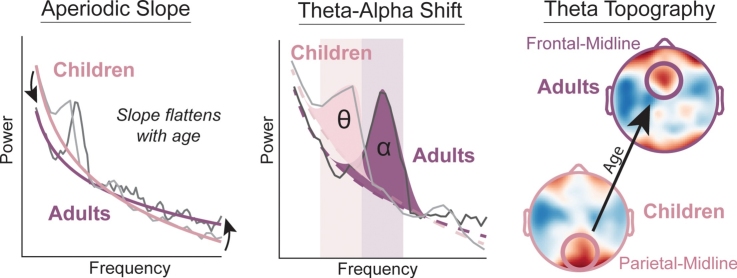
Graphical abstract
Keywords: Alpha oscillations, Aperiodic signal, Development, EEG, Peak frequency, Theta oscillations
Highlights
-
•
Canonical EEG analyses conflate neural oscillations with the aperiodic signal.
-
•
From early childhood to adulthood, the aperiodic signal flattens in slope.
-
•
Dominant posterior oscillations shift from theta to alpha around 7–8 years old.
-
•
Theta oscillations transition from posterior dominant to anterior dominant with age.
Abstract
Intrinsic, unconstrained neural activity exhibits rich spatial, temporal, and spectral organization that undergoes continuous refinement from childhood through adolescence. The goal of this study was to investigate the development of theta (4−8 Hertz) and alpha (8−12 Hertz) oscillations from early childhood to adulthood (years 3–24), as these oscillations play a fundamental role in cognitive function. We analyzed eyes-open, resting-state EEG data from 96 participants to estimate genuine oscillations separately from the aperiodic (1/f) signal. We examined age-related differences in the aperiodic signal (slope and offset), as well as the peak frequency and power of the dominant posterior oscillation. For the aperiodic signal, we found that both the aperiodic slope and offset decreased with age. For the dominant oscillation, we found that peak frequency, but not power, increased with age. Critically, early childhood (ages 3–7) was characterized by a dominance of theta oscillations in posterior electrodes, whereas peak frequency of the dominant oscillation in the alpha range increased between ages 7 and 24. Furthermore, theta oscillations displayed a topographical transition from dominance in posterior electrodes in early childhood to anterior electrodes in adulthood. Our results provide a quantitative description of the development of theta and alpha oscillations.
1. Introduction
Decades of research suggest that rhythmic fluctuations in electrical potentials generated by neuronal activity, known as neural oscillations, are intimately linked to brain function and behavior (Uhlhaas et al., 2010; Voytek and Knight, 2015). Neural oscillations are ubiquitous phenomena in extracellular local field potentials, intracranial electrocorticography, and extracranial electroencephalography (EEG) recordings. Oscillations can be induced by external task manipulations, spontaneously expressed in resting-state or unconstrained conditions, and have been extensively studied in both humans and nonhuman primates (Cohen, 2017). Mechanistically, neural oscillations are known to be generated by circuit mechanisms that operate across temporal and spatial scales. For example, oscillations may reflect interactions between glutamatergic pyramidal neurons and GABAergic inhibitory interneurons (Cardin et al., 2009; Sohal et al., 2009), excitability of a cortical region (Haegens et al., 2011; Iemi et al., 2017), and coordinated neural activity between distributed brain networks (Buschman and Miller, 2007; Gregoriou et al., 2009; Saalmann et al., 2012).
This functional diversity is also reflected in the frequency organization of neural oscillations. Neural oscillations can be partitioned into different bands of frequencies, ranging in adult human EEG from delta (2–4 Hertz), theta (4−8 Hertz), alpha (8−12 Hertz), beta (12–30 Hertz), to gamma-band (30–100 Hertz)— though the specific frequency ranges used for analysis of these oscillatory bands vary greatly between adults and children (Bell and Cuevas, 2012; Marshall et al., 2002). Each frequency band may be driven by a distinct set of cellular or circuit mechanisms and serve different behavioral functions. For instance, theta-band activity has been shown to be involved in top-down control (Cavanagh and Frank, 2014; Riddle et al., 2019) and working memory (Riddle et al., 2020a). Whereas alpha-band power in adults is thought to index the inhibition of task-irrelevant processes that distract attention and working memory functions (Foxe and Snyder, 2011; Klimesch et al., 2007; Riddle et al., 2020b). Thus, neural oscillations can be useful descriptive indices for bridging neural dynamics and brain functions.
Given this significance, clarifying the relationship between neural oscillations and development should be a major goal for developmental cognitive neuroscience. One major step towards achieving this goal is to characterize the development of spontaneous, intrinsic, neural oscillations unconstrained by task conditions. Intrinsic neural activity is known to exhibit an organized spatial structure in the form of functional brain networks, which undergoes continuous refinement from childhood through adolescence (Cui et al., 2020; Marek et al., 2015). Intrinsic activity is organized into spatially distributed functional networks, as measured by resting-state functional magnetic resonance imaging (fMRI), which have proven useful in capturing individual differences and developmental variability related to behavioral phenotypes (Seitzman et al., 2019; Uddin et al., 2010). Similarly, spontaneous neural oscillations reflect dynamic activity at the network level with greater temporal resolution than fMRI (Deco et al., 2011; Hipp et al., 2012). Thus, elucidating the development of spontaneous neural oscillations augments resting-state fMRI studies by elucidating the dynamics of intrinsic brain activity. Such effort may be significant for identifying when and which component of neural dynamics may go awry during atypical development (Uhlhaas et al., 2010; Uhlhaas and Singer, 2011). Characterizing the developmental change of spontaneous neural oscillations is the primary motivation of our study.
To date, many longitudinal and cross-sectional EEG studies have focused on quantifying the spectral power within fixed bands of frequency ranges— e.g., the average power over 4−8 Hertz for studying theta-band oscillations (Arns et al., 2012; Barry and Clarke, 2009; Benninger et al., 1984; Clarke et al., 2001; Cragg et al., 2011; Dustman et al., 1999; Gasser et al., 1988a; Matthis et al., 1980; Orekhova et al., 2006; Segalowitz et al., 2010; Smith, 1938). For example, Clarke et al. (2001) examined age-related differences in theta (defined as 2.5–7.5 Hertz) and alpha (7.5–13.5 Hertz) band in EEG for children ages 8–12 years, and found a relative decrease in theta-band and increase in alpha-band power with age in both frontal and posterior regions. Marshall et al. (2002) analyzed longitudinal data from 5 months to 4 years of age and found that the frequency band with the greatest power transitioned from theta to alpha-band around 10 months of age. Together, previous research suggests that the power of alpha and theta band are critical developmental markers. However, recently improved understanding of the nature of neural oscillations provides new techniques to improve on these early studies (Donoghue et al., 2020).
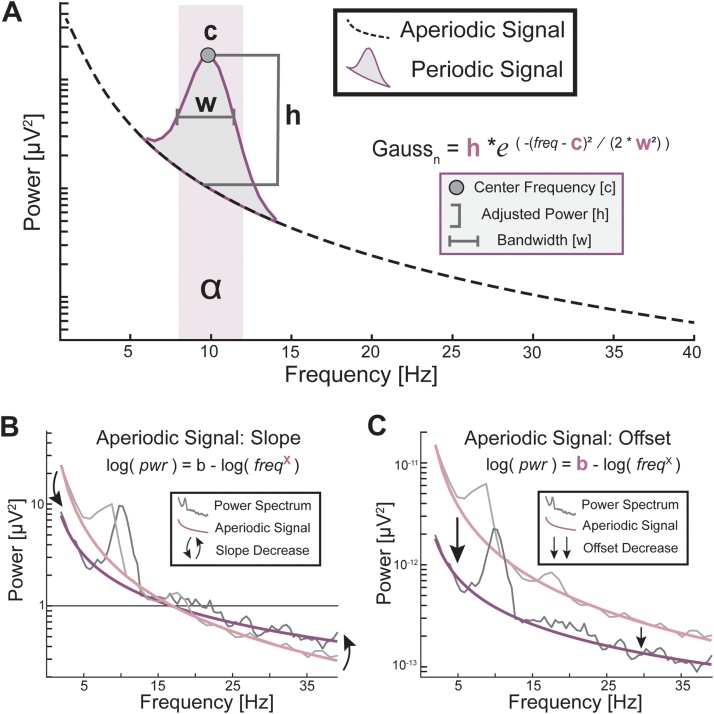
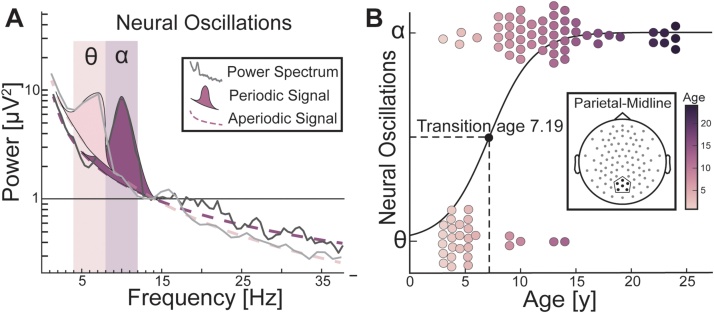
An accurate description of how neural oscillations develop with age demands precision in measuring and interpreting different aspects of the oscillation, as neural oscillation is not a one-dimensional dependent measure, but contains various measurable characteristics (e.g., frequency, power, phase, spectrum). Each of these characteristics may have a different underlying physiology and distinct functional contribution to brain function and behavior. Recent methodological developments offer analytical tools that can measure these oscillatory components with increased precision (Haller et al., 2018). For example, traditional analyses using canonical frequency bands do not separate a commonly observed background activity of the brain—known as the aperiodic signal—from the oscillatory (periodic) signal; nor do they estimate the peak frequency of the oscillation (Fig. 1A). Consequently, what appears as an age-related difference in oscillatory activity might be due to a shift in aperiodic slope (Fig. 1B) or intercept (Fig. 1C) of the power spectrum, but not changes in the power of an oscillation (Donoghue et al., 2020; Haller et al., 2018). Because many prior studies did not account for influences for the aperiodic signal, it is not clear if these findings reflect changes in genuine oscillations, whereases a more parsimonious explanation might be an age-related change in features of the aperiodic signal (e.g., the shift in greater theta to alpha power might just be a change in aperiodic slope). It is therefore critical to separate the aperiodic activity from oscillatory activity to accurately estimate the power of neural oscillations, as this allows the establishment of the presence or absence of an oscillation relative to the aperiodic signal (Donoghue et al., 2020); see Fig. 1 for illustration). Adopting these practices can derive a more accurate description of the development of neural oscillations.
