Abstract
Increased intestinal permeability and hepatic macrophage activation by endotoxins are involved in alcohol-induced liver injury pathogenesis. Long-term alcohol exposure conversely induces endotoxin immune tolerance; however, the precise mechanism and reversibility are unclear. Seventy-two alcohol-dependent patients with alcohol dehydrogenase-1B (ADH1B, rs1229984) and aldehyde dehydrogenase-2 (ALDH2, rs671) gene polymorphisms admitted for alcohol abstinence were enrolled. Blood and fecal samples were collected on admission and 4 weeks after alcohol cessation and were sequentially analyzed. Wild-type and ALDH2*2 transgenic mice were used to examine the effect of acetaldehyde exposure on liver immune responses. The productivity of inflammatory cytokines of peripheral CD14+ monocytes in response to LPS stimulation was significantly suppressed in alcohol dependent patients on admission relative to that in healthy controls, which was partially restored by alcohol abstinence with little impact on the gut microbiota composition. Notably, immune suppression was associated with ALDH2/ADH1B gene polymorphisms, and patients with a combination of ALDH2*1/*2 and ADH1B*2 genotypes, the most acetaldehyde-exposed group, demonstrated a deeply suppressed phenotype, suggesting a direct role of acetaldehyde. In vitro LPS and malondialdehyde-acetaldehyde adducted protein stimulation induced direct cytotoxicity on monocytes derived from healthy controls, and a second LPS stimulation suppressed the inflammatory cytokines production. Consistently, hepatic macrophages of ethanol-administered ALDH2*2 transgenic mice exhibited suppressed inflammatory cytokines production in response to LPS compared to that in wild-type mice, reinforcing the contribution of acetaldehyde to liver macrophage function. These results collectively provide new perspectives on the systemic influence of excessive alcohol consumption based on alcohol-metabolizing enzyme genetic polymorphisms.
Subject terms: Alcoholic liver disease, Monocytes and macrophages
Introduction
Alcohol and its metabolites induce systemic damage such as oxidative stress, hepatocyte injury, and mitochondrial damage. Habitual and excessive alcohol consumption gives rise to alcohol-related liver diseases (ALDs) including steatohepatitis, liver fibrosis, and liver cirrhosis. ALD remains the leading cause of death due to alcohol worldwide.
To date, many studies have tried to elucidate the pathogenesis of ALD from the viewpoint of the gut-liver axis1,2. Alcohol consumption results in high intestinal permeability, the overgrowth of gram-negative bacteria in the proximal small intestine and increased circulating endotoxin following bacterial translocation3. Endotoxins are pathogen-associated molecular patterns (PAMPs) recognized by Toll-like receptors (TLRs). TLR4 and CD14 are essential components for macrophages/monocytes activation by circulating lipopolysaccharides (LPS)4, the major components of the outer membrane of gram-negative bacteria. A major innate immune response of TLR4 binding is NF-kB-mediated activation of pro-inflammatory cytokines such as tumor necrosis factor-alfa (TNF-α) and IL-65–8.
Long-term alcohol consumption has negative effects on host immune functions, which might contribute to suppressed immunity and potential susceptibility to systemic infection9. The negative effects of alcohol and its metabolites on monocytes have been studied both in humans and in murine models. These studies have demonstrated the evidence of reduced pro-inflammatory cytokine production in response to LPS stimulation and dysregulated antigen presentation on monocytes owing to chronic alcohol consumption10–12; however, the precise mechanism and reversibility with alcohol withdrawal still remain unclarified. Human peripheral blood monocytes have been classified according to their surface expression patterns of CD14 and CD16 into the following three major subsets: classical (CD14highCD16−), intermediate (CD14highCD16+), and non-classical (CD14lowCD16+)13. These subsets are functionally distinct and play different roles in various diseases14,15, however, the contribution of each cell subset to ALD pathogenesis has not been well studied.
Acetaldehyde is a well-known highly toxic metabolite of alcohol. Consumed alcohol is mainly metabolized by alcohol dehydrogenases (ADHs) or cytochrome P450 2E1 (CYP2E1) to acetaldehyde, which is then metabolized to acetate by aldehyde dehydrogenases (ALDHs). In East Asians, the fast-metabolizing form of ADH1B is encoded by ADH1B*2 (rs1229984), affecting more than 90% of the Japanese population16. ALDH2 is a major enzyme in acetaldehyde oxidation in humans and its genetic polymorphism (rs671) determines blood or tissue acetaldehyde concentration after alcohol consumption. Individuals with ALDH2 *1/*2 or ALDH2 *2/*2 polymorphisms have much lower activity than those with homozygous ALDH2 *1/*1. Approximately half of the population in East Asia possesses the ALDH2 *2 allele, in contrast to non-Asians, who rarely possess this17. ALDH2*2 allele carriers show high acetaldehyde concentrations after alcohol consumption18, and extensive DNA damage is induced by chronic excessive drinking19,20. Acetaldehyde accumulated in the intestine disrupts the barrier function, which potentially involves the gut–liver axis21,22. In addition to the promotion of ethanol-induced gut barrier dysfunction in mice with ALDH2 deficiency21, acetaldehyde impairs microtubule-dependent protein trafficking pathways leading to hepatocyte ballooning23, results in the formation of immunogenic protein adducts24, and increases hepatic stellate cell activation and production of fibrillar collagen25. In contrast, a meta-analysis of Asian studies has shown a strong protective effect of ALDH2 deficiency against alcoholic liver cirrhosis as well as alcohol dependence26. Furthermore, the results of large surveys of Japanese alcohol-dependent (AD) patients have demonstrated that the inactive ALDH2*1/*2 genotype is associated with a lower risk of alcoholic liver cirrhosis27,28. These conflicting findings suggest that acetaldehyde is associated with both fascinating and protective aspects with respect to the development of alcoholic cirrhosis, which prompted us to examine the role of acetaldehyde as an inflammatory mediator, especially in regulating immune response during alcohol exposure.
In the current study, we examined changes in immunological impairment of monocytes in AD subjects following alcohol withdrawal. We unexpectedly noticed that functional impairment of monocytes was closely associated with genetic polymorphisms in alcohol-metabolizing enzymes and thus explored the potential mechanism involved using in vitro assays and murine models.
Material and methods
Patients and samples
This study included 72 Japanese male AD patients (average age 52.4 years) who were admitted to the National Hospital Organization Kurihama Medical and Addiction Center for the treatment of alcoholism (Table 1). They fulfilled the following criteria: continued to drink more than 60 g/day of ethanol, did not use alcohol-aversive drugs, were not related to another etiology of liver disease (hepatitis B, C, PBC, and AIH), and had never been admitted for abstinence previously. Blood samples were collected early in the morning, after overnight fasting, on the next day of admission, and at the end of the 4-week of hospital stay. A BD vacutainer cell preparation tube™ was used to isolate peripheral blood monocytes (PBMCs), which were stored at − 80 °C. Thirteen PBMC samples as controls were collected from healthy male donors without drinking habits and were processed. The institutional review board of Keio University School of Medicine and National Hospital Organization Kurihama Medical and Addiction Center approved all human studies (No. 20140211) according to the guidelines of the 1975 Declaration of Helsinki (2008 revision). The study subjects were prospectively recruited, and each subject provided prior written informed consent for blood sampling, study participation, and analysis of clinical data.
