shown as mean ± SD (n = 6, scale bar = 50 μm). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
Western blot results showed that, compared with expression levels in the C group, the expression levels of mTOR and p-mTOR in the M group decreased (P < 0.01). In addition, the expression levels of mTOR and p-mTOR in the SZL-L group were increased compared with those in the M group (P < 0.05), whereas the expression levels of mTOR in the Don, SZL-H, and SZL-M groups were significantly increased (P < 0.01) (Fig. 6d, e).
Effect of SZL on the expression of MBP
Compared with expression in the C group, the expression of MBP in the hippocampus of the M group was significantly decreased (P < 0.01). Compared with expression in the M group, MBP expressions in all drug groups except the SZL-L group were significantly increased (P < 0.01) (Fig. 7a, b).
Fig. 7.
Immunohistochemistry, western blot, and RT-qPCR results of MBP in STZ-induced SAD mice. Results are shown as mean ± SD (n = 6, scale bar = 50 μm). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
RT-qPCR results showed that mTOR mRNA expression in the M group was significantly reduced compared with expression in the C group (P < 0.01). Compared with expression in the M group, the expression of mTOR mRNA in the Don and SZL-H groups was increased (P < 0.05) (Fig. 7c).
Western blot results showed that, compared with the expression level in the C group, the expression level of MBP in the M group was decreased (P < 0.01). The expression level of MBP in the SZL-M and SZL-L groups was increased compared with the level in M group (P < 0.05 or P < 0.01) (Fig. 7d).
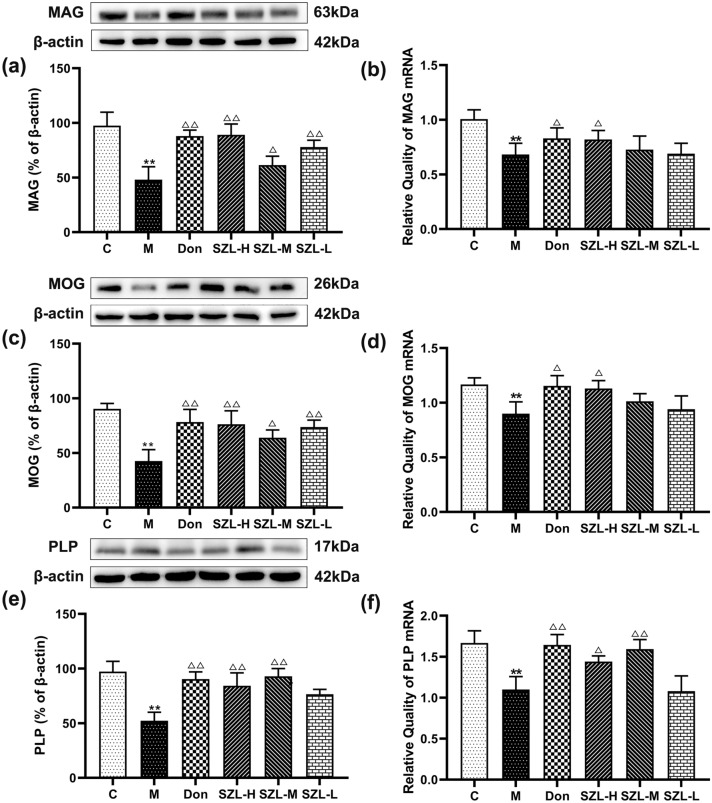
Effect of SZL on myelin-specific proteins
Western blot results showed that the expressions of MAG, MOG, and PLP in the hippocampus in the M group, compared with in the C group, were significantly decreased (P < 0.01) (Fig. 8). Compared with expressions in the M group, the expressions of MAG, MOG, and PLP in the Don and SZL groups were increased (P < 0.05 or P < 0.01) (Fig. 8a, c, e).
Fig. 8.