Fig. 1.
Power spectrum as an aperiodic signal with superimposed periodic signal(s) (A) An illustration of the power spectrum as two separable signal sources: the aperiodic background signal (dashed line), and one or more periodic components (dark purple “bump”). Analysis of the aperiodic and periodic signals separately allows for a more accurate estimate of changes in oscillatory activity with age. The canonical alpha-band (α) is highlighted in light purple. (B, C) Two example participants, one age 4.5 years (light purple line) and the other age 15 (dark purple line), have different aperiodic slopes (B) and offsets (C). The solid grey lines show the raw power spectra. The aperiodic signal slope was estimated as the exponent of the equation in (B) and the offset as the intercept in (C). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
One cardinal characteristic of oscillatory signals is the expression of a prominent peak of oscillatory activity in the power spectrum (Fig. 1A). We will hereon define this oscillatory peak as the “dominant oscillation,” consistent with terminology adopted by the field (Lodder and van Putten, 2011; Marcuse et al., 2008). In adult EEG, the dominant oscillation most commonly falls within the alpha-band range of 8−12 Hz (Berger, 1929; Chiang et al., 2011; Klimesch, 1999), but during early childhood, the dominant oscillation appears to be within the theta-band frequency range (Lodder and van Putten, 2011; Rodríguez-Martínez et al., 2017). Alpha and theta oscillations are developmentally significant because they have both been shown to be closely related to attention (Foxe and Snyder, 2011; Fries et al., 2001; Klimesch, 1999) and working memory (Roux and Uhlhaas, 2014; Sauseng et al., 2009, 2005). These cognitive functions are also known to exhibit significant developmental improvement from childhood through adolescence (Davidson et al., 2006; Luna et al., 2004).
The goal of the present study is to characterize the age-related differences in the dominant oscillation from early childhood to early adulthood. To accomplish this goal, we examined eyes-open resting-state EEG data from two independent samples with participants aged 2.95–24 years old. By accounting for the influence of both aperiodic and periodic signal, our goals were to first identify when genuine theta and alpha oscillations were present during development, second to characterize the developmental trajectories of the peak frequency, relative power, and topography of the dominant oscillation, and finally to determine developmental changes in features of the aperiodic signal.
2. Methods
2.1. Participants
We examined two independent samples. The first was a sample from the School Readiness Study (SRS), an ongoing longitudinal study that consisted of children recruited between ages 36 and 63 months from the community surrounding the University of Iowa. Children were scheduled to return for longitudinal follow-ups with repeated measures in approximately 9-month increments. This sample (abbreviated as the SRS sample hereafter) includes a total of 41 children (19 females), sixteen who entered the study at 36 months of age, nine who entered the study at 45 months of age, six who entered at 54 months, and ten who entered at 63 months. We re-assessed 22 of these 41 children during a subsequent visit 9 months after their initial visit, resulting in 63 total study sessions spanning from 2.95 to 6.13 years of age. All study procedures were approved by the institutional review board at the University of Iowa.
The second sample was acquired from publicly available data from the Child Mind Institute, Multimodal Resource for Studying Information Processing in the Developing Brain project (CMI-MIPDB) (Langer et al., 2017). It consisted of an older range of participants, 8–24 years of age. We isolated a subset of 72 participants from the CMI-MIPDB dataset that were screened for a wide range of clinical diagnostic criteria and had no clinical diagnosis.
2.2. EEG data preprocessing
For both samples, resting-state EEG data was collected using a 128-channel Hydrocel Geodesic SensorNet EEG system. For the CMI-MIPDB sample, resting-state EEG data were collected with alternating eyes-open and eyes-closed periods lasting 20 s and 40 s, respectively. Each of these blocks were repeated five times, for a total of 300 s of data collected. We extracted the eye-open portion of the data for our analysis, and eye-closed data were not included. The sampling rate was 500 Hz. For the SRS sample, participants were instructed to remain as still and quiet as possible while watching child-friendly cartoon video clips on a screen, and each resting-state recording lasted for 180 s, recorded at a sampling rate of 1000 Hz. Thus, our analyses focused on eye-open data from both samples.
All preprocessing was performed with custom Python 3.7 scripts using MNE v0.18.2 (Gramfort et al., 2013). For the SRS sample, each set of continuous data were band-pass filtered at 1 and 40 Hz. Noisy channels were inspected visually and interpolated using spherical spline interpolation (Perrin et al., 1989). Data were re-referenced to the average of all of the available 128 channels. Subsequently, epochs were hand-inspected and noisy epochs excluded from further analyses. We submitted the data to an independent component analysis (ICA) using infomax rotation (Lee et al., 1999). This procedure seeks to identify components that capture muscle and eye-blink related artifacts, and we visually inspected all independent components. Noisy components that were likely motion-related were identified based on the presence of artifacts around the perimeter of the topographic map, or singular noisy channels. Components that were related to blinks and saccades, muscles, and heartbeats were excluded as well. Noise components were then rejected and removed from the data. A final inspection of the epochs was performed after component rejection. An average of 6% of epochs and 30.3 % of independent components per participant were rejected from the SRS sample.
The CMI-MIPDB dataset was preprocessed using identical steps, with the following exceptions. An iterative process of modification was necessary due to greater noise in this sample as identified during independent component analysis and slight differences in testing procedure. Re-referencing was accomplished using an average of the 90 scalp electrodes, excluding external/face electrodes. The rationale for this is that global re-referencing in this dataset introduced excessive noise from facial artifacts and noisy channels. Eyes-open resting state data were extracted and the eyes-closed data were discarded. Epochs and independent components rejected were 15 % and 41 %, respectively, for the CMI-MIPDB dataset.
Four EEG sessions from the SRS sample were excluded from analysis due to an incomplete resting-state EEG file, resulting in a total of 59 resting-state EEG sessions from 39 individuals. Fifteen CMI-MIPDB participants were excluded due to excessive noise identified during preprocessing of EEG data, resulting in a total of 57 participants (24 females, ages 8–24 years old) included. We combined data from the CMI-MIPDB and SRS samples into one dataset of 116 unique visits from 96 individuals, spanning ages 2.95–24 years old.
2.3. EEG cluster selection
To address the multiple comparisons problem of independently testing each electrode, we restricted our analysis to two a priori clusters of electrodes on the scalp. First, a posterior electrode cluster over parietal-occipital cortex was selected based on prior research indicating that the dominant oscillation of the brain typically originates in parietal-occipital cortex (Chiang et al., 2011; Klimesch, 1999; Segalowitz et al., 2010). Second, we selected an anterior electrode cluster over the frontal-midline based on previous research in adults that finds this region as the location of peak power of theta oscillations (Cavanagh and Frank, 2014; Ishii et al., 1999). In the 128-electrode system of Electrical Geodesics, Inc., the electrodes in the parietal-midline cluster were POz and its surrounding electrodes (E62, E67, E71, E72, E76, and E77), and the electrodes in the frontal-midline cluster were Fz and its surrounding electrodes (E4, E5, E11, E12, E16, E18, and E19). Because the dominant oscillation is known to be more prominent in posterior electrodes during resting-state conditions, we focused our age-related analysis on the dominant oscillations in the parietal-midline electrode cluster. Because theta oscillations are also commonly observed in frontal-midline electrodes in adults (Cohen and Donner, 2013; Riddle et al., 2020a), we included the frontal-midline cluster in an analysis exploring age-related differences in topography of the theta oscillation. We hypothesized that signal from the parietal-midline cluster would capture age-related transition in peak frequency of the dominant oscillation. We also compared the frontal-midline cluster to the parietal-midline cluster to test the difference in topology of theta oscillations between adults and children.
2.4. Estimating aperiodic signal and periodic oscillations
Frequency analysis was performed using Welch’s estimation of power spectra (Welch, 1967), then averaged across epochs and electrodes within each cluster. The power spectrum was estimated for frequencies 1 through 40 Hz, with a FFT and Welch segment length of 512 with 45 % overlap to achieve 512 millisecond and 1024 millisecond sliding windows, respectively, for both the SRS and CMI-MIPDB samples. This difference in frequency resolution is a result of the differing sampling rates of each dataset and is not large enough to be of consequence.
Electrophysiology recordings of human brain activity consistently report a prominent 1/f power distribution, often referred to as “background noise” (Bédard et al., 2006; Usher et al., 1995). This 1/f-like power distribution, or aperiodic signal, captures the phenomenon whereby the power at low frequencies is relatively greater. Power is progressively decreased in the higher frequencies, resulting in an overall negatively sloped power spectrum across a wide range of frequencies (e.g., 1–100 Hz). However, the most commonly used time-frequency analyses method (Stroganova et al., 1998) for characterizing the development of neural oscillations is done by averaging power within pre-specified frequency bands and comparing the absolute (total) or relative (proportional) power of each frequency band between age groups (Gmehlin et al., 2011b; Segalowitz et al., 2010; Somsen et al., 1997). This form of analysis introduces a confound where the frequency band is assumed to represent a neural oscillation but in fact contains a superposition of periodic and aperiodic signals. Thus, we adopted an approach that estimates both aperiodic and periodic signals.
We used the open-source, Python-based Fitting Oscillations and One-Over-F (FOOOF) toolbox (Haller et al., 2018) to estimate both the periodic and aperiodic signals. We restricted the FOOOF algorithm to four oscillatory peaks within the 1–40 Hertz range and constrained the minimum peak width to approximately twice the frequency resolution (4 Hz for the SRS sample, and 2 Hz in the CMI-MIPBD sample). We restricted the number of oscillatory peaks estimated by FOOOF to reduce the risk of over overfitting (see Chiang et al. (2011) and Dickinson et al. (2018)). The oscillatory peaks were modeled using Gaussian functions, , where:
With h corresponding to power (referred to as height in Fig. 1A), c corresponding to center frequency, w corresponding to the bandwidth (2*std), and F as a vector of frequency values of the power spectrum in Hertz.
The aperiodic signal, L, is modeled as:
Where b, the intercept, determines the aperiodic offset and the exponent x determines its slope. Hereon, we use the terms slope and offset to refer to the exponent and intercept, respectively. The aperiodic signal L is algorithmically incorporated with oscillatory “bumps” in the power spectrum, modeled as Gaussian curves ():
Where P is the combination of both the periodic and aperiodic signals. The periodic signal(s) are represented here as a sum of N peaks.
To derive a full estimate of the aperiodic and periodic signals that contributed to the power spectrum, the aperiodic signal was first estimated. The estimated aperiodic signal was then removed from the power spectrum and the maximum frequency peaks from the residuals were identified. If a peak was greater than the noise floor (at least 2 standard deviations above the residuals), then the peak was labeled as a genuine neural oscillation and a Gaussian function was fit around the peak frequency. Gaussian peaks that surpassed the noise floor were iteratively estimated and removed from the power spectrum until none were found or the pre-defined number of periodic signals was reached (four). When the iterative Gaussian estimation was complete, Gaussians that overlapped with each other or that were too close to the limits of the power spectrum were dropped, and the remaining Gaussians were used as seeds to re-fit the data to a multi-Gaussian model using a non-linear least squares operation, in effect combining them into a finalized “periodic signal.” Finally, the estimated periodic activity was removed from the original power spectrum and the aperiodic component was re-fitted on the residuals to achieve a more accurate aperiodic estimate. The final outputs of the algorithm included the linear combination of the aperiodic and periodic estimations, the exponent and intercept values of the aperiodic signal, and the bandwidth, power, and center frequencies of the identified oscillatory peaks. Our analysis of aperiodic signal included both the slope and offset. Our analysis of neural oscillations used the center frequency (c) and power of the dominant oscillation, as well as an analysis for the presence or absence of an oscillation within pre-defined band ranges.
2.5. Statistical analysis of the aperiodic signal
From the preprocessing stages described above, we obtained slope and offset estimates for the aperiodic signal of each study session. Our goal was to describe age-related differences in these components. To this end, slope and offset were entered into separate ordinary least squares regression models as the outcome variable, and age as the predictor variable. Regressions were performed by including both frontal-midline and parietal-midline electrode clusters as a within-participant variable to examine its interaction with age. We also ran separate regression models to examine the association with age for each electrode cluster. We corrected for multiple comparisons using the Bonferroni correction, where p-value less than 0.0125 was considered to be statistically significant. In order to account for the subset of participants who were sampled multiple times in the longitudinal SRS study, and to avoid violation of the assumption of independence in multiple regression, we ran our least squares regression with a cluster variable (i.e., clustered regression) to account for inter-participant dependence. We fit clustered regression models using the rms package (Harrell, 2015) in R v3.6.3 (Team, 2020) that calculates robust standard errors using a robust (Huber-White sandwich) estimator of the covariance matrix (Huber, 1967; White, 1980). Sandwich estimators are widely used to account for data dependency in regression models (for an example using sandwich estimators in the context of longitudinal neuroimaging, see Guillaume et al. (2014)).
2.6. Statistical analysis of periodic oscillations
Independently of the aperiodic signal, the center frequency of the dominant oscillation (i.e., the genuine oscillation of highest power) in the 4–12 Hertz range was selected for each participant who showed an oscillation in this range. To determine the age-related change of the peak frequency and the oscillatory power of the dominant oscillation, both of these estimates were entered into separate ordinary least squares regression models as the outcome variable, with age as the predictor variable. This regression included a cluster variable to account for repeated measurements for the same participant in the SRS sample.
To determine whether there was an age-related transition in the dominant oscillation from theta to alpha frequency band, every genuine neural oscillation in the frequency range from 4 to 12 Hz was included in the analysis (N = 78). We fit a logistic regression sigmoid function with the age of the participant as the predictor variable, and the frequency band (theta or alpha) as the categorical outcome variable. The estimated r-value was derived from the McFadden pseudo-R squared values calculated by the maximum likelihood estimation. From the fit of the logistic regression, we derived an inflection point in age where the likelihood that a participant possessed a theta or alpha oscillation equaled 50 %. We used a significance threshold of p < 0.05 for this analysis.
It is also possible that a participant could show no oscillatory peaks at all within the theta or alpha range. Thus, we investigated a difference in the presence or absence of theta and alpha oscillations between “younger,” versus “older” age groups (defined by a median split by age at 6.12 years). We then performed a chi-square test to test whether the observed number of genuine oscillations in a particular band (theta or alpha) significantly differed between age groups. We corrected for multiple comparisons using the Bonferroni method: we set the significance threshold at p < 0.025.
Finally, we investigated whether the dominant oscillation in younger and older participants displayed a relationship with age within that age group and frequency band. To run this analysis, we had to define an inflection point between theta and alpha oscillations in a data-driven way. Participants were split into two groups based off the data-driven age inflection point from the previous analysis separating ‘young’ and ‘old,’ and a logistic regression was run with peak frequency of the dominant oscillation as the predictor variable, with membership to the ‘old’ versus ‘young’ age group as the outcome variable. Then, we used ordinary least squares regression to correlate the peak frequency and power of the dominant theta oscillation with age in younger participants and peak frequency and power of the dominant alpha oscillation with age in older participants. We corrected for multiple comparisons using the Bonferroni method: we set the significance threshold at p < 0.025.
2.7. Statistical analysis of frontal-midline theta oscillations
We found that many younger participants exhibited a dominant oscillation in the theta frequency band. We suspected that the dominant oscillation in the theta band in a young participant would be found in posterior electrodes and that this was distinct from the often reported frontal-midline theta oscillation in adults performing cognitive control tasks (Cavanagh and Frank, 2014), and rather is more functionally similar to posterior alpha-band activity in adults (Orekhova et al., 2001; Saby and Marshall, 2012; Stroganova et al., 1999). If this were the case, then the presence of theta oscillations in older participants would be greater in the frontal-midline electrode cluster than in the parietal-midline electrode cluster. To probe this question of the difference in topography of peak theta power with age, we performed a logistic regression with age as the predictor variable and the cluster location (parietal-midline or frontal-midline) with greatest peak theta power as the outcome variable. The reported r-value was derived from the McFadden pseudo-R squared values calculated by the maximum likelihood estimation. From the fit of the logistic regression, we derived an inflection point in age where the likelihood that theta power of a genuine oscillation was greater in frontal-midline than parietal-midline electrodes was 50 %. We set a significance threshold for this regression analysis at p < 0.05.
3. Results
Using two independent samples with eyes-open resting-state EEG from 116 participants age 2.95–24 years old, we investigated the relationship between age and features of the aperiodic signal (slope and offset), between age and features of the dominant neural oscillation (peak frequency and power). We investigated the development of the frontal-midline theta oscillation as distinct from a dominant oscillation in the theta-band in posterior electrodes in early childhood.
3.1. Aperiodic slope and offset
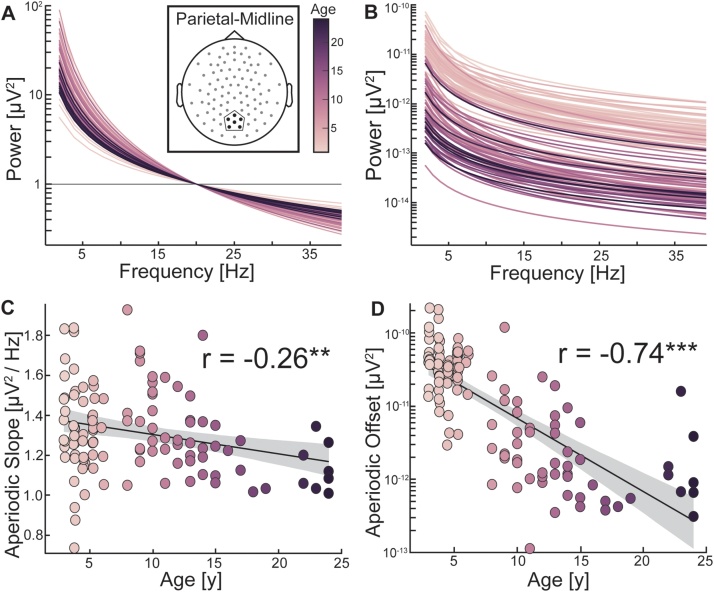
The aperiodic slope is the slope of the aperiodic signal of the power spectra after removal of “bumps” of neural oscillations (Fig. 1B). When the distribution of power is shifted towards higher frequencies, then the aperiodic offset is decreased (Fig. 1C). We performed regression analyses including age as the independent variable and the aperiodic slope and offset as the dependent variables. We found that in parietal-midline electrodes, age was negatively associated with both the slope (β = -0.0097, t(114) = -3.26, R2 = 0.069, p = 0.0015) and the offset (β = -0.0999, t(114) = -9.94, R2 = 0.554, p < 0.0001; Fig. 2A–D). For the frontal-midline electrode cluster, we found a significant negative association between age and offset (β = -0.094, t(114) = -8.93, R2 = 0.530, p < 0.0001), but not between age and slope (β = -0.007, t(114) = -1.83, R2 = 0.025, p = 0.07). We then examined the interaction effects between electrode clusters (frontal-midline versus parietal-midline) and age on slope and offset. We found no significant age by cluster interaction for slope (β = -0.0026, t(228) = -0.76, p = 0.45) and offset (β = -0.006, t(228) = -1.18, p = 0.24). Thus, as age increased, the aperiodic signal flattened in slope and decreased in offset, representing a shift towards more power in higher relative to lower frequencies.
Fig. 2.
Aperiodic signal flattens with age in early childhood (A) The aperiodic signal from the parietal-midline is depicted for all participants with the intercept anchored to a single point at 20 Hz to visualize individual differences in slope varying with age. Younger participants (light colors) have a steeper aperiodic signal than older participants (dark colors). Insert depicts the parietal-midline electrode cluster for all data in this figure. (B) The aperiodic signal is depicted for all participants to illustrate the difference in the aperiodic offset. Younger participants have greater intercepts (offsets) than older participants. (C) Aperiodic slope displayed a significant inverse relationship with age. (D) Aperiodic offset also displayed a significant inverse relationship with age. As participants increased in age, the slope and offset of the aperiodic signal decreased. ** p < 0.005, *** p < 0.0005. Grey area is 95 % confidence interval.
3.2. Age-related change in dominant oscillations
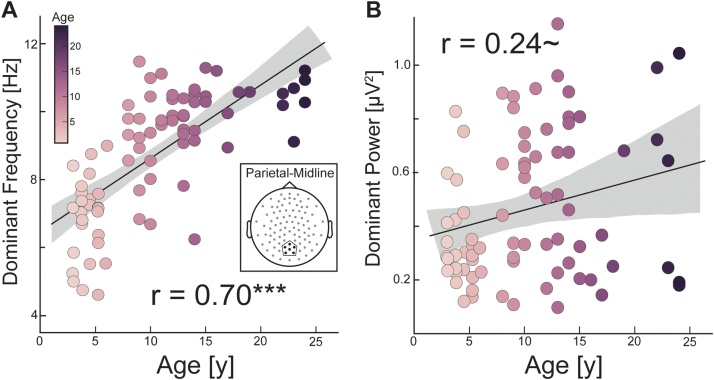
Previous literature (using canonical time-frequency analyses) showed that theta-band power decreased with age while the faster alpha-band power increased with age (Segalowitz et al., 2010), and a few studies found that the peak frequency of the alpha oscillation increases during development (Chiang et al., 2011; Gmehlin et al., 2011b). We examined whether the aperiodic-corrected posterior dominant oscillatory peak (defined for 4–12 Hertz) exhibited signs of age-related differences. In a linear regression with dominant peak frequency as the dependent variable, we found a significant interaction between age and electrode cluster (β = 0.073, t(145) = 2.32, p = 0.02). This suggests that the association between age and peak frequency differed between frontal-midline versus parietal-midline electrodes. Further regression analysis revealed that peak frequency was significantly positively associated with age (β = 0.216, t(76) = 9.32, R2 = 0.49, p < 0.0001) in the parietal-midline cluster (Fig. 3A). This relationship was stronger than the relationship between age and peak frequency in the frontal-midline cluster (β = 0.139, t(69) = 4.28, R2 = 0.20, p < 0.0001; Fig. S2A). When including power of the dominant oscillation as the dependent variable in the regression model, we did not find a significant interaction between electrode cluster and age (β = 0.0049, t(145) = 1.24, p = 0.22). Additional analysis did not find the peak power to change with age in the parietal-midline cluster (β = 0.0112, t(76) = 1.95, R2 = 0.06, p = 0.055; Fig. 3B) or the frontal-midline cluster (β = 0.006, t(69) = 1.32, R2 = 0.046, p = 0.19; Fig. S2B). Thus, peak frequency of the dominant oscillation, particularly in the parietal-midline, increased as a function of age within the low-frequency range from 4 to 12 Hertz.
Fig. 3.
The frequency and power of the dominant oscillation increases with age (A) The peak frequency of the dominant oscillation between 4-12 Hz is displayed in all participants who exhibited an oscillation. The peak frequency of the dominant oscillation from the parietal-midline electrode cluster (insert) increased in frequency with increasing age. (B) Power of the posterior dominant oscillation trended positively with age. Altogether, these results suggest a shifting of the dominant posterior oscillation from slower theta (4-8 Hz) to faster alpha-range (8-12 Hz) peaks. *** p < 0.0001 ∼ p < 0.1 Grey area is 95 % confidence interval.
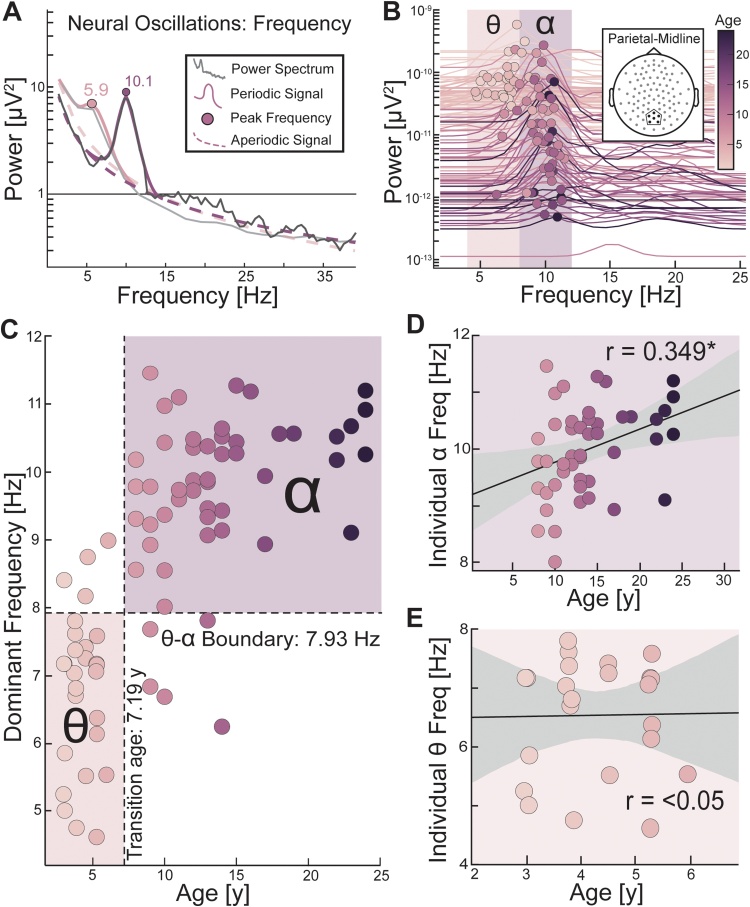
3.3. Theta-alpha transition occurs in early childhood
An advantage conferred by aperiodic correction is the ability to examine whether oscillations within a given frequency band are present or absent. Given the age-related shift in the frequency of individual dominant oscillations, we further hypothesized a transition in the overall presence of oscillations from theta to alpha frequency range in early childhood (Fig. 4A). 78 participants showed genuine (aperiodic-corrected) neural oscillations in the theta or alpha range (Fig. 4B). From this subset, 31 % of participants had only theta peaks, 64 % had only alpha peaks, and 5% had both theta and alpha peaks. We selected the parietal-midline cluster for this analysis, because the parietal-midline cluster displayed a stronger association between age and the dominant peak frequency relative to the frontal-midline cluster (Results 3.2) and based on findings from previous work suggesting that the dominant oscillatory signature is primarily located in posterior cortices (Klimesch, 1999), including in childhood through to adulthood (Chiang et al., 2011). From our logistic regression analysis, we found a significant age-related transition from theta to alpha oscillations present in the parietal-midline (r(81) = 0.678, p < 0.0001, logit coefficient = 0.5095) (Fig. 4B). The inflection point of transition was at 7.19 years of age. Post-hoc chi-square tests for the younger and older participants (median split) confirm that there was a greater presence of theta oscillations in younger participants (younger than 7.19 years; = 15.25, p < 0.0001), and a greater presence of alpha in older participants (older than 7.19 years; = 73.31, p < 0.0001). These findings indicate that the canonical alpha band (8−12 Hertz) in posterior electrodes in adults may not emerge until around age 7.
Fig. 4.
Theta to alpha oscillation transition with age (A) Two hypothetical participants with periodic signals modeled as Gaussian curves above the aperiodic signal. (B) Logistic regression of the age of the participant from which a genuine theta or alpha oscillation (categorical variable) was found. The 50 % probability mark (dashed line) occurs at approximately 7.19 years of age. Dots represent a genuine alpha or theta oscillation and the age of the participant. Dots are jittered along the y-axis for illustration.
Given that the age inflection of 7.19 years fell within the age gap between our two datasets (the oldest participant in the SRS sample was 6.13 years old, while the youngest participant in the CMI-MIPDB dataset was 8 years old), we ran a control analysis modeling dataset membership with an additional grouping variable. As age was entirely confounded with the two datasets, the age-related transition in the presence of theta and alpha oscillations became non-significant (logit coefficient = 0.216, p = 0.187), albeit in the same direction. We address this caveat in the discussion section.
3.4. Peak frequency of the dominant oscillation increases with age
After establishing the development of dominant oscillation from theta-band to alpha-band frequency peaks, we investigated whether the peak frequency of the dominant oscillation in the parietal-midline electrodes linearly increased with age. We correlated the peak frequency (Fig. 5A) of theta and alpha dominant oscillations (Fig. 5B) with age (Fig. 5C). If a positive linear relationship existed for peak theta frequency in young participants and for peak alpha frequency in old participants, then this suggested a smooth linear transition in dominant frequency from theta to alpha oscillation between ages 3 and 24. We found a significant positive relationship between peak alpha frequency and age in participants older than 7.19 years (r(45) = 0.35, p = 0.017; Fig. 5D), but no relationships between peak theta frequency and age in participants younger than 7.19 (r(22) = 0.011, p = 0.96; Fig. 5E). This finding suggests that once the alpha oscillation emerges as the dominant oscillation, then there is a linear increase in its peak frequency with age between 7 and 24 years old. In contrast, the theta frequency dominant oscillation may be categorically distinct in early childhood as it does not change with age between 3 and 7 years old.
Fig. 5.
Analysis of peak theta and alpha frequency in younger and older participants (A) Two example participants with peak frequency of the dominant oscillation highlighted by a colored circle. (B) The power spectra correcting for the slope of the aperiodic signal for all participants is depicted with peak frequency in the theta-band (4-8 Hz) and alpha-band (8-12 Hz) plotted as dots. Younger (light purple) participants tend to have an overall lower alpha peak frequency than older (dark purple) participants. (C) Dominant frequency plotted with age. Logistic regression determined the age and frequency inflection points separating old and young participants and theta and alpha oscillations (dashed lines). Light purple square depicts theta frequency dominant oscillations in the young participants and dark purple square depicts alpha frequency dominant oscillation in the old participants. (D) Linear regression of age to individual alpha frequency in old participants and (E) to individual theta frequency in young participants. * p < 0.05. Grey area is 95 % confidence interval. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
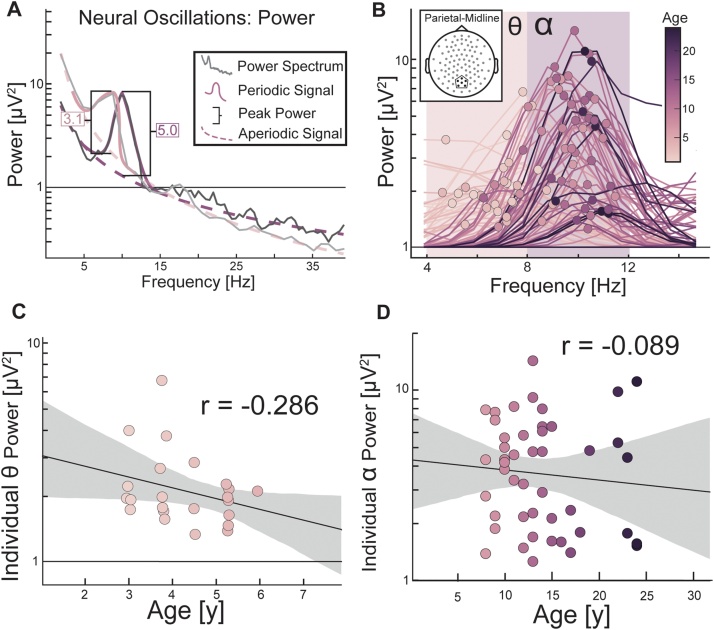
As a control analysis to test for the specificity of these effects, we ran an identical analysis but with the power (Fig. 6A–B) of the dominant oscillations. We found no significant relationship between alpha power and age in older participants (r(45) = 0.089, p = 0.558; Fig. 6C) nor between theta power and age in young participants (r(21) = -0.29, p = 0.0797; Fig. 6D). These findings suggest that the peak frequency of the dominant oscillation changes with age, while the peak power exhibits a non-significant relationship with age.
Fig. 6.
Analysis of peak theta and alpha power in younger and older participants (A) Two hypothetical participants with oscillations that have a similar power prior to aperiodic signal correction. Note that without aperiodic signal correction, the power of the two oscillations in the hypothetical participants appears to be similar. After aperiodic signal correction, the peaks differ in power. (B) Aperiodic signal corrected power spectra for all participants. Dots indicate the peak frequency of each oscillations. (C) Linear regression of age to individual alpha power in younger participants and (D) to individual theta power in older participants. * p < 0.05. Grey area is 95 % confidence interval.
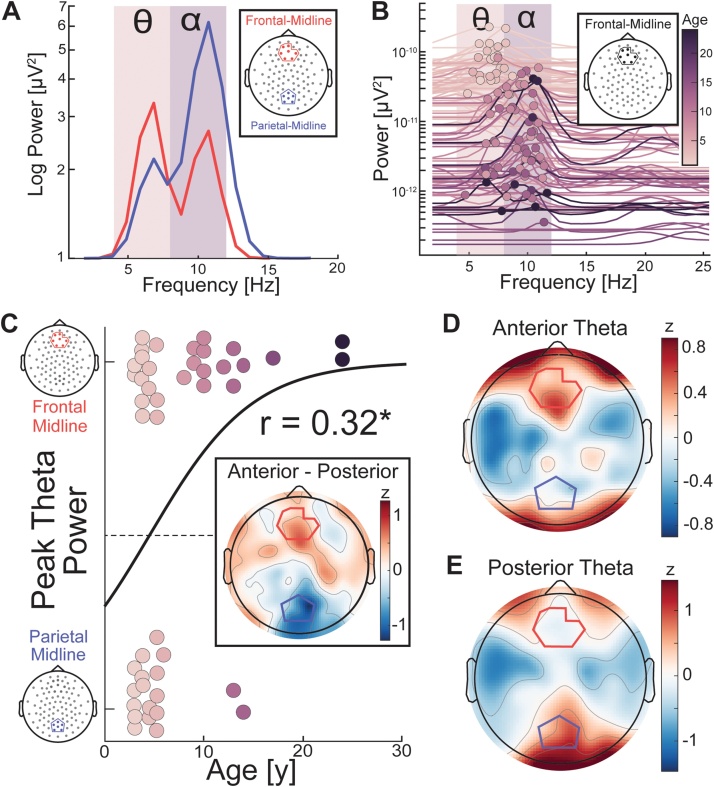
3.5. Frontal-midline theta oscillations emerge with age
We observed the presence of dominant oscillations in the theta frequency range in the young participants. We investigated whether the dominant theta oscillations in early childhood originated from posterior electrodes and were distinct from frontal-midline theta oscillations found in adulthood. This is motivated by findings indicating that posterior theta oscillations found in infants shared functional characteristics with alpha oscillations reported in adults (Gasser et al., 1988a; Saby and Marshall, 2012). Due to volume conduction, neural oscillations recorded in posterior electrodes may reflect the same source driving oscillations in anterior electrodes. We reasoned that the electrode cluster that showed greater theta power likely was closer to the true underlying source of the theta oscillations. Therefore, we examined whether theta power was strongest in the frontal-midline or the parietal-midline electrodes (Fig. 7B). We expected that for older participants, anterior electrodes would show stronger theta power. In contrast, younger participants would show stronger theta power in the posterior electrodes. We ran a logistic regression for the site with the strongest theta oscillations (frontal-midline or parietal-midline) as a function of age (Fig. 7C). We found a significant age-related transition of peak theta power from posterior to anterior electrodes (r(44) = 0.32, p = 0.04). The topographic distribution of older participants with greater theta power in anterior electrodes was clustered around the frontal-midline (Fig. 7D), whereas younger participants with greater theta in posterior electrodes displayed theta oscillations spreading into electrodes over occipital cortex. This finding suggests that frontal-midline theta emerges in development and is distinct from the dominant posterior theta oscillations of early childhood.
Fig. 7.
Emergence of the frontal-midline theta oscillation in development (A) Example participant (age 10) who demonstrated alpha and theta oscillations in frontal-midline and parietal-midline clusters. Theta power was greater in the frontal-midline (red trace), whereas alpha power was greater in the parietal-midline (blue trace). (B) Frontal-midline power spectra for all participants with the slope of the aperiodic signal removed. Dots indicate the peak frequency for each participant. We observed a number of anterior theta peaks in younger participants and anterior alpha peaks in older participants, possibly due to the confounding influence of volume conduction. We therefore conducted our analyses on peak theta power, since this is likely to reflect greater proximity to the true origin of resting theta power in children versus adults. (C) Logistic regression of the site of aperiodic signal corrected peak theta power with age. Spatially-normalized (z-scored) theta topography averaged over participants with greater power in anterior (D) and posterior (E) electrodes. (Insert in C) Difference in z-scored theta power between participants with greater anterior versus posterior power. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
In this study, we aimed to investigate the development of intrinsic neural oscillations from early childhood to adulthood (years 3–24), focusing on characterizing the first prominent oscillatory peak of the spectrum, also known as the “dominant oscillation.” To improve the precision in detecting the dominant oscillation, we separated several different components of intrinsic neural activity: slope and offset of the aperiodic signal, peak frequency and power of the periodic oscillation(s). We then examined the relationship of these components with age. For the aperiodic signal, we found that both the slope and offset decreased with age. For the dominant oscillation, we found that the peak frequency, but not the power, of the dominant oscillation increased with age. Specifically, as age increased, the dominant oscillation increased from theta (4−8 Hz) to alpha (8−12 Hz) frequency range. The association between age and peak frequency was stronger in the parietal-midline cluster than in the frontal-midline cluster, which is consistent with the notion that the dominant oscillation is more strongly expressed in posterior cortices (Berger, 1929; Lodder and van Putten, 2011). Notably, we found two distinct developmental trajectories for theta versus alpha dominant oscillations; there was no age-related increase in the peak frequency of theta-band dominant oscillations in younger participants (age 3–7), but the peak frequency of alpha-band dominant oscillations in older participants (ages 7–24) did increase with age. Given that age-related differences in the aperiodic offset were found in both the frontal-midline and parietal-midline clusters, the aperiodic signal may be a global phenomenon. By contrast, the age-related transition in the dominant frequency was local to the parietal-midline cluster suggesting the development of a specific signal generator. Finally, we found that while participants in early childhood exhibited a theta frequency dominant oscillation that was most pronounced in parietal-midline electrodes, older participants exhibited theta oscillations that were more pronounced in the frontal-midline.
Recent studies suggest that human neurophysiology signals, such as those recorded with EEG, should be interpreted as a linear combination of two distinct types of signals: periodic and aperiodic (Donoghue et al., 2020; Haller et al., 2018). The aperiodic signal is also known as the 1/f phenomenon, where the power is inversely proportional to the frequency of the signal. For example, for a given EEG power spectrum plotted from 1 to 100 Hz, we would observe that the power at low frequencies (1 Hz) is higher than that at high frequencies (100 Hz). Studies demonstrated that the power spectrum of human EEG signals exhibits a negative slope, where the steepness of the slope, as well as its offset, vary between individuals (Ouyang et al., 2020; Voytek et al., 2015) and brain regions (Podvalny et al., 2015). Critically, neural oscillations should be more parsimoniously defined as periodic signals expressing power amplitudes stronger than the background aperiodic signal (Fig. 1A). Conflation of aperiodic and periodic signals could be problematic for two reasons. First, the spectral power is dominated by the aperiodic, non-oscillatory signal (Bullock et al., 2003). Second, Haller et al. (2018) illustrated the potential drawback of not accounting for the aperiodic when estimating the power of oscillations, demonstrating that shifts in the offset or changes in the slope of the aperiodic signal can spuriously change total band-limited power without changes in the periodic oscillation (Fig. 1B–C). This is a critical issue when comparing oscillatory signals between individuals or age groups, because what appears to be individual differences in periodic oscillations could be driven by unaccounted changes of the aperiodic slope and offset. Aperiodic correction of the power spectrum can detect the presence or absence of periodic oscillatory peaks independent of the aperiodic component, and improve the precision in characterizing the development of neural oscillations (Donoghue et al., 2020).
We found that both the aperiodic slope and offset decreased with age. An age-related decrease in aperiodic slope indicates that the 1/f spectrum becomes less steep with age, and an age-related decrease in offset indicates that the overall spectral power decreases with age. Two studies that examined the aperiodic component of human EEG data have also found age related decrease in the aperiodic slope during human lifespan (He et al., 2019; Schaworonkow and Voytek, 2020b; Tran et al., 2020; Voytek et al., 2015). Voytek et al. (2015) found that the aperiodic slope is significantly flatter in older adults (60–70 years old) than in younger adults (20–30 years old), while He et al. (2019) found the aperiodic slope to be flatter in adults (23–58 years old) than children (ages 5.5–10.5 years). Schaworonkow and Voytek (2020a) show a flattening of the aperiodic signal within the first seven months of life. Our finding suggests that this developmental decrease in the aperiodic slope continues between infancy and early adulthood.
The precise neural mechanism that alters the aperiodic slope of intrinsic neural activity remains an active area of research. Critically, active stimuli processing flattens the slope (He et al., 2010; Podvalny et al., 2015), and a flattened slope is correlated with cognitive decline (Tran et al., 2020; Voytek et al., 2015). The few existing studies suggest that a shift in aperiodic slope is indicative of a shift in the ratio of excitation and inhibition (E-I balance) of a neural population. Evidence for this hypothesis comes from propofol-induced anesthesia of macaque monkeys, where propofol, acting on GABAA inhibitory activity, increased the “I” in the E:I balance, causing the aperiodic signal to steepen (Gao et al., 2017). E-I balance is mediated by local and long-range circuits composed in part by inhibitory GABAergic and excitatory glutamatergic neurons (Tatti et al., 2017), and is aberrant in human and animal models of neurological disorders such as autism spectrum disorder (Hegarty et al., 2018; Rubenstein and Merzenich, 2003; Sohal and Rubenstein, 2019; Yizhar et al., 2011), schizophrenia (Lisman, 2012; Uhlhaas and Singer, 2011), and epilepsy (Shao et al., 2019). Recent studies have examined the aperiodic slope in schizophrenia and ADHD and demonstrated systematically steeper aperiodic signals in clinical populations compared to healthy controls (Molina et al., 2020; Robertson et al., 2019). Developmental decreases in the aperiodic slope may therefore indicate maturation of the E-I balance. Furthermore, the aperiodic offset may reflect overall neuronal population firing rates and scale-free brain activity that is dissociable from ongoing oscillations (He, 2014; Ray and Maunsell, 2011). The offset is likely largely reflected in measurements of oscillatory activity (Donoghue et al., 2020), and as such we are left to speculate as to whether the neural origins thought to underlie changes in overall EEG power might lend explanatory power to changes in offset with age. For example, Whitford et al. (2007) investigated age-related differences in brain structure and resting-state electrophysiology using MRI and EEG, respectively, in 138 individuals aged 10–30 years. They found that decreasing grey matter volume with age, hypothesized to reflect synaptic pruning, was paralleled by decreases in EEG power across slow-range frequency bands.
Traditionally, relative band power is a measure of absolute power (power summed over a frequency range) divided by the absolute power of all other frequencies outside that band (Segalowitz et al., 2010). While absolute power likely largely reflects fluctuations in the aperiodic signal, particularly those of the offset, relative power aims to get more directly at nuances between various frequencies. Our finding has implications for interpreting prior studies that reported developmental differences in relative and absolute power of the frequency spectrum (Barry and Clarke, 2009; Bell, 2002; Buchsbaum et al., 1992; Clarke et al., 2001; Cragg et al., 2011; Dustman et al., 1999; Gasser et al., 1988b; Gmehlin et al., 2011a; Klimesch, 1999; Matoušek and Petersén, 1973; Orekhova et al., 1999, 2001; Orekhova et al., 2006; Paulino et al., 2011; Schäfer et al., 2014; Segalowitz et al., 2010; Somsen et al., 1997; Stroganova et al., 1999, 1998; Uhlhaas et al., 2010, 2009; Uhlhaas and Singer, 2011; Whitford et al., 2007; Yordanova and Kolev, 1997). The method commonly adopted in previous studies, however, still incorporates the aperiodic signal into its calculation of oscillatory power rather than separating it from the periodic estimate. For example, Dustman et al. (1999) examined resting-state EEG data from healthy males aged 4–90 years old and found that, while relative theta-band power exhibited a lifelong decrease in power until old age, relative alpha-band power began an increase at age 6, peaked at around age 24, and then steadily declined. In addition, several studies have investigated the potential of using the ratio of theta-to-beta power as a biomarker for neural development and psychopathology (Arns et al., 2012). However, elevated theta-band power and attenuated beta-band power in ADHD might be more parsimoniously explained by changes in the aperiodic slope (Robertson et al., 2019).
After accounting for the aperiodic signal, we found that the peak frequency of the dominant oscillation significantly increased with age. Specifically, we found that participants younger than 7 years tend to exhibit a dominant oscillation in the slower theta frequency range (4–8 Hz), and that peak theta frequency has no significant relationship with age. After age 7, the peak frequency of the dominant oscillation shifted to the faster alpha-band (8–12 Hz), and the peak alpha frequency continuously increased with age. Intrinsic, alpha-band neural oscillations have been shown to be modulated by alertness and attention (Cantero et al., 1999; Pfurtscheller et al., 1996; Romei et al., 2008). Prior EEG studies demonstrated that in toddlers, neural oscillations overlapping with the adult theta range of 4–8 Hertz in posterior electrodes can also be modulated by alertness, attention, and inhibitory control (Tatiana A Stroganova et al., 1999; Whedon et al., 2020). This suggests that theta-band dominant oscillations during early childhood may be functionally similar to alpha neural oscillations (8−12 Hz) observed in adults (Cuevas et al., 2012). Other studies suggest that based on its functional properties, the equivalent of the theta oscillation could overlap with the lower delta-range (2–4 Hertz) during infancy (Orekhova et al., 1999, 2006). These studies suggest that there may be a general pattern of age-related frequency shift for both theta and alpha oscillations (Stroganova and Orekhova, 2007), and researchers often define 6–8 Hertz oscillations as “infant alpha” (Orekhova et al., 1999, 2001, 2006; Stroganova et al., 1999; Stroganova et al., 1998). In the current study, we examined this developmental pattern with a data-driven approach, without defining a priori frequency bands. Specifically, we defined peak frequency of neural oscillations by computing the aperiodic signal correction of the power spectrum. This approach avoids conflating developmental change in genuine neural oscillation with developmental changes in the aperiodic signal, which we have also identified. Relatively few studies have also leveraged a methodology resembling aperiodic correction, but they demonstrated the same pattern (Chiang et al., 2011; Schaworonkow and Voytek, 2020b; Tröndle et al., 2020). In conclusion, our results suggest that during childhood and adolescence development, slower theta oscillations in posterior cortices first transition into alpha oscillations, and the peak frequency continues to increase after the transition.
The development of intrinsic, dominant alpha neural oscillations could be related to age-related development in various cognitive functions. In adults, individual differences in the peak alpha oscillation frequency, also known as the individual alpha frequency (IAF), have been shown to correlate with individual differences in intelligence and cognitive performance, such as on a mental rotation task (Angelakis et al., 2007; Grandy et al., 2013; Klimesch et al., 2003; Mierau et al., 2017). In children, IAF has been shown to increase with age (Marcuse et al., 2008) and correlate with sensorimotor skills in ages 3–6 years (Mierau et al., 2016). IAF has also been studied in a population of 11–70 year-olds, in which it was found to be positively correlated with performance on the reverse digit span task (Richard Clark et al., 2004). The alpha component of auditory evoked potentials during an oddball task has also been shown to increase in amplitude and post-stimulus phase locking between the ages of 6 and 10 years (Yordanova and Kolev, 1997). Another study found that, in comparison of visual evoked potentials between children aged and adults, the delta and theta, but not the alpha, component of the evoked potential were reliably found in children. In adults, alpha oscillations have been implicated in wide range of cognitive functions (Klimesch, 1999), for example selective attention (Foxe and Snyder, 2011; Haegens et al., 2011; Jensen and Mazaheri, 2010; Wolfgang Klimesch et al., 2007), working memory (Riddle et al., 2020b; Sauseng et al., 2009), and distractor inhibition (Bonnefond and Jensen, 2013). Whether peak alpha frequency is correlated with these functions, and therefore could serve as a maturational index for neurocognitive development, remains an open question.
We found that the theta dominant oscillation (4−8 Hz) transitioned into the alpha-band frequency range (8−12 Hz) around 7.19 years. Our findings are consistent with previous research that finds functional similarity between theta oscillations (or “infant alpha”) in infants and alpha oscillations in adults. For example, a study of one year old infants found that when using a blocked design alternating between periods of attending to a person blowing bubbles and an eyes-open baseline, oscillatory power in the theta-band (5.2–6.9 Hz) is lower during the attending condition (Stroganova et al., 1999). This finding is similar to the well-replicated phenomenon that cognitive tasks requiring active visual attention decrease alpha amplitude in adults (Klimesch, 2012). Thus, theta-band activity observed during infancy may be functionally similar to alpha band activity observed in adults, and some described theta oscillation during infancy as “infant alpha”. However, because we did not manipulate attention and alertness in the current study, we are not able to definitely determine whether the theta activity (or “infant alpha”) we observed in early childhood is functionally similar to alpha activity in adults. Future studies should address this question while correcting for the aperiodic signal.
It is important to acknowledge that this age inflection point of 7.19 years old roughly corresponds to the age difference between the two samples. Therefore, we cannot rule out the possibility that age-related differences in theta versus alpha dominant oscillations may be explained by systematic differences between the SRS and CMI studies (e.g. different data collection site and procedure). One notable distinction between the data collection procedures between datasets is that younger participants in the SRS dataset collected eyes-open resting-state during restful fixation on a video, whereas the CMI-MIPDB dataset collected eyes-open resting state during restful fixation on a central point (Langer et al., 2017). In order to further evaluate how these differences in resting-state data collection procedures could potentially affect oscillations between 4 and 12 Hz, we must consider previous investigations on differences in neural activity between the resting-state and passive video watching.
Resting-state studies are often conducted with the goal of investigating spontaneous, unconstrained neural activity that evolves over an extended time period, usually several minutes (Lee et al., 2013; van den Heuvel and Hulshoff Pol, 2010). This activity is presumed to capture trait-like patterns of brain activity that are not constrained by a specific cognitive state (Tavor et al., 2016). In research on young children, the use of video watching to promote unconstrained, spontaneous activity is a common practice due to the practical concerns surrounding acquiring artifact-free data in young children (Bell and Cuevas, 2012; Uddin et al., 2010). Specifically, some studies have used video-watching to collect baseline estimates of functional connectivity (Sonkusare et al., 2019). This is particularly advantageous because video watching has been shown to reduce motion-induced artifacts and to minimize drowsiness (Vandewouw et al., 2021), two known potential confounds when using a fixation rest. These considerations are particularly crucial in pediatric, geriatric, or otherwise difficult-to-study clinical populations who have trouble remaining still during EEG or fMRI experiments (Yerys et al., 2009). In addition to practical considerations, we need to empirically understand how watching a video versus a fixation point differentially influence unconstrained, spontaneous brain activity. In short, some studies suggest that these states are comparable, while others have found slight differences in oscillatory power. For example, one study found that video watching can evoke functional connectivity patterns that closely resemble resting-state networks identified using fMRI (Vanderwal et al., 2015). This suggests that trait-level brain activity is conserved across cognitive states. Recently, a different study found that among typically and atypically developing participants, low-arousal videos evoked elevated 4–7 Hertz power and decreased 7–12 Hertz power when compared to a fixation condition (Vandewouw et al., 2021). While this finding suggests movie watching and fixation-based rest may have different effects on theta and alpha power, it is important to point out that we did not find age-related differences in power, but only in the peak frequency. Whether movie-watching versus fixation rest differentially modulate the dominant peak frequency needs to be addressed by future research.
Another source of systematic difference between the datasets could give rise to saccade-related, motion-related, or muscle-related noise artifacts. One study quantified potential artifacts induced by eye-movements during video-watching in children, and found that theta-band power was higher in artifact-corrupted data (McEvoy et al., 2015). Given that we removed ocular artifacts with ICA and that our analysis was focused on posterior theta signal, it seems unlikely that our results could be explained by ocular artifacts. Furthermore, we conducted topographic analysis of theta power and found that, in adults, frontal-midline theta was clustered around Fz and FCz, distinct from anterior sites that would be more indicative of ocular-artifacts (e.g. Fpz and AFz). Motion-related artifacts are most often manifested as low-frequency power (< 2 Hz) around the edge of the electrode montage. To address motion-related artifacts, large signal transients were removed from our analyses, and we used ICA to remove components with edge artifacts that were indicative of motion-related signals. In regards to muscle-related artifacts, these artifacts are manifested primarily around temporal electrodes close to the jaw or in lateral occipital electrodes near the neck, and most often found in the beta-frequency range (15−30 Hz). Our ICA rejection was designed to remove these signals, and our analyses did not focus on beta frequency oscillations. Thus, it is unlikely that ocular, motion, or muscle artifacts can explain our findings.
An additional factor that we need to consider is the potential impact of combining data collected from different data collection designs. The SRS is a longitudinal study, whereas the CMI-MIPDB is a cross-sectional study. A longitudinal design yields non-independent observations (i.e., multiple observations from the same person over time), whereas a cross-sectional design, in theory, yields independent observations. Ideally, a dataset would assess the same individual multiple times over a long span of their life to capture with maximal granularity age-related changes in the resting-state electrophysiological profile from childhood through adulthood. What may appear as developmental change in a cross-sectional design could reflect cohort effects (i.e., effects specific to people of a given birth year due to their shared experiences) or systematic variation in sampling different age groups (Kraemer et al., 2000). However, longitudinal designs can also be susceptible to cohort effects (Zelinski et al., 2009) and the confounds of repeated measures (Baltes, 1968), whereby practice effects or a child’s general familiarity with methodological procedures result in differences between sessions that are not strictly related to development over age. Thus, data from longitudinal and cross-sectional designs may not be fully comparable. When we combined the SRS and CMI datasets, the majority of study participants in the SRS dataset were only sampled once, and were sampled at regularly spaced age intervals. This feature should minimize practice and time-of-measurement effects and thus make the two datasets more comparable. In addition, we performed nearly identical data processing steps for all participants, which further facilitates our ability to pool participants from the two datasets.
The dominant theta oscillation in posterior electrodes during early childhood is likely functionally distinct from frontal-midline theta long acknowledged to be involved in top-down cognitive control (Cavanagh and Frank, 2014), because we found that theta oscillations were more strongly expressed in frontal-midline electrodes for older participants. Conversely, in younger participants the theta peaks of greatest power were concentrated in the parietal-midline cluster. As described above, posterior theta-band power may shift into alpha frequency as the dominant posterior oscillation (Rodríguez-Martínez et al., 2017), while a frontal theta oscillation may mature separately during development (Adam et al., 2020; Barriga-Paulino et al., 2011). This relatively late emergence of intrinsic, frontal-midline theta oscillation may be related to the development of cognitive functions known to recruit frontal-midline theta oscillations, for example feedback and error processing (Cavanagh and Frank, 2014; Nigbur et al., 2011; Velanova et al., 2008) and hierarchical cognitive control (Riddle et al., 2020c; Unger et al., 2016). Specifically, many cognitive functions, for example working memory and top-down control are known to have a protracted development through adolescence (Luna et al., 2004). Future studies should investigate whether or not the development of these functions is related to the development of intrinsic frontal-midline theta oscillations.
Data_statement
All data used in this analysis is available on the Open Science Framework: https://osf.io/2exkc/.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The study was funded by Grant HD098235 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the Iowa Neuroscience Institute.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100969.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adam N., Blaye A., Gulbinaite R., Delorme A., Farrer C. The role of midfrontal theta oscillations across the development of cognitive control in preschoolers and school‐age children. Dev. Sci. 2020:e12936. doi: 10.1111/desc.12936. [DOI] [PubMed] [Google Scholar]
- Angelakis E., Stathopoulou S., Frymiare J.L., Green D.L., Lubar J.F., Kounios J. EEG neurofeedback: a brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin. Neuropsychol. 2007;21(1):110–129. doi: 10.1080/13854040600744839. [DOI] [PubMed] [Google Scholar]
- Arns M., Conners C.K., Kraemer H.C. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J. Atten. Disord. 2012;17(5):374–383. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- Baltes P.B. Longitudinal and cross-sectional sequences in the study of age and generation effects. Hum. Dev. 1968:145–171. doi: 10.1159/000270604. [DOI] [PubMed] [Google Scholar]
- Barriga-Paulino C.I., Flores A.B., Gómez C.M. Developmental changes in the EEG rhythms of children and young adults: analyzed by means of correlational, brain topography and principal component analysis. J. Psychophysiol. 2011;25(3):143. [Google Scholar]
- Barry R.J., Clarke A.R. Spontaneous EEG oscillations in children, adolescents, and adults: typical development, and pathological aspects in relation to AD/HD. J. Psychophysiol. 2009;23(4):157. doi: 10.1027/0269-8803.23.4.157. [DOI] [Google Scholar]
- Bédard C., Kröger H., Destexhe A. Does the $1/f$ frequency scaling of brain signals reflect self-organized critical states? Phys. Rev. Lett. 2006;97(11):118102. doi: 10.1103/PhysRevLett.97.118102. [DOI] [PubMed] [Google Scholar]
- Bell M.A. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39(4):450–458. doi: 10.1111/1469-8986.3940450. [DOI] [PubMed] [Google Scholar]
- Bell M.A., Cuevas K. Using EEG to study cognitive development: issues and practices. J. Cogn. Dev. 2012;13(3):281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger C., Matthis P., Scheffner D. EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalogr. Clin. Neurophysiol. 1984;57(1):1–12. doi: 10.1016/0013-4694(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das elektroenkephalogramm des menschen. Arch. Fã¼r Psychiatr. Und Nervenkrankh. 1929;87(1):527–570. [Google Scholar]
- Bonnefond M., Jensen O. The role of gamma and alpha oscillations for blocking out distraction. Commun. Integr. Biol. 2013;6(1):e22702. doi: 10.4161/cib.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum M.S., Monsour C.S., Teng D.G., Zia A.D., Siegel B.V., Rice D.M. Adolescent developmental change in topography of EEG amplitude. Schizophr. Res. 1992;7(2):101–107. doi: 10.1016/0920-9964(92)90039-8. [DOI] [PubMed] [Google Scholar]
- Bullock T.H., McClune M.C., Enright J.T. Are the electroencephalograms mainly rhythmic? Assessment of periodicity in wide-band time series. Neuroscience. 2003;121(1):233–252. doi: 10.1016/S0306-4522(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Buschman T.J., Miller E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cantero J.L., Atienza M., Salas R.M., Gómez C.M. Alpha EEG coherence in different brain states: an electrophysiological index of the arousal level in human subjects. Neurosci. Lett. 1999;271(3):167–170. doi: 10.1016/S0304-3940(99)00565-0. [DOI] [PubMed] [Google Scholar]
- Cardin J.A., Carlén M., Meletis K., Knoblich U., Zhang F., Deisseroth K. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang A.K.I., Rennie C.J., Robinson P.A., van Albada S.J., Kerr C.C. Age trends and sex differences of alpha rhythms including split alpha peaks. Clin. Neurophysiol. 2011;122(8):1505–1517. doi: 10.1016/j.clinph.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Clarke A.R., Barry R.J., McCarthy R., Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin. Neurophysiol. 2001;112(5):806–814. doi: 10.1016/S1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Cohen M.X. Where does EEG come from and what does it mean? Trends Neurosci. 2017;40(4):208–218. doi: 10.1016/j.tins.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Donner T.H. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 2013;110(12):2752–2763. doi: 10.1152/jn.00479.2013. [DOI] [PubMed] [Google Scholar]
- Cragg L., Kovacevic N., McIntosh A.R., Poulsen C., Martinu K., Leonard G., Paus T. Maturation of EEG power spectra in early adolescence: a longitudinal study. Dev. Sci. 2011;14(5):935–943. doi: 10.1111/j.1467-7687.2010.01031.x. [DOI] [PubMed] [Google Scholar]
- Cuevas K., Raj V., Bell M.A. A frequency band analysis of two-year-olds’ memory processes. Int. J. Psychophysiol. 2012;83(3):315–322. doi: 10.1016/j.ijpsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Li H., Xia C.H., Larsen B., Adebimpe A., Baum G.L., Satterthwaite T.D. Individual variation in functional topography of association networks in youth. Neuron. 2020;106(2):340–353. doi: 10.1016/j.neuron.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M.C., Amso D., Anderson L.C., Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Dickinson A., DiStefano C., Senturk D., Jeste S.S. Peak alpha frequency is a neural marker of cognitive function across the autism spectrum. Eur. J. Neurosci. 2018;47(6):643–651. doi: 10.1111/ejn.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T., Dominguez J., Voytek B. Electrophysiological frequency band ratio measures conflate periodic and aperiodic neural activity. bioRxiv. 2020 doi: 10.1101/2020.01.11.900977. 2020.2001.2011.900977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman R.E., Shearer D.E., Emmerson R.Y. Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin. Neurophysiol. 1999;110(8):1399–1409. doi: 10.1016/S1388-2457(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Foxe J., Snyder A. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011;2(154) doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P., Reynolds J.H., Rorie A.E., Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gao R., Peterson E.J., Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage. 2017;158:70–78. doi: 10.1016/j.neuroimage.2017.06.078. [DOI] [PubMed] [Google Scholar]
- Gasser T., Jennen-Steinmetz C., Sroka L., Verleger R., Möcks J. Development of the EEG of school-age children and adolescents II. Topography. Electroencephalogr. Clin. Neurophysiol. 1988;69(2):100–109. doi: 10.1016/0013-4694(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Gasser T., Verleger R., Bächer P., Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr. Clin. Neurophysiol. 1988;69(2):91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gmehlin D., Thomas C., Weisbrod M., Walther S., Pfüller U., Resch F., Oelkers-Ax R. Individual analysis of EEG background-activity within school age: impact of age and sex within a longitudinal data set. Int. J. Dev. Neurosci. 2011;29(2):163–170. doi: 10.1016/j.ijdevneu.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Gmehlin D., Thomas C., Weisbrod M., Walther S., Resch F., Oelkers-Ax R. Development of brain synchronisation within school-age – individual analysis of resting (alpha) coherence in a longitudinal data set. Clin. Neurophysiol. 2011;122(10):1973–1983. doi: 10.1016/j.clinph.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D., Strohmeier D., Brodbeck C. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013;7(267) doi: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy T.H., Werkle‐Bergner M., Chicherio C., Schmiedek F., Lövdén M., Lindenberger U. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology. 2013;50(6):570–582. doi: 10.1111/psyp.12043. [DOI] [PubMed] [Google Scholar]
- Gregoriou G.G., Gotts S.J., Zhou H., Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B., Hua X., Thompson P.M., Waldorp L., Nichols T.E., Initiative, A. s. D. N Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S., Nácher V., Luna R., Romo R., Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl. Acad. Sci. 2011;108(48):19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M., Donoghue T., Peterson E., Varma P., Sebastian P., Gao R. Parameterizing neural power spectra. bioRxiv. 2018:299859. doi: 10.1101/299859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell F.E., Jr . Springer; 2015. Regression Modeling Strategies: with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. [Google Scholar]
- He B.J. Scale-free brain activity: past, present, and future. Trends Cogn. Sci. 2014;18(9):480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66(3):353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Donoghue T., Sowman P.F., Seymour R.A., Brock J., Crain S. Co-increasing neuronal noise and beta power in the developing brain. bioRxiv. 2019:839258. doi: 10.1101/839258. [DOI] [Google Scholar]
- Hegarty J.P., Weber D.J., Cirstea C.M., Beversdorf D.Q. Cerebro-cerebellar functional connectivity is associated with cerebellar excitation–inhibition balance in autism spectrum disorder. J. Autism Dev. Disord. 2018;48(10):3460–3473. doi: 10.1007/s10803-018-3613-y. [DOI] [PubMed] [Google Scholar]
- Hipp J.F., Hawellek D.J., Corbetta M., Siegel M., Engel A.K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 2012;15(6):884–890. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P.J. The behavior of maximum likelihood estimates under nonstandard conditions. Paper Presented at the Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1967 [Google Scholar]
- Iemi L., Chaumon M., Crouzet S.M., Busch N.A. Spontaneous neural oscillations bias perception by modulating baseline excitability. J. Neurosci. 2017;37(4):807–819. doi: 10.1523/jneurosci.1432-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R., Shinosaki K., Ukai S., Inouye T., Ishihara T., Yoshimine T. Medial prefrontal cortex generates frontal midline theta rhythm. NeuroReport. 1999;10(4) doi: 10.1097/00001756-199903170-00003. https://journals.lww.com/neuroreport/Fulltext/1999/03170/Medial_prefrontal_cortex_generates_frontal_midline.3.aspx Retrieved from. [DOI] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29(2):169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur. J. Neurosci. 2003;17(5):1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kraemer H.C., Yesavage J.A., Taylor J.L., Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am. J. Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Langer N., Ho E.J., Alexander L.M., Xu H.Y., Jozanovic R.K., Henin S. A resource for assessing information processing in the developing brain using EEG and eye tracking. Sci. Data. 2017;4(1):170040. doi: 10.1038/sdata.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-W., Girolami M., Sejnowski T.J. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 1999;11(2):417–441. doi: 10.1162/089976699300016719. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Smyser C.D., Shimony J.S. Resting-state fMRI: a review of methods and clinical applications. Am. J. Neuroradiol. 2013;34(10):1866. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr. Opin. Neurobiol. 2012;22(3):537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder S.S., van Putten M.J.A.M. Automated EEG analysis: characterizing the posterior dominant rhythm. J. Neurosci. Methods. 2011;200(1):86–93. doi: 10.1016/j.jneumeth.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Marcuse L.V., Schneider M., Mortati K.A., Donnelly K.M., Arnedo V., Grant A.C. Quantitative analysis of the EEG posterior-dominant rhythm in healthy adolescents. Clin. Neurophysiol. 2008;119(8):1778–1781. doi: 10.1016/j.clinph.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Matoušek M., Petersén I. Automation of Clinical Electroencephalography. Raven Press; New York: 1973. Frequency analysis of the EEG in normal children and in normal adolescents; pp. 75–102. [Google Scholar]
- Matthis P., Scheffner D., Benninger C., Lipinski C., Stolzis L. Changes in the background activity of the electroencephalogram according to age. Electroencephalogr. Clin. Neurophysiol. 1980;49(5-6):626–635. doi: 10.1016/0013-4694(80)90403-4. [DOI] [PubMed] [Google Scholar]
- McEvoy K., Hasenstab K., Senturk D., Sanders A., Jeste S.S. Physiologic artifacts in resting state oscillations in young children: methodological considerations for noisy data. Brain Imaging Behav. 2015;9(1):104–114. doi: 10.1007/s11682-014-9343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau A., Felsch M., Hülsdünker T., Mierau J., Bullermann P., Weiß B., Strüder H.K. The interrelation between sensorimotor abilities, cognitive performance and individual EEG alpha peak frequency in young children. Clin. Neurophysiol. 2016;127(1):270–276. doi: 10.1016/j.clinph.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Mierau A., Klimesch W., Lefebvre J. State-dependent alpha peak frequency shifts: experimental evidence, potential mechanisms and functional implications. Neuroscience. 2017;360:146–154. doi: 10.1016/j.neuroscience.2017.07.037. [DOI] [PubMed] [Google Scholar]
- Molina J.L., Voytek B., Thomas M.L., Joshi Y.B., Bhakta S.G., Talledo J.A. Memantine effects on electroencephalographic measures of putative excitatory/inhibitory balance in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5(6):562–568. doi: 10.1016/j.bpsc.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigbur R., Cohen M.X., Ridderinkhof K.R., Stürmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J. