Abstract
Background
Scalp hair loss (alopecia) in women is a common ageing and senescing condition. It usually presents as androgenetic alopecia (AGA) or telogen effluvium (TE) and often has pronounced psychological consequences. ALRV5XR is a novel treatment aiming to regenerate a normal hair phenotype by targeting multiple molecular pathways linked to hair growth promotion and hair follicle stem cell activation. The primary objectives of this 24-week trial were to evaluate the safety and efficacy of ALRV5XR in terminal hair (TH) regrowth in women with AGA or TE.
Methods
This randomised, double-blind, placebo-controlled trial was performed in a USA community clinic. Healthy women 18-65 years of age with AGA or TE of Ludwig classification I-II and Fitzpatrick skin type I-VI were enrolled. They were allocated in a 1:1 ratio into ALRV5XR or placebo treatment groups using a random number table. Masked dermatologist assessments, phototrichograms and blood samples were obtained at baseline, 12 and 24 weeks. Subjects were given a masked treatment regimen of oral capsules, shampoo, conditioner and follicle serum for daily administration. Main outcomes were absolute and per cent changes in TH density and response rates. The trial was registered with clinicaltrials.gov (NCT04450602) and is completed.
Findings
46 subjects (23 ALRV5XR, 23 placebo) were enrolled between April 3 and October 20, 2018. Five subjects dropped out and two were non-compliant. Thirty-nine subjects completed the trial (18 ALRV5XR, 21 placebo). At 24 weeks, the absolute change in TH density improved by 30·1THs/cm2 (95% CI: 15·1–45·1; p=0·0002), and the relative density increased by 19·7% (95% CI: 8·0%–31·4%; p=0·0016). The odds ratio for being a responder (≥0 change) was 2·7. Efficacy increased 133% from week 12 to 24. Efficacy outcomes were similar in AGA and TE subjects. 66·7% of the ALRV5XR group responded by regrowing 40THs/cm2 or more hair. No adverse events were reported.
Interpretation
In women with AGA or TE, ALRV5XR treatment significantly increased hair regrowth without adverse events. ALRV5XR displayed a multi-fold improved efficacy and response rate when compared to published trials of standard therapy. Progressive acceleration of TH regrowth suggests regeneration of the structure and function of non-productive telogen follicles and prolonged treatment may restore a normal hair phenotype.
Keywords: Androgenetic alopecia, Telogen effluvium, Female pattern hair loss, Hair restoration, Minoxidil, Finasteride, Botanical, Women's health, Menopause, Senescence, Aging, Wnt/beta-catenin, Multi-targeting, Stem cell, Hair regeneration, Regenerative medicine, COVID-19, PRP
Research in context.
Evidence before this study
Hair loss (HL) is a sign of normal ageing. It is associated with impaired quality of life in women, metabolic syndrome and increased mortality. Most pharmaceuticals or botanical, vitamin and mineral (BVM) formulations use high-dose compounds targeting few pathways, which lead to limited effectiveness and adverse effects. Similarly, minoxidil, the only FDA approved treatment for HL in women, is effective in only 13-40% of women. A literature review of hair follicle (HF) biology, compounds influencing hair growth and regrowth, and clinical trials, showed that HF regeneration and maintenance is associated with the expression of more than 1,000 genes, and is dependent on HF stem cell (HFSC) activation. It is also influenced by a complex, rhythmic behaviour of multiple pathways that often have opposing influences. Therefore, a single pathological cause of HL is unlikely, and present perspectives suggest that the next generation of HL therapies should aim to address multiple targets by systemic, topical or a combination delivery regimen. While activation of the Wnt/β-Catenin signalling pathway appears to be a dominant factor for hair regrowth and maintenance, the modulation of certain cytokines, growth factors and hormones are also key components of hair physiology. We researched the mechanisms of many BVMs relevant to HF physiology. After screening candidate BVMs in HFSCs in vitro and HFs in vivo, we developed ALRV5XR from multiple BVMs for oral or topical administration, with the aim of modulating >20 of the accepted pathways involved in healthy HF physiology.
Added value of this study
ALRV5XR, studied as an oral and topical regimen in women with androgenetic alopecia (AGA) or telogen effluvium (TE), demonstrated a 4-fold improvement in terminal hair regrowth and response rates when compared to published results of trials using standard therapy, with no side effects. ALRV5XR's multi-molecular targeting BVM approach induces homeostasis in the ageing HF and might restore a woman's normal scalp hair phenotype.
Implications of all the available evidence
The multi-targeting compound ALRV5XR might potentially become a new standard of care for women with HL. Future research should focus on evaluation of its long-term safety and efficacy. Moreover, further elucidation of the mechanisms underlying ALRV5XR action might justify its utility in other ageing tissues.
Alt-text: Unlabelled box
1. Introduction
Alopecia or hair loss (HL) of the scalp is a common clinical finding associated with ageing and cellular senescence [1,2]. It is emotionally distressing for both sexes, but women are psychologically more affected than men as suggested by parameters of self-esteem, body image and quality of life [3]. The most common forms of female hair loss are androgenetic alopecia (AGA), also called female-pattern hair loss (FPHL), and telogen effluvium (TE) [1]. AGA is thought to have many commonalities with senescence of the hair follicle (HF) and 758 expressed genes were found common to both AGA and senescent alopecia[2,4]. AGA is a heritable, age-dependent process that results in a progressive decline in scalp hair density in a sex-dependent pattern [5]. While onset of hair thinning in women begins between the ages of 12 and 40, it becomes noticeable in about half of the population by the fifth decade [6]. Alopecia has been related to hormonal disturbances, chronic medical conditions, use of certain drugs, topical scalp treatments, and chemo and radiotherapy [1]. It has also been linked to sudden weight loss, low protein intake, stress and pollution [1]. Miscellaneous hair disorders and alopecia have also been associated with deficiencies in minerals such as copper, iron, selenium, and zinc as well as of vitamins such as niacin and vitamin D [7].
The healthy adult scalp has an area of approximately 700 cm2 with a total hair count ranging from 100,000 – 150,000 hairs [1,8] Ninety per cent of scalp hairs are large, pigmented, and are known as terminal hairs (TH) [9]. They have a shaft diameter of 30 – 150 µm [9]. The remaining ten per cent are called vellus hairs (VH), and are barely visible, depigmented hairs [9]. Individual hairs are produced by HFs, which are epithelial skin mini-organs that modulate and sustain cyclic hair regrowth over repeated hair cycles [2]. The human scalp has the greatest density of HFs on the body, ranging from 118 to 350 HFs per cm2 [9]. Hair density in women is highest from age 20 – 30 years and decreases thereafter at an increasing rate [10].
AGA and TE in women have different clinical features and prognoses [1]. AGA is diagnosed by a high diversity in hair shaft diameter, while TE is diagnosed by an excess of telogen follicles [1,11]. Although most women with AGA have diffuse thinning of the crown, the frontal hairline remains intact, and they rarely become bald [1]. AGA is linked to the metabolic syndrome and is an independent predictor of mortality from diabetes mellitus and heart disease [12]. TE is a medical sign related to underlying conditions rather than a discrete disorder, and can resolve spontaneously in healthy women [1]. Triggering events for TE include acute physical stressors such as surgery, illness, or weight loss, hormonal imbalances, psychological stress, post-partum state, drugs, nutritional deficiencies and scalp inflammation [1]. Interestingly, cases of TE onset have been reported 3 weeks to 3 months after diagnosis of COVID-19 [13]. In some individuals, TE has been associated with senescent thinning, autoimmune disease, or nutritional deficiencies, all conditions which may confuse its proper distinction from subjects affected by AGA [1].
There is increasing evidence that AGA is the consequence of organ-specific premature ageing of HFs [1]. Indeed, age-related hair regrowth reduction and diminished hair shaft diameter, along with abnormalities of the hair cycle, including decreased duration of the growth phase (anagen), increased time between the loss of hair (exogen) in the resting stage (telogen), and the emergence of a replacement hair (neogen) have all been reported [14]. Affected HFs miniaturize, thereby leading to a gradual replacement of THs by VHs, eventually resulting in HF deletion [15,16].
A literature review on HF biology, compounds influencing hair growth and in pathology studies, showed at the molecular level, that hair growth and the physiological HF cycle are influenced by a complex and rhythmic behaviour of multiple intricate signalling pathways, often with opposing influences, including those of various cytokines, hormones, prostaglandins, and growth factors [17]. This implies that it is unlikely to ascertain a single and discrete cause of HL. Consequently, and to improve efficacy, the next generation of HL therapies will likely be a combination therapy consisting of agents that maintain normal growth and promote regrowth, combined with agents that delay catagen entry, while relieving negative pressure on the follicle [18]. Most relevant and as a dominant physiological influence, the canonical Wnt/β-Catenin signalling in HF stem cells controls hair cycle induction and maintenance [2]. This signalling cascade is less active in aged vs. young telogen HF stem cells; [2] moreover, inactivation of the Wnt/β-Catenin signalling pathway has been implicated in AGA [17,18].
At present, most available pharmaceutical and non-pharmaceutical hair regrowth treatments target a single or a limited number of pathways by using high-dose active ingredients. They have relatively limited efficacy or are effective only in a minority of women, while associated adverse effects occur at non-negligible incidence rates [18,19], possibly due to the elevated doses needed to attain effective results. Topical minoxidil, at concentrations of 2% and 5%, was approved for treatment of AGA in women in 1991 and 2014, respectively. It remains as the only FDA approved pharmaceutical to treat women affected with this hair disorder. Currently, there is no FDA approved treatment for TE. The average improvement in hair density (minoxidil vs. placebo) in published trials in women with AGA ranged from 12·4 to 15·1 THs/cm2 after 48 weeks of treatment with 2% and 5% minoxidil, respectively [[19], [20], [21]]. Only 13–40% of female subjects with AGA responded to minoxidil treatment [21,22]. While various adverse events were observed, increased facial hair growth was reported by 22% and 46% of participants using 2% and 5% minoxidil, respectively [21]. Minoxidil is a potassium channel opener and has been associated with several mechanisms of actions (MOAs) such as increased HGF, PGE and VEGF expression, EGF inhibition and is also thought to stimulate canonical Wnt/β-Catenin signalling [18,19,23]. Yet despite these multiple MOAs, TH density changes in AGA subjects treated with minoxidil tend to peak at 12-16 weeks, declining thereafter [24]. Of note, following treatment cessation, there is rapid regression to a density lower than at baseline [24]. This treatment response has been explained as a triggering of the HF in the latent part of telogen into anagen without a prolongation of anagen or reversal of follicular miniaturization [24]. Anti-androgen therapy using finasteride, which inhibits the conversion of testosterone to dihydrotestosterone, while effective in men, has shown no efficacy in randomised placebo-controlled trials (RCTs) in women and is contraindicated in women of childbearing potential [19]. Nevertheless, several uncontrolled studies in women have reported improvements in hair growth [19]. Recombinant human insulin-like growth factor-I (rhIGF-I) treatment has induced the appearance of normal hair and recovery of hairline in subjects with growth hormone insensitivity and concomitant IGF deficiency [25]. Combination therapies of topical minoxidil with pharmaceuticals and supplements have been studied in women, showing no significant difference in TH density changes to those of minoxidil monotherapy [19]. RCT's of Platelet-Rich Plasma (PRP) for the treatment of AGA resulted in significant increases in hair density in men [19]. While for women no significant efficacy has yet been demonstrated for PRP in RCTs, several non-RCTs showed promising results [19]. Combination therapies of PRP supplemented by autologous stem cell transplants, micro-needle wounding and low level laser therapy (LLLT), in men as well as in women, have reported significant improvements in hair regrowth [26]. RCTs with anti-androgen molecules, vitamins, minerals, botanical extracts, collagen and various marine extracts have shown limited efficacy in TH regrowth in women [19]. As it can be appreciated from these experiences, there is an unmet need for safe and effective proven therapies for a wide range and severity of hair loss in women.
ALRV5XR is a novel multi-molecular targeting agent aimed to restore the normal biological homeostasis of the HF. The agent is composed of specific compounds obtained from standardized botanical extracts, vitamins and minerals (BVM), and can be delivered orally as a dietary supplement, or topically. In this RCT, and to maximize HF exposure to the treatment, we administered ALRV5XR, both orally and topically as a regimen of dietary supplementation, shampoo, conditioner, and follicle serum. ALRV5XR was designed to support normal biochemical signalling, thereby contributing to normal HF physiology. ALRV5XR is intended to prolong anagen of HFs and induce neogen in involuted HFs. This combination of actions should aid to regenerate the normal scalp hair phenotype. ALRV5XR was designed to modulate, directly or indirectly, several molecular pathways of the HF, which are associated with hair growth and regrowth promotion as well as hair loss inhibition. Among them, activation of the Wnt/β-Catenin cascade in HF stem cells and dermal papilla cells appears to be the dominant required effect for new hair formation and maintenance of growth [2,17,18,26,27]. Inhibition of targets linked to reducing hair growth is also relevant, as is the case of BMP-4, DHT and androgen regulator 5α -Reductase [17,18]. In addition, increase of VEGF signalling, aimed to enhance blood flow to HFs, is also linked to promotion of hair growth [17,18]. Similarly the regulation of other growth factors such as FGF-7, FGF-18, HGF, IGF, KGF, and cytokines such as mTORC1, PKC, PPARγ, Shh, COX, INF-α, INF-γ, Interleukins 1b, 6 and 12, NF-κβ, Prostaglandin D2, TNF-α, and TGF-β are likely to be influenced directly or indirectly by ALRV5XR [17]. Our hypothesis is that ALRV5XR, a multi-molecular targeting approach designed to modulate multiple pathways, is a highly effective treatment in regenerating and maintaining TH in women.
2. Methods
2.1. Study design
This 24-week randomised, double-blind, placebo-controlled trial was conducted in San Francisco, California, USA in a single centre community clinic. The study protocol was granted ethics approval on March 22, 2018, by IRB Services, registered with the FDA #IRB00000776. The trial was registered with ClinicalTrials.gov (NCT04450602), and is completed. A CONSORT checklist has been completed and we declare adherence.
2.2. Sample size calculation
While designing this study we determined that a sample size of 36 subjects, 18 in each group (before dropout), was required to achieve a power of 80% and a significance level of 5%. This sample size should be able to demonstrate a clinically meaningful difference between the ALRV5XR and placebo groups, as reflected in the increase in total hair count from baseline to Week 24. Based on previous studies of minoxidil vs placebo, we calculated this sample size using a two-sample t test power calculation for a one-sided hypothesis test based on a mean difference of 20 hairs/cm2, and a standard deviation of 23 hairs/cm2. We anticipated a dropout rate of 20% and therefore adjusted the sample size to 23 per group, for a total sample size of 46.
2.3. Study participants
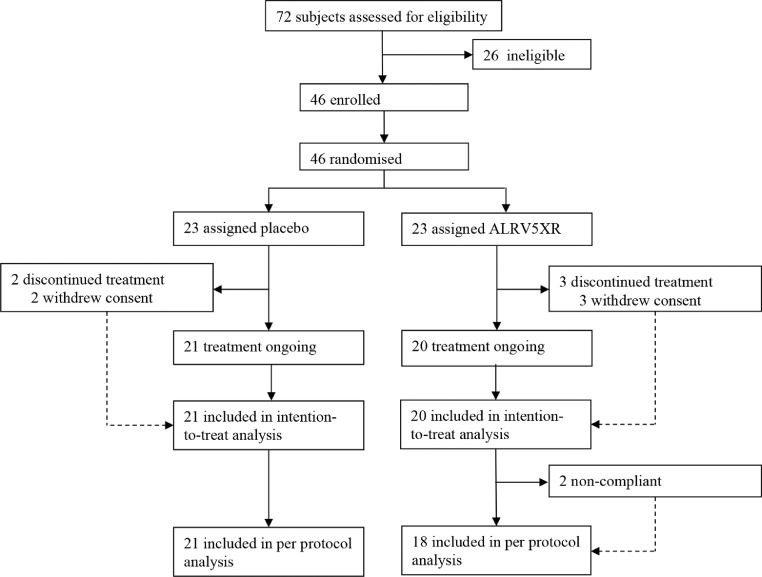
Eligible subjects were healthy adult females, 18 – 65 years of age with AGA, TE, or with self-reported hair thinning, or hair loss, occurring for more than 3 months prior to screening. Hair loss was confirmed by physical examination performed by a certified dermatologist, who eliminated ineligible types of alopecia, hair or scalp conditions, and assigned a clinical diagnosis of AGA or TE, which was later confirmed by phototrichometry. AGA was determined clinically by observing hair thinning and miniaturisation in the region of the scalp that is consistent with Ludwig classification. AGA diagnosis was confirmed by phototrichogram analysis of diversity of TH diameters and graded with the Anisotrichosis score (the ratio of one standard deviation of TH diameters to the mean TH diameter) >20%. TE was diagnosed by a combination of subject history, reports of self-reported thinning hair, shedding and by evaluating signs of general thinning or diffuse hair loss of the scalp, and directly ruling out underlying treatable causes. Phototrichogram analysis of TH telogen rate >20% was used to confirm the TE diagnosis. Luwdig classification was verified from global photographs. Any differences in diagnosis between the dermatologist and phototrichometry were resolved with the available evidence. For remaining subjects with dubious ascertainment, the diagnosis given by the dermatologist was used. For purposes of this study, only a primary diagnosis of AGA or TE was assigned. 72 women were assessed for eligibility and 46 were enrolled in the trial after AGA or TE diagnoses. All subjects signed IRB approved Informed Consent Forms prior to participation (See Fig. 1).
Fig. 1.
Profile of Randomised Controlled Trial. 72 subjects were assessed for eligibility which resulted in 21 subjects evaluated in the placebo group and 18 subjects evaluated in the ALRV5XR (treatment) group.
Exclusion criteria included scarring forms of alopecia, chemical or physical burns and traumatic lesions. Psoriasis, scaling, and follicular dermatitis. Fungal or bacterial infection, lice, and flea infestation of the scalp were also cause for exclusion. In addition, subjects with unusual thinning patches, traction alopecia or trichokryptomania, trichothiodystrophy, pili annulati, monilethrix or clear signs of trichodysmorphia, undernutrition or poor hygiene were excluded. Subjects with abnormalities such as skin damage in or around assessment areas, and scalp hair loss on treatment surfaces due to disease, injury, or medical therapy as well as those with history of surgical correction of scalp hair loss, hair transplants, and hair weave were also excluded.
Participating women were not allowed to use depilatories, razors, or wax on the scalp if the investigators considered that it might interfere with study assessments. Pregnant or planning to become pregnant women, those who were breastfeeding, or not using recommended contraceptives for the duration of the study, were excluded. Participation of subjects diagnosed with diabetes, endocrine, cardiovascular, renal, or liver disease, hypertension, and those with neurological disease such as Parkinson's disease, stroke, and traumatic brain injury was not allowed. Similarly, women who have recently started (<6 months) hormone replacement therapy were not included.
Women were not allowed to use any medications within 30 days prior to the baseline visit, including natural health products, if these were known to affect hair growth. Subjects with history of malignancy in the past 5 years or undergoing chemo or radiation therapy, and those with a known history of autoimmune disease (e.g., HIV/AIDS, systemic lupus erythematosus, inflammatory bowel disease, alopecia areata, alopecia totalis) were not included. Subjects with uncontrolled thyroid disease or any other disorders that may interfere with the study treatment were also excluded. Evidence of hepatic or renal dysfunction were additional reasons for exclusion.
Eligible subjects with a diagnosis of AGA or TE, Fitzpatrick skin type I-VI, Ludwig hair loss classification I, II or III as clinically established by a certified dermatologist, were enrolled if willing to follow study requirements and procedures, and after signing an IRB approved informed consent form.
2.4. Randomisation and masking
Randomisation and masked allocation into two blinded groups were done as follows: All screened subjects were allocated a sequential screening number by one dermatologist. A second dermatologist assigned the screening numbers of the eligible subjects sequentially to a biostatistician generated simple randomisation table. This resulted in subjects being randomised in a 1:1 ratio into one of two blinded treatment groups: Group A or B. Both dermatologists were masked from subject identity, characteristics, and diagnosis, to avoid potential bias during the randomisation process.
Study materials and label codes were masked for study subjects, site dermatologists, site personnel, tricho-analyst, and biostatistician. Upon completion of the biostatistician's report, the trial was unblinded and group A became the Placebo group and Group B the ALRV5XR group. An assessment of masking success was evaluated by various factors including baseline characteristics statistics and global photographs, test material dispensing records and inventory, and by observing positive responders in the placebo group.
2.5. Procedures
Similar looking test materials for both groups included oral capsules, shampoo, conditioner, and follicle serum. The ALRV5XR treatment contained botanical extracts, vitamins, and minerals as listed in Table S2 of the supplementary materials. Placebo test preparations contained identical vehicle compounds as those in the active ALRV5XR group products. ALRV5XR and placebo were provided by Arbor Life Labs.
All subjects were required to ingest one oral supplement capsule twice per day, along with daily use of a shampoo, conditioner, and follicle serum for a period of 24 weeks. Subjects were required to maintain a diary of study agent use and report any adverse events.
The duration of the study was approximately 28 weeks, including a screening period designed for eligibility assessment, a baseline visit, and visits at 12 and 24 weeks during the 24-week comparison period.
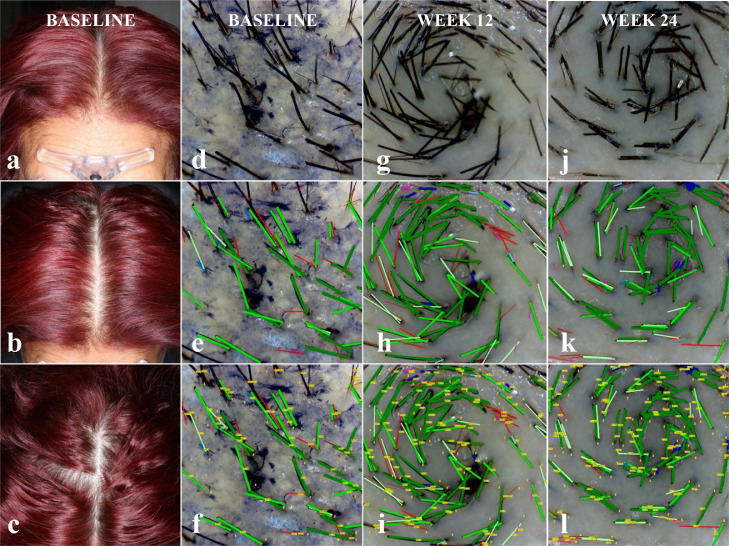
Subjects were evaluated by a certified dermatologist for eligibility, diagnosis of the hair loss condition, and hair loss pattern classification. At weeks 12 and 24, subjects were assessed for changes in health and safety parameters. A 35 mm Nikon digital camera mounted on a Canfield Scientific stereotactic device was used to take 8MP resolution global photographs of the frontal, mid-scalp and vertex of the subject's head, at 0, 45 and 90 degrees (See Fig. 2).
Fig. 2.
| Hair Density | Baseline | 12 Weeks | 24 Weeks |
|---|---|---|---|
| Total Hairs (per cm2) | 211 | 250 | 289 |
| Terminal Hairs (%/per cm2) | 73/152 | 76/191 | 73/211 |
| Vellus Hairs (%/per cm2) | 28/59 | 24/60 | 27/78 |
Subject: F008; Sex: Female; Age: 54; Ethnicity: Hispanic; Ethnic Hair Type: Latin/Indian/Semitic; Fitzpatrick Skin Type: III; Diagnosis: Androgenetic Alopecia; Ludwig Class-Type: I-3; Phototrichoscopy Area of View: 5.51mm2; Phototrichoscopy Magnification: 35x.
Target area hair counts (TAHC) were performed at baseline, and at week 12 and 24. At baseline, a circular area at the centre of the trichoglyph, located on the vertex region of the scalp was chosen for the TAHC. A permanent ink dot tattoo was placed for precise localization of the target area on subsequent evaluations. Hairs in the target area were clipped to less than 0·5 mm and then dyed. Mineral oil was applied to the target area.
Phototrichoscopic images of the target area were taken at each visit, using a Firefly DE330T Trichoscope with 2MP resolution at 35X magnification, and a 55·10 mm2 field of view. A cover glass was fixed to the lens of the trichoscope to orient the hair shafts in the target area, and at 90 degrees to the lens.
Phototrichoscopy images were evaluated by a blinded tricho-analyst using the TrichoSciencePro version 1.6 computer software. Terminal (non-vellus) hair density was determined by counting hairs in the field of view and by measuring their thickness. It was required that hairs be fully visible from the trimmed end to the exiting position of a scalp pore. THs were identified by a hair diameter greater than or equal to 40 µm, while VHs were those with a diameter of less than 40 µm. Digital images were automatically computer analysed to determine hair count and to measure hair shaft diameter. This was repeated and compared using semi-automatic and manual detection, and by diameter measurement performed by a tricho-analyst. TH density was calculated based on the number of THs in the TAHC per cm2. (See Fig. 2).
2.6. Outcomes
The purpose of this study was to evaluate the efficacy and safety of ALRV5XR used for hair regrowth in women with AGA, TE, or self-reported thinning hair, over a 24-week period. The primary efficacy objective was measurement of the terminal (non-vellus) hair density change from baseline to week 24, as measured by terminal hair count (THC) and evaluation of TH regrowth per cent change. Secondary objectives included evaluation of the TH density change from baseline to week 12 and assessment of ALRV5XR response rates, as measured in graduated intervals of TH regrowth changes at weeks 12 and 24. Non-responders were individuals whose TH density declined from baseline. Quality of life (QoL) questionnaires were completed by subjects at each visit. Baseline QoL data were recorded using an instrument that was not compatible with the instrument used for weeks 12 and 24. Therefore, only changes in QoL between weeks 12 and 24 were evaluable. Safety was reviewed by dermatologist assessment, subject reporting, and laboratory testing of blood and urine samples.
2.7. Statistical analysis
Baseline comparisons of demographic and physical characteristics of treatment and placebo groups were performed using Welch's two sample t-test which allows comparison of group means with differing variances. This method was also used to compare changes within each treatment group from baseline to week 12 and from baseline to week 24, and then confirmed with analysis of variance (ANOVA) using Fisher's F-test. For comparison of categorical variables between treatment groups at baseline, Pearson's chi-squared test (with simulated p-value) was used [28,29].
Longitudinal hair variations over time were assessed using a linear mixed effects model which examines the change in THs from baseline to week 24; adjusting for all covariates, and incorporating the demographic and physical subject characteristics (age, weight, ethnicity, skin type, diagnosis and Ludwig classification) [30,31]. All statistical analyses were performed using the R software version 4.0.3 (2020-10-10) [32].
2.8. Role of the funding source
The funder of the study had no role in the recruitment of subjects or data collection. The funder had a role in study design, data analysis, data interpretation and writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Study participants
A total of 46 women were enrolled in the study and 23 were randomly assigned to each group between April 3 and October 20, 2018. After enrolment, 2 subjects in the placebo and 3 in the ALRV5XR group withdrew consent during baseline assessments and were therefore excluded from the study. The intent to treat population (ITT) data originated from the 41 remaining subjects and was used for baseline characteristics and safety analyses. There were 21 subjects receiving placebo and 20 subjects receiving ALRV5XR. Two non-compliant subjects in the ALRV5XR group were excluded from the efficacy analyses. In summary, data from 21 subjects receiving placebo and 18 receiving ALRV5XR materials were analysed, as per protocol, for primary and secondary efficacy parameters. (See Fig. 1)
Baseline demographic and physical characteristics were similar between the ALRV5XR and placebo groups. No significant differences between the groups were found in categorical variables, such as age, height, weight, body mass index (BMI), blood pressure, TH density, ethnicity, skin type, diagnosis, and Ludwig classification (See Table 1).
Table 1.
Baseline characteristics by treatment group (ITT Population).
| Placebo (N=21) | ALRV5XR (N=20) | |
|---|---|---|
| Age (years) | ||
| Mean (±SD) | 51·6 (±10·7) | 50·1 (±9·1) |
| Range | 25 - 64 | 24 - 62 |
| Anthropometrics | ||
| Height (cm) | 159·0 (±4·5) | 161·1 (±8·2) |
| Weight (kg) | 63·0 (±12·0) | 63·9 (±11·9) |
| BMI (kg/m2) | 24·9 (±4·6) | 24·6 (±4·3) |
| Blood Pressure (mmHg) | 118·1/74·7 (±10·8/5·7) | 118·8/76·5 (±8·3/7·0) |
| Trichometry | ||
| Terminal Hair Density (per cm2) | 141·6 (±32·5) | 147·0 (±31·3) |
| Vellus Hair Density (per cm2) | 47·9 (±22·6) | 33·1 (±20·2) |
| Ethnicity | ||
| Caucasian | 3 (14%) | 1 (5%) |
| Chinese | 12 (57%) | 13 (65%) |
| Filipino | 0 (0%) | 1 (5%) |
| Hispanic | 4 (19%) | 3 (15%) |
| Japanese | 2 (10%) | 1 (5%) |
| Mongolian | 0 (0%) | 1 (5%) |
| Fitzpatrick Skin Type | ||
| I | 3 (14%) | 4 (20%) |
| II | 8 (38%) | 8 (40%) |
| III | 7 (33%) | 8 (40%) |
| IV | 2 (10%) | 0 (0%) |
| VI | 1 (5%) | 0 (0%) |
| Diagnosis | ||
| AGA | 11 (52%) | 16 (80%) |
| TE | 10 (48%) | 4 (20%) |
| Ludwig Classification-Type | ||
| I-1 | 3 (14%) | 2 (10%) |
| I-2 | 4 (19%) | 11 (55%) |
| I-3 | 9 (43%) | 5 (25%) |
| I-4 | 1 (5%) | 1 (5%) |
| II-2 | 0 (0%) | 1 (5%) |
| Missing | 4 (19%) | 0 (0%) |
Abbreviations: ± or SD=standard deviation.
3.2. Outcomes
Results are summarized in Tables 2–4. They depict the mean changes in THs/cm2 and by per cent, and are stratified per visit, per treatment group, and by response rates.
Table 3.
Summary of efficacy: mean of changes in terminal hair.
| Placebo (N=21) |
ALRV5XR (N=18) |
Efficacy (95% CI) |
Effect Size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Period | Mean | SD | Min | Max | Mean | SD | Min | Max | ∆-Mean | SD | p-value | Odds Ratio |
| Terminal (Hairs/cm2) | ||||||||||||
| Week 0-12 | 3·1 | ±7·5 | -7 | 22 | 16·1 | ±16·6 | -9 | 41 | 12·9 | ±8·2 | 0·0028 | 1·8 |
| Week 12-24 | 0·7 | ±9·5 | -9 | 32 | 17·9 | ±15·5 | -5 | 38 | 17·2 | ±8·2 | 0·0001 | 10·7 |
| Week 0-24 | 3·9 | ±15·8 | -9 | 54 | 33·9 | ±29·8 | -6 | 79 | 30·1 | ±15·0 | 0·0002 | 2·7 |
| Terminal Hairs (%) | ||||||||||||
| Week 0-12 | 2·6% | ±6·3% | -5·3% | 18·3% | 10·9% | ±12·5% | -6·0% | 36·3% | 8·3% | ±6·3% | 0·0110 | 1·8 |
| Week 12-24 | 0·8% | ±8·0% | -8·1% | 26·7% | 12·1% | ±11·2% | -3·2% | 33·6% | 11·4% | ±6·3% | 0·0007 | 10·7 |
| Week 0-24 | 3·4% | ±13·5% | -8·1% | 45·0% | 23·1% | ±22·2% | -4·9% | 69·9% | 19·7% | ±11·7% | 0·0016 | 2·7 |
Abbreviations: Week 0 = baseline; N=Per Protocol Evaluable Population; SD=standard deviation; ∆-Mean=Difference of Means between ALRV5XR and Placebo.
Table 2.
Summary of terminal hair counts per visit (Hairs/cm2)
| Placebo N=21 |
ALRV5XR N=18 |
|||
|---|---|---|---|---|
| Week | Mean | SD | Mean | SD |
| 0 | 141·6 | ±32·5 | 145·8 | ±32·9 |
| 12 | 144·7 | ±31·5 | 161·9 | ±39·2 |
| 24 | 145·4 | ±32·9 | 179·8 | ±48·9 |
Abbreviations: Week 0 = baseline; N=Per Protocol Evaluable Population; SD or ± =standard deviation.
Table 4.
Response Rates by Absolute and Relative Change in Terminal Hair Density. For the ALRV5XR treatment group, this table shows a significant shift of subjects’ Response Rates by Change in Terminal Hairs/cm2 from a relatively even distribution of 0-40 TH/cm2 at week 12 to higher values of 30 THs/cm2 and above at week 24. Response Rates by % Change in Terminal Hairs for the ALRV5XR treatment group, shows a significant shift of subjects’ hair % changes at week 12 to higher percentage ranges at week 24.
| Placebo |
ALRV5XR |
|||||
|---|---|---|---|---|---|---|
| Week 0-12 | Week 12-24 | Week 0-24 | Week 0-12 | Week 12-24 | Week 0-24 | |
| Positive Responders % | 67·7% | 42·9% | 42·9% | 77·8% | 88·9% | 66·7% |
| Change in Terminal Hairs/cm2 | ||||||
| <0 | 33·3% | 57·1% | 57·1% | 22·2% | 11·1% | 33·3% |
| 0-10 | 47·6% | 33·3% | 28·6% | 16·7% | 22·2% | - |
| 10-20 | 9·5% | - | 4·8% | 16·7% | 11·1% | - |
| 20-30 | 9·5% | 4·8% | - | 16·7% | 27·8% | - |
| 30-40 | - | 4·8% | - | 16·7% | 27·8% | 5·6% |
| 40-50 | - | - | 4·8% | 11·1% | - | 22·2% |
| ≥50 | - | - | 4·8% | - | - | 38·9% |
| Change in Terminal Hairs % | ||||||
| <0% | 33·3% | 57·1% | 57·1% | 22·2% | 11·1% | 33·3% |
| 0-10% | 52·4% | 33·3% | 28·6% | 22·2% | 27·8% | - |
| 10-20% | 14·3% | - | 4·8% | 33·3% | 33·3% | 11·1% |
| 20-30% | - | 4·8% | - | 16·7% | 22·2% | 11·1% |
| 30-40% | - | 4·8% | 4·8% | 5·6% | 5·6% | 27·8% |
| 40-50% | - | - | 4·8% | - | - | 11·1% |
| ≥50% | - | - | - | - | - | 5·6% |
| Max Regrowth Response | ||||||
| Terminal Hairs/cm2 | 22·0 | 32·0 | 54·0 | 41·0 | 38·0 | 79·0 |
| Terminal Hairs % | 18·3% | 26·7% | 45·0% | 36·3% | 33·6% | 69·9% |
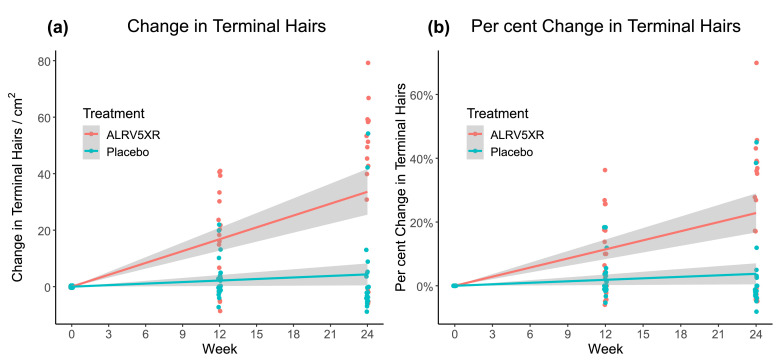
Fig. 3 depicts a strong and significant correlation of ALRV5XR efficacy with treatment duration. The ALRV5XR group showed a significant change in THC from baseline over time (33·9 THs/cm2 in the 24-week study period). After adjusting for categorical variables (age, weight, ethnicity, diagnosis, Ludwig classification and skin type), none of the covariates affected the outcome in a statistically significant manner. Differences in QoL changes from 12 to 24 weeks between the ALRV5XR and placebo groups were not significant.
Fig. 3.
Changes in Terminal Hair from Baseline Over 24 Weeks. Treatment group ALRV5XR was significantly superior to placebo group for both (a) Absolute Change in Terminal Hairs (P=0·0002) and (b) Per Cent Change in Terminal Hairs (P=0·0016). Linear regressions are shown as lines with 95% confidence intervals shown in grey.
3.3. Primary outcomes
For the primary outcome at 24 weeks, efficacy of ALRV5XR over placebo induced significant TH regrowth of 30·1 THs/cm2 with an Odds Ratio (OR) of 2·7 [95%CI: 15·1 – 45·1; (p=0·0002)] and 19·7% with OR of 2·7 [95%CI: 8·0% – 31·4%; (p=0·0016)]. Also, 12 (66·7%) of 18 subjects in the ALRV5XR group, compared to nine (42·9%) of 21 subjects in the placebo group, responded favourably.
An ANOVA analysis for the overall trial of ALRV5XR vs placebo found F = 32·3 (p<0·0001).
An ITT analysis of the primary outcome, at 24 weeks, including the non-compliant subjects, showed an efficacy of ALRV5XR over placebo of 26·3 THs/cm2 with an OR of 2·0 [95%CI: 11·2 – 41·4; (p=0·0011)] and 17·1% with an OR of 2·0 [95%CI: 5·5% – 28·7%; (p=0·0049)], with 12 (60·0%) of 20 subjects in the ALRV5XR group compared with 9 (42·9%) of 21 subjects in placebo group responding favourably.
Treatment with ALRV5XR was safe, well-tolerated and no adverse effects were observed. Concordantly, no significant changes were observed in physical examinations, blood or urine laboratory parameters (for more details see Tables S4a, S4b and S4c in the supplemental materials).
3.4. Secondary outcomes
At 12 weeks efficacy of ALRV5XR over placebo was 12·9 THs/cm2 with an OR of 1·8 [95%CI: 4·8 – 21·1; (p=0·0028)] and 8·3% with an OR of 1·8 [95%CI: 2·0% – 14·6%; (p=0·0110)], with 14 (77·8%) of 18 in the ALRV5XR group responding favourably compared to 14 (66·7%) of 21 subjects in the placebo group.
From 12-24 weeks efficacy was 17·2 THs/cm2 with an OR of 10·7 [95%CI: 9·0 – 25·4; (p=0·0001)] and 11·4% with an OR of 10·7 [95%CI: 5·1% – 17·6%; (p=0·0007)], with 16 (88·9%) of 18 responding favourably in the ALRV5XR group compared with nine (42·9%) of 21 subjects in the placebo group.
At 12-weeks, 11·1% of the ALRV5XR group had increased their TH density by more than 40 THs/cm2, increasing to 61·1% at 24-weeks with all responders increasing TH by more than 30 THs/cm2. Comparable results were achieved when measuring per cent change in TH density: 5·6% of the ALRV5XR group increased their TH density by 30% after week 12 while 44·5% had attained these results after 24-weeks. Moreover, at 12-weeks, the greatest increases in THC in the ALRV5XR group was 41·0 THs/cm2 and 36·3%. At 24-weeks, the greatest increases seen were 79·0 THs/cm2 and 69·9% respectively. Specifics can be found in Table 4, with additional responder information in Supplementary Table S5 and Figure S1.
Efficacy of total hair counts including all TH and VH at 24 weeks of ALRV5XR vs. placebo was 33·9 hairs/cm2 with OR 4·2 [95%CI:18·1 – 49·8; (p=0·0001)]. Total hair count change within the ALRV5XR group at 24 weeks was 37·9 hairs/cm2 (p<0·0001). There was no statistical significance in changes to VH or TH/VH ratios between or within any of the groups at 24 weeks (see Table S3 in the supplemental materials).
3.5. AGA and TE cohort outcomes
Efficacy for the AGA cohort was similar to the overall trial at 24 weeks, with efficacy of ALRV5XR vs. placebo of 31·4 more THs/cm2 with an OR of 3·2 [95%CI: 10·3 – 52·6; (p=0·0053)]. Efficacy for the TE cohort was also similar to the overall trial and that of the AGA cohort at 24 weeks with an efficacy of ALRV5XR vs. placebo of 29·0THs/cm2 with an OR of 3·0 [95%CI: 3·3 – 54·7; (p=0·0301)]. An ANOVA analysis showed that differences between the AGA and TE cohorts for efficacy and rate of change of efficacy were not significant.
4. Discussion
During this 24-week clinical trial, ALRV5XR was well-tolerated by all female subjects, and displayed an identical safety profile to that of placebo. Compliance with the treatment regimen of one oral supplement capsule twice per day, along with daily use of a shampoo, conditioner, and follicle serum was greater than 95% with only two non-compliant subjects. ALRV5XR's efficacy demonstrated superiority vs. placebo in the treatment of AGA or TE. Efficacy in the AGA and TE cohorts was similar to that observed in the overall trial. The effect size of the efficacy found in all groups, and presented as odds ratios, was largely in favour of the ALRV5XR treatment.
When compared to placebo, the use of ALRV5XR in subjects affected with AGA or TE significantly increased THC and per cent change of TH from baseline to week 12. The larger effects observed at week 24 suggested cumulative effects of the ALRV5XR treatment over time. These positive effects were observed regardless of age, weight, ethnicity, diagnosis, skin type or hair loss classification. Baseline trichometric differences between study groups were not statistically or clinically relevant and did not affect the study outcome, especially considering that the balance of the differences had no influence in results in either group.
The response profile of the placebo group remained generally low at weeks 12 and 24. In sharp contrast, the ALRV5XR group showed a rather uniform spread of responses at week 12 and a bimodal effect at week 24 (66·7% responders vs. 33·3% non-responders). This bimodal response was not dependent on absolute THC or per cent THC change. In the responders, a noticeable acceleration of hair regrowth from week 12 to week 24 was documented.
When comparing efficacy of ALRV5XR vs. placebo over time, there was a significant improvement from 12·9 THs/cm2 in the first 12-week period, with an additional 17·2 THs/cm2 in the second 12-week period culminating in the overall 30·1 THs/cm2 efficacy at week 24. This impressive 133% increase in efficacy in the second 12-week period over the first 12-week period indicates that the accelerated regrowth induced by the ALRV5XR treatment in responders may continue well beyond week 24. This finding suggests that longer and sustained exposure to ALRV5XR may continue to induce anagen in involuted (telogen) HFs and might induce almost complete regeneration of the normal scalp hair phenotype.
Considering that the average human scalp has approximately 700 cm2 of hair coverage, the efficacy observed at week 24 (30·1 THs/cm2), indicates a regrowth potential of an additional 21,000 THs for the individual using ALRV5XR. Hypothetically, if the regrowth rate of the ALRV5XR group at week 24 is maintained, 91·6% of responders may gain more than 28,000 new THs, and 58·3% of responders more than 35,000 new THs.
There were two outliers in the placebo group with unexpected high positive responses at 24 weeks where a subject with AGA increased their THC by 42 THs/cm2 (39%) and a TE subject increased theirs by 54 THs/cm2 (45%). These subjects were included in the final analysis. There are few trials studying regrowth in healthy TE subjects and no treatments are approved for TE. Given the clinical view that TE in a healthy subject typically self resolves, there was a risk that the TE placebo group might diminish or confound the efficacy of the overall trial. The randomisation table assigned approximately equal proportions of AGA subjects to the ALRV5XR and placebo groups, however the TE cohort had by chance, a disproportionate assignment to the placebo group, which may have further confounded the trial outcome. The similar efficacy in each of the AGA and TE cohorts, and its similarity to that of the overall trial, suggest that TE is a treatable condition and ALRV5XR is an effective option for women with TE.
In published clinical trials and meta-analyses, the current standard therapy (minoxidil) after 48 weeks of treatment, resulted in average gains of hair density of 12·4 (2% minoxidil) to 15·1 (5% minoxidil) THs/cm2 [19–21]. The efficacy of ALRV5XR reported in this study compares favourably to these results. At 12 weeks, with an efficacy of 12·9 THs/cm2, ALRV5XR had similar efficacy than that of 2% minoxidil at 48 weeks. At 24 weeks, with an efficacy of 30·1 THs/cm2, ALRV5XR reached gains that almost double those induced by 5% minoxidil treatment at 48 weeks. Furthermore, the 66·7% responder rate seen with ALRV5XR is far superior to the 13-40% reported for minoxidil [21,22]. In addition, ALRV5XR has a clearly improved safety profile when compared to that of minoxidil, which was associated to a miscellaneous range of adverse events in the reported studies [19,21].
Interestingly, when compared to published RCTs of standard approved therapy, the overall efficacy of ALRV5XR indicates that 2% minoxidil requires 4 times longer treatment to achieve the same efficacy of ALRV5XR. Similarly, ALRV5XR doubles the efficacy of 5% minoxidil in half the time, and the per cent of responders to ALVR5XR approximately quadruples the number of responders in minoxidil trials.
In the pursuit of improving the efficacy of hair regrowth in women, various studies have investigated combination therapies including one or more topical and/or oral agents with and without topical minoxidil, reporting unremarkable improvements [19]. ALRV5XR is consistent with these combination approaches, yet with its multi-molecular targeting, designed to modulate >20 of the accepted pathways involved in healthy HF biology, it has achieved a synergistic effect as documented by its superior outcome.
When evaluating the totality of the evidence in the literature and the results of this clinical trial, our hypothesis of ALRV5XR, proved to be a highly effective treatment in regenerating and maintaining TH in women. The significant clinical effect achieved in this trial, without side effects, and the significant and accelerating increases in TH density from week 12 to 24, can be explained clinically as prolonging anagen while also inducing anagen in involuted or non-productive telogen hair follicles. These clinical effects have been explained in the literature as HF regenerative and homeostatic physiological effects of activating HF stem cells via the Wnt/β-catenin signalling pathway, and by regulation and/or modulation of multiple cytokines, growth factors and hormone mediators, and likely relieving pressure on the HF [2,[16], [17], [18],25-27] These mechanisms of action are similar to those observed in PRP scalp treatments which have been linked to multiple similar pathways [26]. Future research should focus on elucidating the main mechanisms of action to refine ALRV5XR delivery and applications in other ageing tissues. Other interesting aspects of research should aim to explore the potentiating and longevity effects of ALRV5XR as a combination treatment with LLLT, PRP, or transplantation of stem cells and follicle units. More importantly, additional and longer-term studies of ALRV5XR as topical, oral or combination treatments, are required to evaluate various regimen options to maintain adherence for optimal efficacy and the long-term potential in regeneration of a woman's normal scalp hair phenotype of the third decade [10].
ALRV5XR appears to provide a viable option for the unmet need of an effective, safe, and well-tolerated treatment for women of all ages, ethnicities and skin types with premature ageing or senescent hair loss associated with AGA and TE. The ALRV5XR multi-molecular targeting approach for hair regrowth significantly increased TH density and appeared to regenerate the normal structure and function of involuted and non-productive hair follicles and prolong anagen. Besides having an improved safety profile over the current FDA approved standard of care, ALRV5XR achieved a multi-fold improvement in efficacy and response rates. While longer studies are needed to demonstrate the complete potential of ALRV5XR, progressive acceleration of TH regrowth over 24 weeks suggests that ALRV5XR may be a highly effective long-term treatment for hair loss in women, while reasonably expecting that a prolonged ALRV5XR treatment might restore a normal hair phenotype.
5. Funding
This clinical trial was funded by Arbor Life Labs, Toronto, Ontario, Canada.
6. Contributors
PRF and DB contributed to the study concept and design. PRF obtained funding. BMM contributed to recruitment of subjects, investigation, supervision, data acquisition and data curation. PRF, KMF, CP, BMM and JGA contributed to data analysis and interpretation. PRF, KMF and CP contributed to the validation, statistical analysis and visualization. CP and KMF drafted the manuscript. PRF, KMF, CP, DB, DJC and JGA critically revised the manuscript for important intellectual content.
7. Data sharing
Data collected for the study, including individual participant data and a data dictionary, will be made available. Data available includes de-identified participant data, study protocol and informed consent form. These data will be available beginning 6 months and ending 3 years after publication. Data will be shared with investigator support to researchers who provide a methodologically sound proposal with a signed data access agreement. Contact corresponding author.
Declaration of Competing Interest
PRF is the director of Arbor Life Labs (ALL), PRF, DJC and DB are coinventors of patents filed for ALRV5XR and have a share in ALL. PRF, KMF, CP, BMM, DJC and JG, received honoraria from ALL and proposed submission for publication. PRF, DB and DJC have a share in ALL. All authors had full access to the data in the study.
Acknowledgment
The authors express their profound appreciation and acknowledgement to Dr Luciano Eugenio Marra, PhD for his contribution to developing ALRV5XR – we dedicate this publication to Dr Marra, who died shortly after the commencement of this trial; Dr Kurt Stenn MD for reviewing the data and his guidance on reporting and interpreting results; Dr Elizabeth Renouf, PhD for Biostatistical analysis; and Deborah Cahan, without whom ALRV5XR would not have been developed due to her persistent and complex alopecia which dramatically changed her life. This trial was funded by Arbor Life Labs.
Footnotes
Funding: Arbor Life Labs.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100978.
Contributor Information
Peter R Feldman, Email: petefeldman@gmail.com.
Klaus M Fiebig, Email: klausfiebig@gmail.com.
Appendix. Supplementary materials
References
- 1.McMichael A.J., Hordinsky M.K. CRC Press; Boca Raton: 2018. Hair and scalp disorders: medical, surgical, and cosmetic treatments, second edition. [Google Scholar]
- 2.Matsumura H., Mohri Y., Binh N.T. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351(6273):aad4395. doi: 10.1126/science.aad4395. [DOI] [PubMed] [Google Scholar]
- 3.Cash T.F., Price V.H., Savin R.C. Psychological effects on androgenetic alopecia on women: comparisons with balding and female control subjects. J Am Acad Dermatol. 1993;29(4):568–575. doi: 10.1016/0190-9622(93)70223-g. [DOI] [PubMed] [Google Scholar]
- 4.Karnik P., Shah S., Dvorkin-Wininger Y., Oshtory S., Mirmirani P. Microarray analysis of androgenetic and senescent alopecia: comparison of gene expression shows two distinct profiles. J Dermatol Sci. 2013;72(2):183–186. doi: 10.1016/j.jdermsci.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norwood O.T. Incidence of female androgenetic alopecia (female pattern alopecia) Dermatol Surg. 2001;27(1):53–54. [PubMed] [Google Scholar]
- 6.Price V.H. Treatment of hair loss. N Engl J Med. 1999;341(13):964–973. doi: 10.1056/NEJM199909233411307. [DOI] [PubMed] [Google Scholar]
- 7.Guo E.L., Katta R. Diet and hair loss: effects of nutrient deficiency and supplement use. Dermatol Pract Concept. 2017;7:1–10. doi: 10.5826/dpc.0701a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen EA, Salt C.D., II A new take on the Severity of Alopecia tool (SALT) for determining percentage scalp hair loss. J Am Acad Dermatol. 2016;75(6):1268–1270. doi: 10.1016/j.jaad.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Whiting DA. Fairfield; NY: Canfield: 2004. The structure of the human hair follicle: light microscopy of vertical and horizontal sections of scalp biopsies. [Google Scholar]
- 10.Robbins C, Mirmirani P, Messenger AG. What women want - quantifying the perception of hair amount: an analysis of hair diameter and density changes with age in caucasian women. Br J Dermatol. 2012;167(2):324–332. doi: 10.1111/j.1365-2133.2012.11010.x. [DOI] [PubMed] [Google Scholar]
- 11.Trüeb RM. Springer Nature; Switzerland: 2020. Nutrition for healthy hair: guide to understanding and proper practice. [Google Scholar]
- 12.Su LH, Chen LS, Lin SC, Chen HH. Association of androgenetic alopecia with mortality from diabetes mellitus and heart disease. JAMA Dermatol. 2013;149(5):601–606. doi: 10.1001/jamadermatol.2013.130. [DOI] [PubMed] [Google Scholar]
- 13.Olds H, Liu J, Luk K, Lim HW, Ozog D, Rambhatla PV. Telogen effluvium associated with COVID-19 infection. Dermatol Ther. 2021:e14761. doi: 10.1111/dth.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtois M, Loussouarn G, Hourseau C, Grollier JF. Ageing and hair cycles. Br J Dermatol. 1995;132(1):86–93. doi: 10.1111/j.1365-2133.1995.tb08630.x. [DOI] [PubMed] [Google Scholar]
- 15.Messenger AG, Sinclair R. Follicular miniaturization in female pattern hair loss: clinicopathological correlations. Br J Dermatol. 2006;155(5):926–930. doi: 10.1111/j.1365-2133.2006.07409.x. [DOI] [PubMed] [Google Scholar]
- 16.Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37(8-9):981–990. doi: 10.1016/s0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 17.Bernard BA. Advances in understanding hair growth. F1000Res. 2016;5 doi: 10.12688/f1000research.7520.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasserot AP, Geyfman M, Poloso NJ. Androgenetic alopecia: combing the hair follicle signaling pathways for new therapeutic targets and more effective treatment options. Expert Opin Ther Targets. 2019;23(9):755–771. doi: 10.1080/14728222.2019.1659779. [DOI] [PubMed] [Google Scholar]
- 19.Kanti V, Messenger A, Dobos G. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32(1):11–22. doi: 10.1111/jdv.14624. [DOI] [PubMed] [Google Scholar]
- 20.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141. doi: 10.1016/j.jaad.2017.02.054. e135. [DOI] [PubMed] [Google Scholar]
- 21.Lucky AW, Piacquadio DJ, Ditre CM. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50(4):541–553. doi: 10.1016/j.jaad.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Roberts J, Desai N, McCoy J, Goren A. Sulfotransferase activity in plucked hair follicles predicts response to topical minoxidil in the treatment of female androgenetic alopecia. Dermatol Ther. 2014;27(4):252–254. doi: 10.1111/dth.12130. [DOI] [PubMed] [Google Scholar]
- 23.Kwack MH, Kang BM, Kim MK, Kim JC, Sung YK. Minoxidil activates beta-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J Dermatol Sci. 2011;62(3):154–159. doi: 10.1016/j.jdermsci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 25.Guevara-Aguirre J, Torres C, Pena G. IGF-I deficiency and enhanced insulin sensitivity due to a mutated growth hormone receptor gene in humans. Mol Cell Endocrinol. 2021;519 doi: 10.1016/j.mce.2020.111044. [DOI] [PubMed] [Google Scholar]
- 26.Gentile P, Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with Minoxidil(R), Finasteride(R), and Adult stem cell-based therapy. Int J Mol Sci. 2020;21(8):2702. doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman A, Herman AP. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia. 2016;114:18–25. doi: 10.1016/j.fitote.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Dupont WD. Cambridge Press; New York: 2002. Statistical modeling for biomedical researchers: a simple introduction to the analysis of complex data. [Google Scholar]
- 29.Venables WN., Ripley BD. 4th edition. Springer- Verlag; New York: 2002. Modern applied statistics with S. [Google Scholar]
- 30.Fitzmaurice GM., Laird N., Ware JH. 2nd ed. Wiley; New Jersey: 2011. Applied longitudinal analysis. [Google Scholar]
- 31.Lang W. CRC Press; New York: 2010. Mixed effects models for complex data. [Google Scholar]
- 32.R Core Team . R foundation for statistical computing; Vienna, Austria: 2012. A language and environment for statistical computing.http://cran.r-project.org available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.