Therapeutic treatment with SZL enhances myelin protein expression in STZ-induced SAD mice. Expression of myelin-specific proteins (MAG, MOG, and PLP) in the CA1 region of the hippocampus after 3 months of intragastric administration was determined by western blot (a, c, e). Gene expression of myelin-specific proteins (MAG, MOG, and PLP) in the CA1 region of the hippocampus after 3 months of intragastric administration was determined by RT-qPCR (b, d, f). Results are shown as mean ± SD (n = 6). *P < 0.05, **P < 0.01 versus Control group, *P < 0.05, **P < 0.01 versus Model group; ANOVA
RT-qPCR results showed that MAG, MOG, and PLP levels in the hippocampus of the M group were significantly decreased compared with levels in the C group (P < 0.01). Compared with levels in the M group, MAG and MOG expression levels in the Don and SZL-H groups were increased (P < 0.05); PLP expression in the SZL-M group was increased (P < 0.05); and PLP expression in the Don and SZL-L groups was significantly increased (P < 0.01) (Fig. 8b, d, f).
Discussion
SZL, a prescription product approved by the China Food and Drug Administration for the treatment or prevention of mild-to-moderate AD, is composed of Codonopsis pilosula, Cassia twig, Radix Paeoniae Alba, Glycyrrhrizae Radix (processed with honey), Poria, Zingiberis Rhizoma, Cortex et Radix Polygalae, Rhizoma Acori Graminei, Os Draconis, and Concha Ostreae (Xing et al 2017). Our previous research indicated that SZL contained paeoniflorin, liquiritin, cinnamic acid, and glycyrrhizic acid (Zhenhong L et al 2020). The major chemical components of SZL have neuroprotective effects. Paeoniflorin may activate PI3K/Akt signaling pathway to protect against nerve cell injury induced by Aβ25-35 (Ling Liu et al 2014). Liquiritin has shown a neuroprotective effect against glutamate toxicity, predominantly through the Akt/GSK-3β pathways (Teng et al 2014). Cinnamic acid has decreased Aβ plaques in a murine model of AD model (Chandra et al 2019). Glycyrrhizic acid has exhibited a neuroprotective effect on cognitive function in scopolamine-induced cognitive impairment (Ban et al 2020).
Relevant studies have shown that ICV-STZ increases the expression of Aβ and promotes hyperphosphorylation of tau protein (Chu and Qian 2005; Kang and Cho 2014). In this study, C57BL/6 wild-type mice were injected with STZ to induce SAD. Mice in the ICV-STZ model showed cognitive and spatial memory dysfunction in the MWM tests in this study. After 4 months of modeling, the model mice showed white-matter microstructure damage.
Additionally, our experiment showed that the brain slices of mice in the M group had obvious amyloid plaques, with a significantly higher level of Aβ42 than that of mice in the C group. Therefore, we can infer that ICV-STZ successfully induces impaired memory function in the mouse model. White matter abnormalities, in myelin and oligodendrocytes in particular, could be mechanistically important in AD pathology (Nasrabady et al 2018). Myelin is a multilayer membrane that covers nerve fibers, and it is necessary for rapid impulse transmission (Motavaf, 2020).
Demyelination is myelin damage characterized by loss of the nerve sheath and relative fatigue of the nerve sheath and axons. Small changes in the myelin sheath thickness can result in substantial changes in conduction velocity, which may alter the function of neural circuits (Wang et al 2018). In this study, the changes in G-ratio of the mice in each group were consistent with the changes of myelin sheath ultrastructure observed under transmission electron microscopy, which also confirms that SZL has a protective effect on the myelin sheath in the mice with STZ-induced SAD. Oligodendrocytes form the myelin that ensheath the central nervous system (CNS) axons, which is essential for neuronal signaling (Butt et al 2019). Impairment of oligodendrocyte function disrupts myelin homeostasis, resulting in myelin loss and impaired neurotransmission, plasticity, and cognitive ability (Tong et al 2017). In this study, the oligodendrocytes were severely damaged and the surrounding myelin lamellar structure was obviously damage in the M group. Different doses of SZL could improve this situation to different degrees.
Myelin consists of multiple layers of lipids and proteins, including MAG, MBP, MOG, and PLP. They are involved in myelin formation, and MBP is the basic myelin protein that accounts for the majority of total myelin protein (Liu et al 2019). Abnormal PLP results in abnormal myelin and impaired CNS function. In an experimental optic nerve model in rats, chemical deacetylation of the fatty acid part of PLP led to decomposition of the myelin and dysfunction of the nerve (Pottie et al 2011). MAG is a highly glycosylated transmembrane protein that is selectively expressed on the myelin membrane near axons, and it exerts bidirectional regulation on axon growth. Some studies have shown that MAG can promote axon maturation, maintain myelin sheath integrity, and inhibit axon abnormality and excessive growth, thereby playing a neuroprotective role (Lossos et al 2015). MOG contributes to the maintenance and organization of the myelin sheath in neurons; it is a membranous component of the CNS that forms the insulating lipid layer around neurons. In addition to being the main structural proteins of myelin sheath, MBP and MOG are also surface markers of oligodendrocyte maturation (Guo et al 2010; Scolding et al 1989). In our study, myelin structural damage was accompanied by decreased expression of various myelin-specific proteins (MBP, MAG, MOG, and PLP) in STZ-induced SAD mice. After drug treatment, myelin damage was repaired to different degrees in each group of mice.
Donepezil has been widely used to treat AD in China and may affect AD biomarkers, such as hippocampal atrophy, Aβ plaques, tau-protein neurofibrillary tangles, and oxidative stress (Batarseh and Kaddoumi 2018; Goschorska et al 2018; Kim et al 2016; Zhang and Gordon 2018). Beyond those effects, donepezil improves nerve injury and ameliorates the histological structures of both the spinal cord and sciatic nerves as it reduced the swelling of myelin sheath in mice with STZ-induced DM (Atef et al 2019). Thus, donepezil was used as a positive drug in our experimental group.
The processes of sheath formation and myelin regeneration are strictly regulated by several coordinated signal transduction pathways, such as the Wnt/3-catenin, PI3K/Akt-mTOR, and ERK/MAPK pathways. Research has revealed that PI3K/Akt-mTOR signaling directs peripheral nervous system myelination at multiple critical levels, including at the onset of myelination and myelin growth (Figlia et al 2017). The results of this study indicated that donepezil and SZL can improve the expression of the key proteins and mRNA in the PI3K/Akt-mTOR pathway.
Collectively, SZL had similar or even better neuroprotective effects than donepezil in the mice with STZ-induced SAD in this study. Our results showed that the neuroprotective effect of SZL is related to improvements in the ultrastructure of the myelin sheath, oligodendrocytes, and expression of myelin-specific proteins (MAG, MBP, MOG, and PLP). We also found that SZL could affect the PI3K/Akt-mTOR signaling pathway. Therefore, we inferred that the neuroprotective effect of SZL on mice with SAD was related to the improvement of myelin injury, and its potential mechanism was related to the PI3K/Akt-mTOR signaling pathway. These findings provide experimental data support for SZL as an AD treatment in clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 81573927) and Special Fund for Basic Scientific Research Operating Fees of Central Universities (university-level projects, No. 2019-BUCMXJKY018).
Author’s contribution
GQ, YW, LM, ZL, and SH conducted experiments. GQ, YW, and ZL performed data analysis. GQ, YW, and ZL wrote or contributed to the writing of the manuscript. PW was responsible for experimental design and fund support, and approved the final version for publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Gaofeng Qin, Yahan Wang and Zhenhong Liu should be considered joint first author
References
- Atef MM, El-Sayed NM, Ahmed AAM, Mostafa YM. Donepezil improves neuropathy through activation of AMPK signalling pathway in streptozotocin-induced diabetic mice. Biochem Pharmacol. 2019;7(159):1–10. doi: 10.1016/j.bcp.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Ban JY, Park HK, Kim SK. Effect of glycyrrhizic acid on scopolamine-induced cognitive impairment in mice. Int Neurourol J. 2020;5(24):48–55. doi: 10.5213/inj.2040154.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh YS, Kaddoumi A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-beta load and related toxicity in a mouse model of Alzheimer’s disease. J Nutr Biochem. 2018;55(01):113–123. doi: 10.1016/j.jnutbio.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, De La Rocha IC, Rivera A. Oligodendroglial cells in Alzheimer’s disease. Adv Exp Med Biol. 2019;1175(2):325–333. doi: 10.1007/978-981-13-9913-8_12. [DOI] [PubMed] [Google Scholar]
- Chandra S, Roy A, Jana M, Pahan K. Cinnamic acid activates PPARα to stimulate lysosomal biogenesis and lower amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol Dis. 2019;4(124):379–395. doi: 10.1016/j.nbd.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WZ, Qian CY. Expressions of Abeta1-40, Abeta1-42, tau202, tau396 and tau404 after intracerebroventricular injection of streptozotocin in rats. Di Yi Jun Yi Da Xue Xue Bao. 2005;2(25):168–170. [PubMed] [Google Scholar]
- Figlia GA-O, Norrmén C, Pereira JA, Gerber D, Suter UA-O. Dual function of the PI3K-Akt-mTORC1 axis in myelination of the peripheral nervous system. Elife. 2017;7(6):e29241. doi: 10.7554/eLife.29241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol. 2016;283(1):501–511. doi: 10.1016/j.expneurol.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschorska MA-O, Gutowska I, Baranowska-Bosiacka IA-O, Piotrowska K, Metryka EA-O, Safranow K, Chlubek DA-O. Influence of acetylcholinesterase inhibitors used in Alzheimer’s disease treatment on the activity of antioxidant enzymes and the concentration of glutathione in thp-1 macrophages under fluoride-induced oxidative stress. Int J Environ Res Public Health. 2018;16(1):1–25. doi: 10.3390/ijerph16010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol. 2016;53(3):1741–1752. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia de Souza EST, do Val de Paulo MEF, da Silva JRM, da Silva Alves A, Britto LRG, Xavier GF, Lopes Sandoval MR, Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon. 2020;6(2):e03281. doi: 10.1016/j.heliyon.2020.e03281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Eviatar-Ribak T, Fau - Miskimins R Miskimins R Sp1 phosphorylation is involved in myelin basic protein gene transcription. J Neurosci Res. 2010;88(15):3233–3242. doi: 10.1002/jnr.22486. [DOI] [PubMed] [Google Scholar]
- Hou Y, Wang Y, Zhao J, Li X, Cui J, Ding J, Wang Y, Zeng X, Ling Y, Shen X, Chen S, Huang C, Pei G. Smart soup, a traditional Chinese medicine formula, ameliorates amyloid pathology and related cognitive deficits. PLoS One. 2014;9(11):e111215. doi: 10.1371/journal.pone.0111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes MR, Fang R, Houghton PJ. Effect of chinese herbal medicine on Alzheimer’s disease. Int Rev Neurobiol. 2017;135(1):29–56. doi: 10.1016/bs.irn.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Jung W, Lee J, Shin HG, Nam Y, Zhang H, Oh SH, Lee J. Whole brain g-ratio mapping using myelin water imaging (MWI) and neurite orientation dispersion and density imaging (NODDI) Neuroimage. 2018;182(15):379–388. doi: 10.1016/j.neuroimage. [DOI] [PubMed] [Google Scholar]
- Kang EB, Cho JY. Effects of treadmill exercise on brain insulin signaling and beta-amyloid in intracerebroventricular streptozotocin induced-memory impairment in rats. J Exerc Nutrition Biochem. 2014;18(1):89–96. doi: 10.5717/jenb.2014.18.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Kim HV, Lee DK, Yang SH, Kim Y. Rapid and sustained cognitive recovery in APP/PS1 transgenic mice by co-administration of EPPS and donepezil. Sci Rep. 2016;6(9):1–9. doi: 10.1038/srep34165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang S-Y, Wang J-G. Role of PI3K/Akt pathway in effect of paeoniflorin against Aβ25-35-induced PC12 cell injury. Zhongguo Zhong Yao Za Zhi. 2014;39(20):4045–4049. [PubMed] [Google Scholar]
- Liu BA-O, Xin W, Tan JR, Zhu RP, Li T, Wang D, Kan SS, Xiong DK, Li HH, Zhang MM, Sun HH, Wagstaff W, Zhou C, Wang ZJ, Zhang YG, He TC. Myelin sheath structure and regeneration in peripheral nerve injury repair. Proc Natl Acad Sci USA. 2019;116(44):22347–22352. doi: 10.1073/pnas.1910292116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossos A, Elazar N, Lerer I, Schueler-Furman O, Fellig Y, Glick B, Zimmerman BE, Azulay H, Dotan S, Goldberg S, Gomori JM, Ponger P, Newman JP, Marreed H, Steck AJ, Schaeren-Wiemers N, Mor N, Harel M, Geiger T, Eshed-Eisenbach Y, Meiner V, Peles E. Myelin-associated glycoprotein gene mutation causes Pelizaeus-Merzbacher disease-like disorder. Brain. 2015;138(9):2521–2536. doi: 10.1093/brain/awv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mana L, Feng H, Dong Y, Wang Y, Shi J, TianWangPA-Ohoo J. Effect of Chinese herbal compound GAPT on the early brain glucose metabolism of APP/PS1 transgenic mice. Int J Immunopathol Pharmacol. 2019;33(12):1–13. doi: 10.1177/2058738419841482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri MT, NaghizadehFau - Ghorbanzadeh BGhorbanzadeh B Fau - FarboodFarbood Y Fau - SarkakiSarkaki A Fau - BavarsadBavarsad BYAKK. Gallic acid prevents memory deficits and oxidative stress induced by intracerebroventricular injection of streptozotocin in rats. Pharmacol Biochem Behav. 2013;10(111):90–96. doi: 10.1016/j.pbb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Motavaf M, Sadeghizadeh MA-Ohoo, Babashah S. Dendrosomal nanocurcumin promotes remyelination through induction of oligodendrogenesis in experimental demyelination animal model. J Tissue Eng Regen Med. 2020;10(04):1–13. doi: 10.1002/term.3110. [DOI] [PubMed] [Google Scholar]
- Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018;6(1):22. doi: 10.1186/s40478-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neha SRK, Jaggi AS, Singh N. Animal models of dementia and cognitive dysfunction. Life Sci. 2014;109(2):72–86. doi: 10.1016/j.lfs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Pan WA-O, Wang Q, Kwak S, Song Y, Qin B, Wang M, Yamamoto Y. Shen-zhi-ling oral liquid improves behavioral and psychological symptoms of dementia in Alzheimer’s disease. Evid Based Complement Alternat Med. 2014;2014(2014):913687. doi: 10.1155/2014/913687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SA. A common pathogenic mechanism linking type-2 diabetes and Alzheimer’s disease: evidence from animal models. J Clin Neurol. 2011;7(1):10–18. doi: 10.3988/jcn.2011.7.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Saarimaki H, Bastin ME, Londono AC, Pettit L, Lopera F, Della Sala S, Abrahams S. Memory binding and white matter integrity in familial Alzheimer’s disease. Brain. 2015;138(8):1355–1369. doi: 10.1093/brain/awv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie IR, Higgins EA, Blackman RA. Cysteine thioesters as myelin proteolipid protein analogues to examine the role of butyrylcholinesterase in myelin decompaction. ACS Chem Neurosci. 2011;2(3):151–160. doi: 10.1021/cn100090g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkovic-Petrisic M, Osmanovic-Barilar J, Knezovic A, Hoyer S, Mosetter K, Reutter W. Long-term oral galactose treatment prevents cognitive deficits in male wistar rats treated intracerebroventricularly with streptozotocin. Neuropharmacology. 2014;4(77):68–80. doi: 10.1016/j.neuropharm. [DOI] [PubMed] [Google Scholar]