Table 1.
Characteristics of patients with alcohol dependence.
| Background characteristics | Mean ± SD | |||
|---|---|---|---|---|
| Age, yrs | 52 ± 4 | |||
| Cirrhosis (Y/N) | 8/64 | |||
| Child Pugh score (A/B/C) | 5/3/0 | |||
| AST, IU/L | 86 ± 16 | |||
| ALT, IU/L | 51 ± 8 | |||
| γ-GTP, IU/L | 370 ± 61 | |||
| Alb, g/dL | 4.1 ± 0.6 | |||
| T-bil, mg/dL | 1.0 ± 0.4 | |||
| PT-INR | 1.02 ± 0.22 | |||
| Type IV collagen, ng/mL | 240 ± 20 | |||
| FBS, mg/dL | 92 ± 10 | |||
| WBC, × 103/μL | 6.0 ± 0.3 | |||
| Hb, g/dL | 13.8 ± 0.2 | |||
| Plt, × 104/μL | 20.3 ± 1.1 | |||
| TC, mg/dL | 182 ± 28 | |||
| HDL-C, mg/dL | 58 ± 9 | |||
| TG, mg/dL | 132 ± 14 |
| Ethanol (g/day) | n | |||
|---|---|---|---|---|
| 0–49 | 2 | |||
| 50–99 | 20 | |||
| 100–149 | 32 | |||
| 150–199 | 8 | |||
| 200–249 | 3 | |||
| 250–299 | 3 | |||
| 300–349 | 4 |
| ALDH2 (Glu487Lys) | ADH1B (His47Arg) | |||
|---|---|---|---|---|
| *1/*1 | *1/*2 | *2/*2 | Total | |
| *1/*1 | 14 | 13 | 17 | 44 |
| *1/*2 | 7 | 11 | 10 | 28 |
| Total | 21 | 24 | 27 | 72 |
ADH1B and ALDH2 genotypes
The DNA of each subject was extracted from their blood samples using a QIAamp DNA Blood Mini Kit (Qiagen, USA). Polymerase chain reaction–restriction fragment length polymorphism methods were used to analyze lymphocyte DNA samples from all subjects, without knowledge of their status, to determine their ADH1B and ALDH2 genotypes29.
Isolation of human PBMCs
Human PBMCs were isolated by density centrifugation. Human blood was collected into BD Vacutainer CPT (BD, USA) tubes and centrifuged at 470× g for 20 min at room temperature. PBMCs were collected at the interphase, washed, and resuspended in FACS buffer.
Flow cytometric analysis
We performed cell surface staining to characterize the cell populations in each sample. Briefly, cells were incubated with specific fluorescence-labeled monoclonal antibodies at 4 °C for 30 min. CD14 and CD16 were used to identify the monocyte subpopulation. CD3, CD56, BDCA2, and CD123 were used for T cells, NK cells, NKT cells, and plasmacytoid dendritic cells. Events were acquired with a FACS Canto II (Becton Dickinson, USA) and analyzed with FlowJo software v.10.3 (Tree Star Inc., USA) (https://www.flowjo.com/solutions/flowjo). The following antibodies were used for cell surface staining: PE-Cy7 Mouse Anti-Human CD14 (BD Pharmingen), APC-Cy7 Mouse Anti-Human CD16 (BD Pharmingen), FITC Mouse Anti-Human CD3 (BD Pharmingen), PE-Cy7 Mouse Anti-Human CD56 (BD Pharmingen), APC anti-human CD303 (BDCA-2) Antibody (BioLegend), and PerCP/Cyanine5.5 anti-human CD123 Antibody (BioLegend).
Isolation of CD14+ monocytes and cell stimulation
CD14+ monocytes were isolated by immunomagnetic positive selection using the EasySep™ Human CD14 Positive Selection Kit (STEMCELL technologies, Canada). Acquired CD14+ monocytes were seeded in 24-well plates at 5 × 104 cells/well and stimulated with 5 ng/mL LPS for 24 h at 37 °C.
RT-qPCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, USA), as per the manufacturer’s protocol. Complementary DNA was synthesized by reverse transcription using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, USA). To measure the quantity, real-time PCR was performed using the SYBR green RT-qPCR kit with the predesigned primers. The level of target gene expression was normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression in each sample. The following primers were used for SYBR green assays (BIO-RAD, Japan): TNF (qHsaCEP0040184), IRAK-1 (qHsaCEP0057865), IRAK-3 (IRAK-M; qHsaCIP0031947), CD274 (PD-L1;qHsaCIP0039192), and PDCD1LG2 (PD-L2; qHsaCID0015625).
Quantification and analysis of cytokines
Cytokine production in cell supernatants was measured using the BD™ Cytometric Bead Array (CBA) Human Th1/Th2/Th17 cytokine kit (BD, USA) and were acquired on the BD FACS Canto™ II flow cytometer. Acquired data were analyzed using FCAP Array™ software v.3.0 (BD, USA) (https://www.bdbiosciences.com/jp/applications/research/bead-based-immunoassays/analysis-software/fcap-array-software-v30/p/652099).
Fecal sample collection and DNA extraction
Fresh fecal samples were collected using stool collection tubes and an anaerobiosis generator was added to the samples to favor the preservation of anaerobic bacteria at the outpatient clinic of Keio University Hospital. We selected four AD patients who achieved the recovery from diminished cytokines production following alcohol abstinence. The samples were processed immediately and frozen at − 80 °C for bacterial preservation. Bacterial DNA was isolated as described previously30. In brief, bacterial DNA was isolated by the enzymatic lysis method using lysozyme (Sigma-Aldrich, USA) and achromopeptidase (Wako). DNA samples were then purified by treating with ribonuclease A (Wako, Japan), followed by precipitation with 20% polyethylene glycol solution (PEG6000 in 2.5 M sodium chloride). DNA was then pelleted by centrifugation, rinsed with 75% ethanol, and dissolved in tris–EDTA buffer.
16S rRNA metagenomic analysis
The hypervariable V3–V4 region of the 16S gene was amplified using Ex Taq Hot Start (TAKARA Bio Inc., Japan) and subsequently purified using AMPure XP (Beckman Coulter, USA). Mixed samples were prepared by pooling approximately equal amounts of each amplified DNA sample and sequenced using the Miseq Reagent Kit V3 (600 Cycle) and Miseq sequencer (Illumina, USA), according to the manufacturer’s instructions. Sequences were analyzed using the QIIME2 software package v.2019.10 (https://qiime2.org) 31,32. Paired-end sequences were joined using a fastq-join tool in the ea-utils software package (https://doi.org/10.2174/1875036201307010001). High-quality sequences per sample (15,000) were randomly chosen from quality filter-passed sequences. After trimming both primer sequences using cutadapt (https://doi.org/10.14806/ej.17.1.200) followed by chimera detection by the USEARCH de novo method33, the sequences were assigned to operational taxonomic units (OTUs) using the UCLUST algorithm34 with a sequence identity threshold of 96%. Taxonomic assignments of each OTU were made by similarity searching against the publicly available 16S (RDP version. 10.27 and CORE update 2 September 2012) and NCBI genome database using the GLSEARCH program. Data were rarefied to 10,000 sequences per sample, as determined by the rarefaction curves. Relative abundances of the community members were determined using the rarefied data. UniFrac analysis was performed as described previously35. To determine bacterial taxonomy that explained differences between conditions, the linear discriminant analysis effect size method was used36.
Preparation of MAA-Alb and in vitro stimulation
As previous reported, MAA-Alb was prepared by reacting 1.0 mM acetaldehyde and 1.0 mM malondialdehyde with 2 g/L of bovine serum albumin in 0.1 M phosphate buffer containing 2 mM diethylenetriaminepentaacetic acid and 2 mM phytic acid at 37 °C for 3 days and ultrafiltration of the phosphate buffer37. CD14+ monocytes collected from healthy donors were stimulated with LPS (1 ng/mL), MAA-Alb (10–25 µg/mL), or the combination for 18 h in vitro. In some experiments, CD14+ monocytes following the first treatment were further stimulated with 5 ng/mL LPS for 24 h in vitro, followed by cytokine quantification in supernatants.
Animal experiments
Animals and chronic-plus-binge ethanol feeding
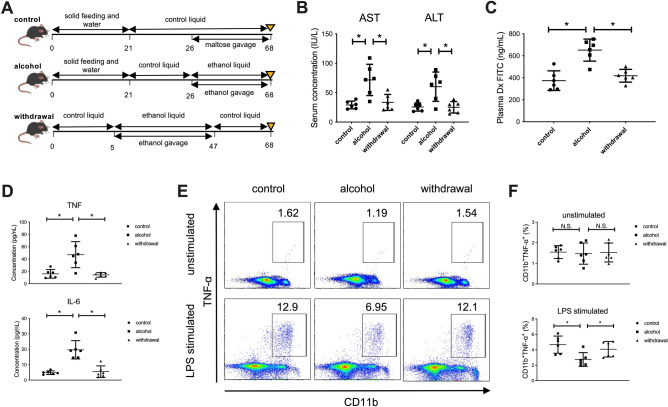
We used 6-week-old male C57BL/6 J (wild-type; WT) mice weighing between 17 and 19 g. Mice were obtained from CLEA Japan Inc. The mice were randomly divided into three groups, the control, alcohol group, and withdrawal groups (Fig. 5A), treated differently as follows:
The control group received the control Lieber-DeCarli diet38 ad libitum for 6 weeks. Maltose solution was administered via gavage twice per week.
The alcohol group received the control Lieber-DeCarli diet ad libitum for the first week and the ethanol Lieber-DeCarli diet containing 5% (v/v) ethanol for the following 6 weeks. Ethanol solution was administered via gavage twice per week.
The withdrawal group was treated the same as that in the alcohol group for 6 weeks and received the control Lieber-DeCarli diet for the following 3 weeks.
Figure 5.
Increased intestinal permeability and the subsequent influx of PAMPs mediated by continuous alcohol administration to mice result in suppressed production of inflammatory cytokines from CD11b+ macrophages in the liver. (A) Study design. Control mice (n = 6) received a control liquid diet for 26 days. They were gavaged with isocaloric maltose dextrin twice per week for the last 6 weeks. Alcohol-treated mice (n = 6) received the control liquid diet for 5 days and the 5% ethanol liquid diet for 6 weeks. Withdrawal-group mice (n = 6) received the control liquid diet for 5 days and 5% ethanol liquid diet for 6 weeks, followed by feeding with the control liquid diet for 3 weeks as a withdrawal period. (B) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. (C) Intestinal permeability evaluated by measuring the amount of fluorescence from dextran-FITC (Dx FITC) at 4 h after gavage. (D) Serum levels of TNF-α and IL-6. (E) Representative surface CD11b and intracellular TNF-α staining of liver mononuclear cells derived from the indicated mice, either unstimulated (upper) or stimulated with LPS for 4 h (lower). (F) Frequency of hepatic TNF-α producing CD11b+ macrophages from the indicated mice either unstimulated (upper) or stimulated with LPS for 4 h (lower). Data represent the mean ± SD. *p value < 0.05 by one-way ANOVA. N.S.: not significant. Data are representative from three independent experiments.
To ensure the same amounts of ethanol intake, each mouse was housed individually and a consistent ethanol Lieber-DeCarli diet containing 5% (v/v) ethanol was provided every day. We prepared a 31.5% (v/v) ethanol solution equivalent to 0.25 g/mL ethanol and 45.0% (w/v) maltose dextrin solution for the gavage. The gavage dose was 20 µL/g body weight, and each mouse received 5 g ethanol/kg body weight or isocaloric maltose. We performed gavage between 7:00 and 9:00, and mice were sacrificed 24 h after the last gavage38.
ALDH2*2 transgenic (TG) mice generated as described in prior studies were kindly provided by the Department of Orthopedic Surgery, Keio University School of Medicine39. Four WT mice and four TG mice were included in the alcohol group for 6 weeks and their serum and liver were collected and analyzed. All experiments were approved by the regional animal study committees (Keio University, Tokyo, Japan) and were performed in accordance with the ARRIVE guidelines and institutional guidelines and Home Office regulations.
Serum transaminase
As a marker of liver injury, serum aspartate aminotransferase and alanine aminotransferase were measured by a simple colorimetric method using the Fuji Dri-Chem 3500™.
Intestinal permeabilization in vivo
We prepared 125 mg/mL fluorescein isothiocyanate-dextran (FITC-Dx) solution. After 4 h fasting, mice were gavaged with the FITC-Dx solution at 4 µL/g body weight (500 mg FITC-Dx/kg body weight). Blood samples were collected 4 h after gavage, and serum FITC-Dx levels were measured at an excitation wavelength of 485 nm and emission wavelength of 528 nm.
Serum cytokine concentrations
The serum cytokine concentrations were measured using a BD™ Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 cytokine kit. Data acquisition and analysis were performed as that used for human samples, described previously herein.
Intracellular cytokine production and cell population
Mononuclear cells were isolated from the liver and spleen by Percoll density gradient centrifugation. They were seeded in 24-well plates at 5 × 105 cells/well and stimulated with 100 ng/mL LPS for 4 h at 37 °C, in the dark. Before permeabilization, the cells were labeled with CD11b, CD11c, Ly6C, Ly6G, and F4/80. Labeled cells were permeabilized with the BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit, and intracellular staining was performed using Fixable Viability Dye (FVD) eFluor™ 780 for TNF-α. To evaluate the subpopulations of T cells, TCR-β, CD4, CD8, and CD1d-tetramer were separately used for cell surface staining. Prepared samples were acquired with the flow cytometer, BD FACS Canto™ II flow cytometer.
Statistical analysis
For comparison of two groups, Student’s paired or unpaired t-test was used. For comparison of three or more groups, one-way analysis of variance (ANOVA) was used. For categorical variables, the χ2 test was used. Data are presented as the mean ± SD with p < 0.05 considered significant.
Results
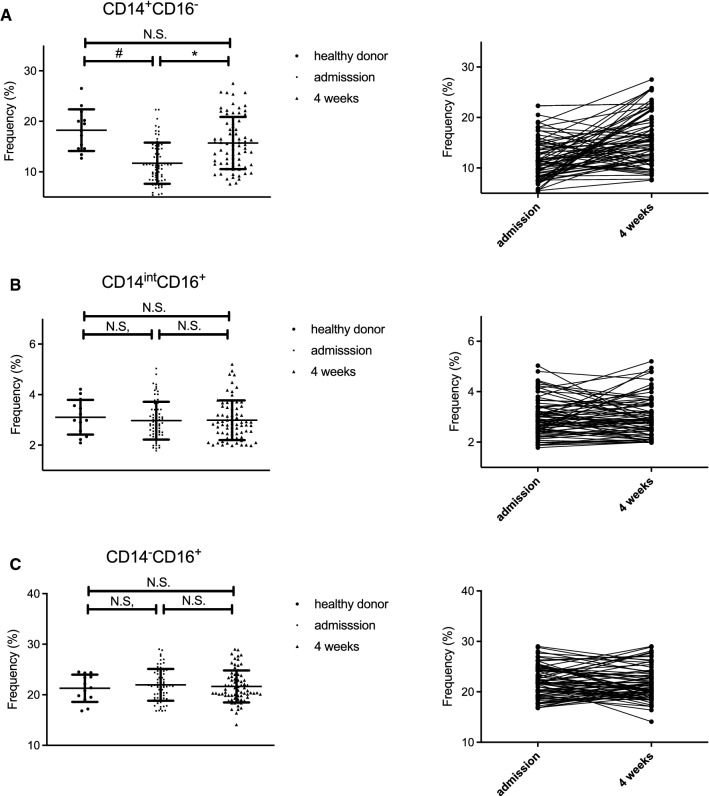
The number of peripheral CD14+CD16− monocytes is decreased in alcohol-dependent patients and partially recovered following alcohol abstinence for 4 weeks
We obtained blood samples from AD patients both at admission and at 4 weeks after abstinence and compared them with those of healthy controls. The clinical characteristics, alcohol intake, and genetic polymorphisms in the alcohol-metabolizing enzymes of patients and controls are shown in Table 1. PBMCs were isolated and the surface expression of CD14 and CD16 was analyzed by flow cytometry (Supplementary Fig. 1A). The frequency of CD14+CD16− monocytes in the PBMCs of AD patients at the time of admission was significantly lower than that in healthy donors and was recovered following alcohol abstinence for 4 weeks (Fig. 1A). However, the difference between AD patients and healthy controls was not observed for other immune cells including CD14int CD16+ monocytes (Fig. 1B), CD14−CD16+ monocytes (Fig. 1C), CD123+BDCA2+ plasmacytoid DCs, CD3+CD56− T cells, CD3−CD56+ NK cells, and CD3+CD56+ NKT cells (Supplementary Fig. 1B–G).
Figure 1.
The number of peripheral CD14+CD16− monocytes is decreased in alcohol-dependent patients, and partially recovered following alcohol abstinence for 4 weeks. Blood samples were collected from healthy male donors (n = 13) and alcohol dependent patients (n = 72). Left; frequency of CD14+CD16− monocytes (A), CD14intCD16+ monocytes (B), and CD14−CD16+ monocytes (C) in the indicated groups. Data represent the mean ± SD. #p < 0.05 by Student’s unpaired t-test. Right; individual change in the frequency of CD14highCD16− monocytes (A), CD14highCD16+ monocytes (B), and CD14lowCD16+ monocytes (C) following abstinence. *p < 0.05 Student’s paired t-test (admission vs 4 weeks).
Peripheral CD14+CD16− monocytes from alcohol-dependent patients produce less TNF-α and IL-6 after in vitro stimulation with LPS, and this defect is partially recovered following alcohol abstinence for 4 weeks.
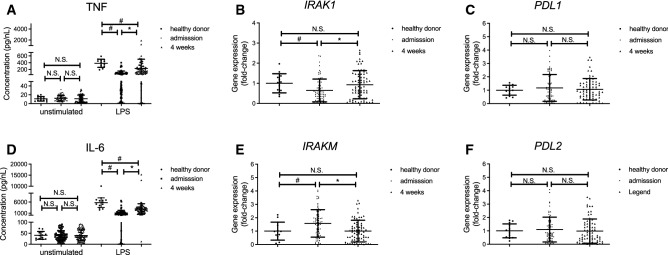
Given that CD14+CD16− monocytes were the main cell subset for which numbers were affected by alcohol abuse, we examined the functional profile of these cells. Sorted peripheral CD14+ monocytes from ADs and healthy controls produced low amounts of the inflammatory cytokines, TNF (Fig. 2A) and IL-6 (Fig. 2B) without stimulation. In contrast, after in vitro stimulation with LPS for 24 h, which induced robust inflammatory cytokines production, CD14+ monocytes from AD patients produced significantly lower amounts of these inflammatory cytokines compared to those from healthy controls (Fig. 2A,B). Importantly, the potential to produce inflammatory cytokines after LPS stimulation was partially recovered following alcohol abstinence for 4 weeks (Fig. 2A,B). CD14+ monocytes from AD patients expressed lower IRAK-1 and higher IRAK-M levels at the time of admission, but their levels were recovered to a similar level as those of healthy donors (Fig. 2C,D), whereas the expression of PD-L1 and PD-L2 was not different between healthy controls and ADs (Fig. 2E,F). Of note, both cytokine production in response to LPS stimulation and the recovery following alcohol abstinence were not affected by any clinical parameter such as age, average ethanol intake before admission, serum transaminase, and type IV collagen level on admission (Supplementary Fig. 2). We initially hypothesized that the altered composition of gut microbiota mediated by alcohol abstinence affected the recovery of monocyte function; however, metagenomics analysis of fecal samples from four AD patients whose monocytes demonstrated a substantial recovery from diminished cytokine production suggested that short-term alcoholic abstinence did not result in a remarkable change in the gut microbiota composition (Supplementary Fig. 3).
Figure 2.
Peripheral CD14+CD16− monocytes from alcohol-dependent patients produce less TNF-α and IL-6 after in vitro stimulation with LPS, and the defect is partially recovered following alcohol abstinence for 4 weeks. (A,B) Concentration of TNF-α (A) and IL-6 (B) in the culture supernatants of CD14+ monocytes isolated from each individual in the indicated groups stimulated with medium or LPS for 24 h in vitro. Data represent the mean ± SD. (C–F) Gene expression of (C) IRAK-1, (D) IRAK-M, (E) PD-L1, and (F) PD-L2 in peripheral blood mononuclear cells (PBMCs) isolated from each individual in the indicated groups. Data represent the mean ± SD. #p < 0.05 by Student’s unpaired t-test. N.S.: not significant.
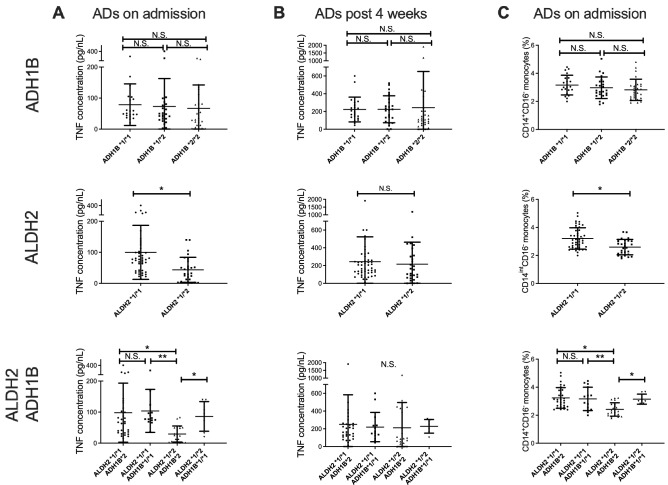
CD14+CD16− monocytes in AD patients with ADH1B*2 and ALDH2*1/*2 genotypes demonstrate a deeply suppressed phenotype
As both ethanol and its metabolite acetaldehyde have been reported to alter the intestinal permeability, in addition to their direct cytotoxic effect21, we hypothesized that genetic factors of alcoholic metabolism might affect the function of these monocytes. For this, patients were classified according to ADH1B or ALDH2 genotypes and reanalyzed. The ability of CD14+ monocytes to produce inflammatory cytokines in response to LPS stimulation did not differ by ADH1B polymorphism; however, we noticed that CD14+ monocytes from AD patients with the ALDH2*1/*2 genotype produced significantly lower amounts of TNF-α compared to those in AD patients with ALDH2*1/*1 on admission (Fig. 3A). Furthermore, CD14+ monocytes from AD patients who carried the combination of ALDH2*1/*2 and ADH1B*2 genotypes, the most acetaldehyde-exposed group, produced the lowest amount of TNF-α in response to LPS stimulation compared to that in patients with the three other genotypic patterns on admission (Fig. 3A). The difference was abolished following alcohol abstinence for 4 weeks (Fig. 3B). We also confirmed that the frequency of CD14+ monocytes was also affected by the combination of the alcohol-metabolizing genes (Fig. 3C). Of note, the daily ethanol consumption of ADs before admission did not differ according to the genetic polymorphisms (Supplementary Fig. 4A). These results collectively suggest that functional impairment in the monocytes of AD patients might be regulated by acetaldehyde and not by the simple amount of alcohol consumption. Regardless of the functional difference in monocytes according to the combination of alcohol-metabolizing genes, clinical parameters including serum transaminase levels, and fibrosis markers were comparable between the groups (Table 2 and Supplementary Fig. 4B–G).
Figure 3.
CD14+CD16− monocytes in alcohol-dependent (AD) patients with ADH1B*2 and ALDH2*1/*2 genotypes demonstrate a deeply suppressed phenotype. (A–C) Concentration of TNF-α in the culture supernatants of CD14+ monocytes isolated from AD patients on admission (A) and at 4 weeks post-abstinence (B) according to the genetic polymorphism of ADH1B (upper), ALDH2 (middle), and the combination (lower). (C) Frequency of CD14+CD16− monocytes isolated from AD patients on admission according to the genetic polymorphism in ADH1B (upper), ALDH2 (middle), and the combination (lower). Data represent the mean ± SD. *p < 0.05 by Student’s paired t-test for two groups or by one-way ANOVA for three or four groups. N.S.: not significant.
Table 2.
Characteristics according to the genetic polymorphisms of alcohol metabolizing enzymes.
| Background characteristics | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | P-valuea |
|---|---|---|---|---|---|
| ADH1B*2 ALDH2*1/*1 | ADH1B*1/*1 ALDH2*1/*1 | ADH1B*2 ALDH2*1/*2 | ADH1B*1/*1 ALDH2*1/*2 | ||
| Number of subjects | 30 | 14 | 21 | 7 | – |
| Age, yrs | 49 ± 5 | 53 ± 5 | 53 ± 4 | 51 ± 7 | 0.292 |
| Cirrhosis (Y/N) | 2/28 | 2/12 | 3/18 | 1/6 | – |
| Child Pugh score (A/B/C) | 2/0/0 | 1/1/0 | 1/2/0 | 1/0/0 | – |
| AST, IU/L | 90 ± 35 | 87 ± 26 | 79 ± 16 | 85 ± 24 | 0.278 |
| ALT, IU/L | 56 ± 17 | 47 ± 10 | 46 ± 8 | 50 ± 11 | 0.450 |
| γ-GTP, IU/L | 381 ± 79 | 369 ± 61 | 357 ± 59 | 403 ± 98 | 0.502 |
| Alb, g/dL | 4.2 ± 0.5 | 3.9 ± 1.1 | 3.9 ± 0.9 | 4.0 ± 1.4 | 0.523 |
| T-bil, mg/dL | 0.8 ± 0.2 | 0.7 ± 0.4 | 1.1 ± 0.6 | 1.3 ± 0.8 | 0.413 |
| PT-INR | 1.03 ± 0.36 | 0.91 ± 0.42 | 1.15 ± 0.30 | 0.96 ± 0.37 | 0.324 |
| Type IV collagen, ng/mL | 237 ± 39 | 234 ± 33 | 250 ± 41 | 232 ± 47 | 0.354 |
| FBS, mg/dL | 100 ± 6 | 90 ± 10 | 84 ± 7 | 96 ± 9 | 0.372 |
| WBC, × 103/μL | 6.1 ± 0.4 | 6.6 ± 0.6 | 5.5 ± 0.5 | 5.7 ± 0.8 | 0.405 |
| Hb, g/dL | 14.2 ± 0.4 | 13.7 ± 0.5 | 13.2 ± 0.5 | 13.6 ± 0.7 | 0.561 |
| Plt, × 104/μL | 20.8 ± 1.7 | 21.3 ± 2.9 | 19.1 ± 2.2 | 20.4 ± 3.2 | 0.412 |
| TC, mg/dL | 177 ± 30 | 187 ± 38 | 180 ± 29 | 191 ± 39 | 0.333 |
| HDL-C, mg/dL | 61 ± 9 | 56 ± 11 | 59 ± 13 | 56 ± 14 | 0.420 |
| TG, mg/dL | 134 ± 19 | 127 ± 21 | 135 ± 17 | 131 ± 19 | 0.303 |
aHomogeneity among the four groups based on one-way analysis of variance (ANOVA).
MAA-Alb enhances LPS stimulation of CD14+ monocytes, and cells exposed to MAA-Alb produce less TNF-α upon secondary LPS stimulation
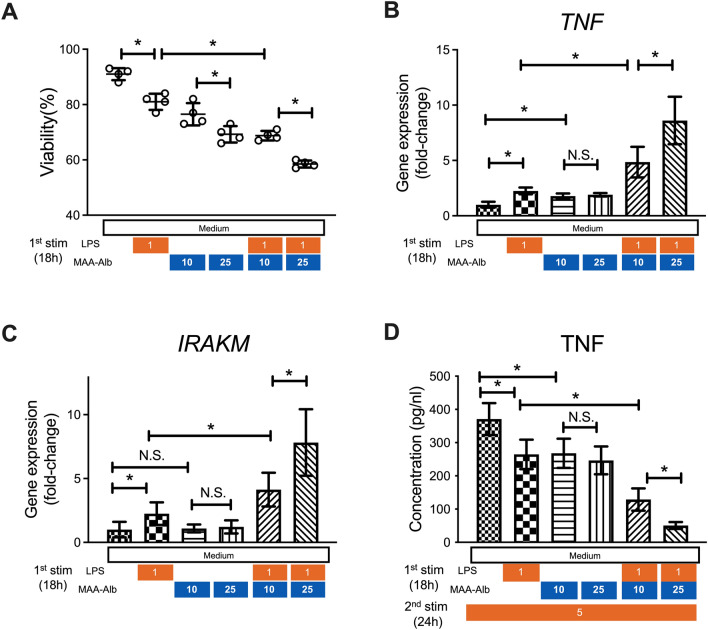
CD14+ monocytes from the blood of healthy donors were obtained and stimulated with LPS in the presence of acetaldehyde in vitro to examine the direct effect of acetaldehyde on the function of peripheral monocytes. CD14+ monocytes demonstrated lower viability after incubation with LPS and MAA-Alb in a dose-dependent manner compared to that in cells without MAA-Alb (Fig. 4A). Monocytes incubated with LPS and MAA-Alb showed higher expression of TNF-α and IRAK-M than those stimulated with LPS alone (Fig. 4B,C). Conversely, monocytes incubated with MAA-Alb and LPS produced lower amounts of TNF-α following secondary LPS stimulation (Fig. 4D). These results collectively reinforce the direct contribution of acetaldehyde to the immune response of monocytes upon continuous LPS stimulation.
Figure 4.
MAA-Alb enhances LPS stimulation of CD14+ monocytes, and cells exposed to MAA-Alb produce less TNF-α upon secondary LPS stimulation. CD14+ monocytes collected from healthy donors were stimulated in the presence of LPS (1 ng/mL) and Alb (10–25 µg/mL) or MAA-Alb (10–25 µg/mL) for 18 h in vitro. (A–C) Viability of (A) and gene expression of TNF-α (B) and IRAK-M (C) in monocytes following stimulation. Cells were stimulated with 5 ng/mL LPS for 24 h. (D) Concentration of TNF-α in the culture supernatants of the monocytes following the second LPS stimulation for an additional 24 h in vitro. Data represent the mean ± SD. *p < 0.05, as determined by one-way ANOVA, N.S.: not significant.
Continuous alcohol administration to mice results in suppressed production of inflammatory cytokines from CD11b+ macrophages in the liver
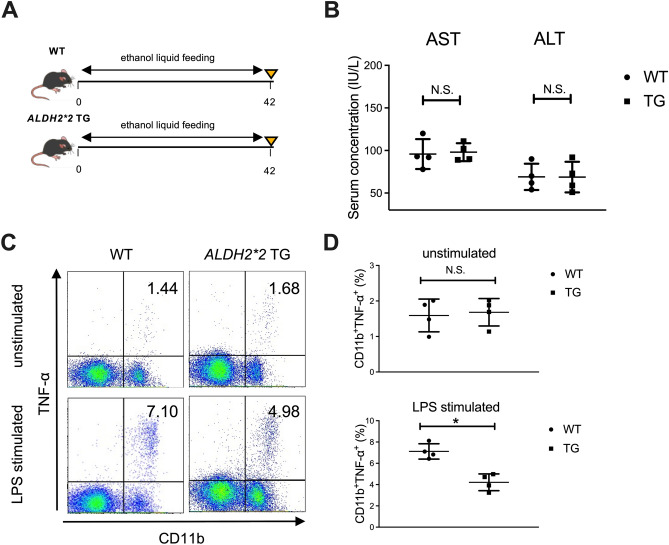
We finally used murine models to examine the effect of continuous alcohol administration and abstinence on the function of macrophages in the liver. Mice were divided into the following three groups: alcohol group, orally administered ethanol gavage for 42 days; withdrawal group, orally administered ethanol gavage for 42 days followed by control liquid feeding for 21 days; control group (Fig. 5A). As expected, alcohol-group mice developed mild liver injury both serologically and histologically, and alcohol abstinence for 3 weeks recovered the damage to the control level (Fig. 5B). Importantly, intestinal permeability, assessed by FITC dextran administration and the level of inflammatory cytokines in the serum, was significantly increased by continuous alcohol administration and was recovered by alcohol abstinence (Fig. 5C,D). Conversely, the production of TNF-α by hepatic CD11b+ macrophages after in vitro LPS stimulation was significantly decreased in the alcohol group and was partially recovered in the withdrawal group, consistent with the results shown in human subjects (Fig. 5E,F). Finally, we used ALDH2*2 transgenic mice to examine the direct contribution of alcohol-metabolizing enzymes to the function of liver macrophages in response to LPS stimulation (Fig. 6A). Although serum transaminase levels were comparable to those of WT mice (Fig. 6B), hepatic CD11b+ macrophages of ALDH2*2 transgenic mice chronically fed ethanol showed significantly lower production of TNF-α in response to in vitro LPS stimulation than those from WT mice (Fig. 6C,D).
Figure 6.
Productivity of inflammatory cytokines from hepatic macrophages in response to LPS stimulation is significantly suppressed in ALDH2*2 transgenic (TG) mice chronically fed ethanol. (A) Study design. WT mice (n = 4) or ALDH2*2 TG mice (n = 4) received a control liquid diet for 5 days and 5% ethanol liquid diet for 6 weeks. (B) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. (C) Representative surface CD11b and intracellular TNF-α staining of liver mononuclear cells derived from the indicated mice either unstimulated (upper) or stimulated with LPS for 4 h (lower). (D) Frequency of hepatic TNF-α producing CD11b+ macrophages from the indicated mice either unstimulated (upper) or stimulated with LPS for 4 h (lower). Data represent the mean ± SD. *p value < 0.05 by Student’s paired t-test. N.S.: not significant. Data are representative of two independent experiments.
Discussion
In this study, we showed that chronic alcohol consumption induces hypo-reactivity of peripheral monocytes to LPS in Japanese male AD patients, and short-term abstinence partially restored this reactivity. Furthermore, we obtained similar results with a murine model of chronic alcohol feeding and alcohol withdrawal. Notably, we demonstrated that the immunological defects in the peripheral monocytes of AD patients are partially regulated by genetic polymorphisms in alcohol-metabolizing enzymes and that the most acetaldehyde-exposed patients carrying the combination of ALDH2*1/*2 and ADH1B*2 showed the most impaired function of peripheral monocytes regardless the similar amounts of alcohol intake.
Clinical observations and experimental data have revealed that excessive alcohol use has significant inhibitory effects on the immune system. From the viewpoint of the gut–liver axis, habitual ethanol consumption induces altered composition of the gut microbiota, termed dysbiosis, and subsequent increased intestinal permeability40. This results in elevated LPS levels in circulation and subsequent activation of NF-κβ-mediated transcription of proinflammatory cytokines as the first line of defense against foreign bacteria or metabolites in the liver41. In contrast, continuous exposure to bacteria and the subsequent immune active state give rise to an immune paralytic state with a higher susceptibility to infection in a specific condition of ALD9. In the current study, the number of CD14+ CD16– monocytes was significantly decreased in the PBMCs of AD patients, whereas that of other subsets was not affected. Furthermore, these cells were functionally impaired as the production of TNF-α and IL-6 in response to LPS was significantly decreased compared to that in cells of healthy controls. As a potential mechanism of immunological impairment, previous studies have shown that the IL-1-receptor-associated kinase (IRAK) family plays important roles in ALD pathogenesis and the compromised status of alcoholism. In the immune response via TLR signaling, IRAK-M, also known as IRAK-3, inhibits inflammatory cytokine production to prevent excessive inflammation and tissue damage42. At the early stage of LPS stimulation to monocytes, the expression of IRAK-1 is predominant and the production of inflammatory cytokines is promoted; however, the expression of IRAK-M gradually becomes dominant and the cytokine productivity per monocyte is decreased, termed LPS tolerance43,44. A previous study has demonstrated that IRAK-M-deficient mice show enhanced intestinal permeability, higher serum endotoxin levels, and worse liver injury after ethanol administration, suggesting that IRAK-M negatively regulates the innate immune response with alcoholic liver injuries. In our study, CD14+ monocytes derived from AD patients expressed higher levels of IRAK-M and produced fewer inflammatory cytokines in response to LPS than those from healthy controls. These results suggest that macrophages and monocytes in AD individuals are in a state of LPS tolerance, and this condition is related to the insufficient production of inflammatory cytokines, leading to the relief of excessive inflammation in the liver, while contributing to an infectious state. Recent papers have reported the contribution of immune-inhibitory receptors, such as PD-1 and TIM-3, to the immunological defects in patients with acute alcoholic hepatitis45; however, the gene expression of PD-L1 was not affected in this study, possibly due to the different state of ALD.
Since alcohol abstinence is still one of the major therapeutic options for ALD, it is critical to determine whether alcohol withdrawal could restore the immunological impairment. We confirmed that the impaired function of inflammatory cytokine production in response to LPS stimulation of circulating monocytes was reversible following short-term abstinence for 4 weeks. As mentioned, gut microbiota affects the intestinal barrier and subsequent immune responses both during the initiation and progression of ALD46. Thus, we initially hypothesized that the altered composition of gut microbiota induced by long-term alcohol consumption could be recovered by alcohol withdrawal, leading to improvements in the impaired immune responses. However, the individual changes following short-term abstinence were not evident in our small cohort.
Rather, it is possible that ethanol or its metabolites directly affect the immunological function of monocytes via TLR signaling. Regarding this point, we noticed that the production of inflammatory cytokines by peripheral monocytes in response to LPS stimulation differed according to the combination of ALDH2/ADH1B gene polymorphisms in AD patients. Interestingly, we confirmed that AD patients with the ALDH2 *1/*2 and ADH1B*2 genotype combination, affecting approximately 30% of the Japanese population16, showed the lowest number of and most impaired cytokine production by peripheral CD14+ monocytes. A recent report demonstrated that the number of circulating monocytes is elevated in AD individuals compared to that in healthy controls47. Considering that the majority of patients included in this study were Caucasian, patient characteristics based on different alcohol metabolism-related genes and the extent of alcohol consumption might explain the conflicting result. The ALDH2*1/*2 genotype is a major determinant of high blood acetaldehyde exposure after alcohol intake. Although an ADH1B*2 genotype has little effect on blood acetaldehyde levels after alcohol challenge tests using moderate doses of ethanol in non-AD patients48, previous studies have suggested that blood acetaldehyde exposure is the highest in drinkers with the ALDH2*1/*2 and ADH1B*2 genotype combination for the following reasons: the slope of the increase in blood acetaldehyde levels according to the increase in blood ethanol levels were found to be steepest for intoxicated AD individuals belonging to this group49, the highest levels of N2-ethylidene-dG, an acetaldehyde-DNA adduct, were detected in the leukocytes of AD individuals belonging to this group19, and the most severe macrocytic anemia and leukocytopenia50 and the slowest recoveries after the cessation of drinking51 were observed among AD individuals belonging to this group, suggesting the strongest bone marrow suppression as a result of the highest blood acetaldehyde exposure. In contrast, the direct effect of acetaldehyde on the function of immune cells has not been elucidated to data. We confirmed that peripheral monocytes of healthy controls stimulated with LPS and MAA produced lower amounts of TNF-α in response to the second LPS stimulation in parallel with the upregulation of IRAK-M gene expression. Although we did not examine the immunological aspect in human livers, we demonstrated that hepatic CD11b+ macrophages of ALDH2*2 transgenic mice chronically fed ethanol were functionally impaired in response to LPS stimulation, similar to the results in humans. Of note, another study demonstrated that patients with ALDH2*1/*2 or Aldh2-deficient mice had much higher levels of blood acetaldehyde and glucocorticoids, leading to immune suppression and the attenuated liver injury by inhibiting T-cell glucose metabolism52. These findings together might explain the regulated liver injuries regardless of continuous exposure to a high concentration of acetaldehyde following alcohol consumption in this specific population, as reported in large cross-sectional studies of Japanese AD patients27,28.
Collectively, our findings suggest a previously unrevealed mechanism of how chronic excessive alcohol intake induces immunological tolerance of monocytes. Strikingly, acetaldehyde directly affects the immune response, which is reversible, following alcohol abstinence. Although further investigations with a large cohort validation are needed, the results of this study provide a new perspective on the systemic influence of excessive alcohol consumption according to the genetic polymorphisms of alcohol-metabolizing enzymes in Japanese AD patients.
Supplementary Information
Acknowledgements
We thank S. Chiba for technical assistance. We would like to thank Editage (www.editage.jp) for English language editing. This study was supported in part by Keio University Academic Development Funds for Individual Research from Keio University Medical Fund.
Author contributions
S.S. helped design the study, performed experiments, analyzed the data, and wrote the paper; N.N. conceived and designed the study, analyzed the data, and wrote the paper; P-S.C., K.O., N.T., A.Y., R.M., T. Katayama, A.Y., R.A., T.T., T.S., and S.H. performed experiments;T.M. provided transgenic mice; A.Y. designed the study and wrote the paper; T.Kanai helped conceive and supervise the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nobuhiro Nakamoto, Email: nobuhiro@z2.keio.jp.
Takanori Kanai, Email: takagast@z2.keio.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93086-y.
References
- 1.Lieber CS. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34(1):9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut Liver. 2014;8(3):237–241. doi: 10.5009/gnl.2014.8.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enomoto N, Schemmer P, Ikejima K, Takei Y, Brenner D, Thurman R. Long-term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin. Exp. Res. 2001;25(9):1360–1367. doi: 10.1111/j.1530-0277.2001.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 5.Donnadieu-Rigole H, Mura T, Portales P, Duroux-Richard I, Bouthier M, Eliaou JF, et al. Effects of alcohol withdrawal on monocyte subset defects in chronic alcohol users. J. Leukoc. Biol. 2016;100(5):1191–1199. doi: 10.1189/jlb.5A0216-060RR. [DOI] [PubMed] [Google Scholar]
- 6.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J. Immunol. 2001;166(7):4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 7.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. Alcohol Clin. Exp. Res. 2006;30(4):720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 8.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J. Gastroenterol. Hepatol. 2012;27(Suppl 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barros FR, Castro-Faria-Neto HC, Castro CL, Aguiar Nemer AS, Rocha EM, Silva Fonseca VA. Effects of chronic ethanol consumption in experimental sepsis. Alcohol Alcohol. 2012;47(6):677–682. doi: 10.1093/alcalc/ags081. [DOI] [PubMed] [Google Scholar]
- 10.Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. doi: 10.1155/2013/495156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J. Gastroenterol. Hepatol. 2007;22(Suppl 1):S53–S56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Yokoyama H, Higuchi S, Hara S, Kato S, Ishii H. Acetaldehyde accumulation suppresses Kupffer cell release of TNF-Alpha and modifies acute hepatic inflammation in rats. J. Gastroenterol. 2004;39(2):140–147. doi: 10.1007/s00535-003-1278-5. [DOI] [PubMed] [Google Scholar]
- 13.Mandl M, Schmitz S, Weber C, Hristov M. Characterization of the CD14++CD16+ monocyte population in human bone marrow. PLoS ONE. 2014;9(11):e112140. doi: 10.1371/journal.pone.0112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi) /CD16(neg) monocytes: Expansion of CD14(hi) /CD16(pos) and contraction of CD14(lo) /CD16(pos) monocytes in acute liver failure. Cytometry A. 2012;81(10):823–834. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. 2007;81(3):584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo K, Wakai K, Hirose K, Ito H, Saito T, Tajima K. Alcohol dehydrogenase 2 His47Arg polymorphism influences drinking habit independently of aldehyde dehydrogenase 2 Glu487Lys polymorphism: Analysis of 2,299 Japanese subjects. Cancer Epidemiol. Biomarkers Prev. 2006;15(5):1009–1013. doi: 10.1158/1055-9965.EPI-05-0911. [DOI] [PubMed] [Google Scholar]
- 17.Goedde H, Agarwal D, Fritze G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum. Genet. 1992;88(3):344–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- 18.Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T, Harada S. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol. Alcohol. 1994;29(6):707–710. [PubMed] [Google Scholar]
- 19.Yukawa Y, Muto M, Hori K, Nagayoshi H, Yokoyama A, Chiba T, et al. Combination of ADH1B*2/ALDH2*2 polymorphisms alters acetaldehyde-derived DNA damage in the blood of Japanese alcoholics. Cancer Sci. 2012;103(9):1651–1655. doi: 10.1111/j.1349-7006.2012.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda T, Yabushita H, Kanaly R, Shibutani S, Yokoyama A. Increased DNA damage in ALDH2-deficient alcoholics. Chem. Res. Toxicol. 2006;19(10):1374–1378. doi: 10.1021/tx060113h. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhry KK, Samak G, Shukla PK, Mir H, Gangwar R, Manda B, et al. ALDH2 deficiency promotes ethanol-induced gut barrier dysfunction and fatty liver in mice. Alcohol. Clin. Exp. Res. 2015;39(8):1465–1475. doi: 10.1111/acer.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, et al. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60(1):146–157. doi: 10.1002/hep.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuma D, Smith S, Sorrell M. Acetaldehyde and microtubules. Ann. NY Acad. Sci. 1991;625(Suppl 1):786–792. doi: 10.1111/j.1749-6632.1991.tb33920.x. [DOI] [PubMed] [Google Scholar]
- 24.Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, Arico S, et al. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31(4):878–884. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 25.Greenwel P. Acetaldehyde-mediated collagen regulation in hepatic stellate cells. Alcohol. Clin. Exp. Res. 1999;23(5):930–933. doi: 10.1111/j.1530-0277.1999.tb04206.x. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Zhao H, Gelernter J. Strong protective effect of the aldehyde dehydrogenase gene (ALDH2) 504lys (*2) allele against alcoholism and alcohol-induced medical diseases in Asians. Hum. Genet. 2012;131(5):725–737. doi: 10.1007/s00439-011-1116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama A, Mizukami T, Matsui T, Yokoyama T, Kimura M, Matsushita S, et al. Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol. Clin. Exp. Res. 2013;37(8):1391–1401. doi: 10.1111/acer.12108. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama A, Taniki N, Nakamoto N, Tomita K, Hara S, Mizukami T, et al. Associations among liver disease, serum lipid profile, body mass index, ketonuria, meal skipping, and the ADH1B and ALDH2 genotypes in Japanese men with alcohol dependence. Hepatol. Res. 2019;50:565. doi: 10.1111/hepr.13475. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama A, Muramatsu T, Omori T, Yokoyama T, Matsushita S, Higuchi S, et al. Alcohol and aldehyde dehydrogenase gene polymorphisms and oropharyngolaryngeal, esophageal and stomach cancers in Japanese alcoholics. Carcinogenesis. 2001;22(3):433–439. doi: 10.1093/carcin/22.3.433. [DOI] [PubMed] [Google Scholar]
- 30.Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23(2):125–133. doi: 10.1093/dnares/dsw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinform. 2011;36:107. doi: 10.1002/0471250953.bi1007s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 35.Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y, et al. Influence of proton-pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin. Transl. Gastroenterol. 2015;6:e89. doi: 10.1038/ctg.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiele GMD, Willis MS, Sorrell MF, Tuma DJ, Klassen LW. Malondialdehyde-acetaldehyde (MAA) modified proteins induce pro-inflammatory and pro-fibrotic responses by liver endothelial cells. Comp. Hepatol. 2004;14(3):1. doi: 10.1186/1476-5926-2-S1-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat. Protoc. 2013;8(3):627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, et al. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ. Res. 2009;105(11):1118–1127. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- 40.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung H, Kim SW, Hong M, Suk KT. Microbiota-based treatments in alcoholic liver disease. World J. Gastroenterol. 2016;22(29):6673–6682. doi: 10.3748/wjg.v22.i29.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi KHL, Galán JE, Janeway CA, Jr, Medzhitov R. Flavell RA IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/S0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 43.van Veer C, van den Pangaart PS, van Zoelen MA, de Kruif M, Birjmohun RS, Stroes ES, et al. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J. Immunol. 2007;179(10):7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 44.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 2009;183(2):1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148(3):590–602. doi: 10.1053/j.gastro.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 46.Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 2012;26(6):911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47(phox)-oxidative stress pathway in neutrophils. Gut. 2017;66(4):705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenase in individual variation of alcohol metabolism. Alcohol. Alcohol. 1994;29(6):707–710. [PubMed] [Google Scholar]
- 49.Yokoyama A, Tsutsumi E, Imazeki H, Suwa Y, Nakamura C, Yokoyama T. Polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and the blood and salivary ethanol and acetaldehyde concentrations of Japanese alcoholic men. Alcohol. Clin. Exp. Res. 2010;34(7):1246–1256. doi: 10.1111/j.1530-0277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama A, Brooks PJ, Yokoyama T, Mizukami T, Matsui T, Kimura M, et al. Blood leukocyte counts and genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 in japanese alcoholic men. Alcohol. Clin. Exp. Res. 2016;40(3):507–517. doi: 10.1111/acer.12983. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama A, Brooks PJ, Yokoyama T, Mizukami T, Shiba S, Nakamoto N, et al. Recovery from anemia and leukocytopenia after abstinence in Japanese alcoholic men and their genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2. Jpn. J. Clin. Oncol. 2017;47(4):306–312. doi: 10.1093/jjco/hyw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Y, Zhou Z, Ren T, Kim SJ, He Y, Seo W, et al. Alcohol inhibits T-cell glucose metabolism and hepatitis in ALDH2-deficient mice and humans: Roles of acetaldehyde and glucocorticoids. Gut. 2019;68(7):1311–1322. doi: 10.1136/gutjnl-2018-316221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.