- Scolding NJ, FrithFau - Linington C S, Linington C Fau - Morgan BP, Morgan BpFau - Campbell AK, Campbell AkFau - Compston DA, Compston DA. Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J Neuroimmunol. 1989;22(3):169–176. doi: 10.1016/0165-5728(89)90014-3. [DOI] [PubMed] [Google Scholar]
- Shinohara M, FujiokaFau - Murray ME S, Murray Me Fau – Wojtas A, et al. Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer’s disease. Brain. 2014;137(5):1533–1549. doi: 10.1093/brain/awu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Hur BE, Bokara KK, Yang W, Cho HJ, Park KA, Lee WT, Lee KM, Lee JE. Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced alzheimer rat model. Yonsei Med J. 2014;55(3):689–699. doi: 10.3349/ymj.2014.55.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorial ME, El Sayed N. Protective effect of valproic acid in streptozotocin-induced sporadic Alzheimer’s disease mouse model: possible involvement of the cholinergic system. Naunyn Schmiedebergs Arch Pharmacol. 2017;6(390):581–593. doi: 10.1007/s00210-017-1357-4. [DOI] [PubMed] [Google Scholar]
- Teng L, Meng Q, Lu J, Xie J, Wang Z, Liu Y, Wang D. Liquiritin modulates ERK- and AKT/GSK-3β-dependent pathways to protect against glutamate-induced cell damage in differentiated PC12 cells. Mol Med Rep. 2014;10(2):818–824. doi: 10.3892/mmr.2014.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Leão R, Vimbela GV, Yalcin EB, Kay J, Krotow A, de la Monte SM. Altered temporal lobe white matter lipid ion profiles in an experimental model of sporadic Alzheimer’s disease. Mol Cell Neurosci. 2017;82(1):23–34. doi: 10.1016/j.mcn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristão Pereira C, Diao Y, Yin T, da Silva AR, Lanz B, Pierzchala K, Poitry-Yamate C, Jelescu IO. Synchronous nonmonotonic changes in functional connectivity and white matter integrity in a rat model of sporadic Alzheimer’s disease. Neuroimage. 2020;225(15):117498–117514. doi: 10.1016/j.neuroimage. [DOI] [PubMed] [Google Scholar]
- Wang SS, Zhang Z, Zhu TB, Chu SF, He WB, Chen NH. Myelin injury in the central nervous system and Alzheimer’s disease. Brain Res Bul. 2018;140(18):162–168. doi: 10.1016/j.brainresbull. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen F, P. W, Potential synaptic plasticity based Shenzhiling oral liquid for a SAD mouse model. Brain and Benavior. 2019;9(9):1385–1395. doi: 10.1002/brb3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Fu H, Je Z. Gene profiling in the dynamic regulation of the lifespan of the myelin sheath structure in the optic nerve of rats. Mol Med Rep. 2014;10(1):217–222. doi: 10.3892/mmr.2014.2227. [DOI] [PubMed] [Google Scholar]
- Xing S, Shen D, Chen C, Wu B, Chi H. Effect of the herbal formulation Shen-Zhi-Ling on an APP/PS1 mouse model of Alzheimer’s disease by modulating the biliverdin reductase/heme oxygenase 1 system. Exp Ther Med. 2017;14(3):1961–1966. doi: 10.3892/etm.2017.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamini P, Ray RS, Chopra K. Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer’s disease. Inflammopharmacology. 2018;26(1):39–55. doi: 10.1007/s10787-017-0372-x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Gordon ML. Clinical efficacy and safety of donepezil in the treatment of Alzheimer’s disease in Chinese patients. Clin Interv Aging. 2018;13(1):1963–1970. doi: 10.2147/CIA.S159920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhenhong L, Gaofeng Q, M. L Shenzhiling oral liquid protects STZ-injured oligodendrocyte through PI3K/Akt-mTOR pathway. Evidence Based Complementary and Alternative Medicine. 2020;2020(3):1–13. doi: 10.1155/2020/4527283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.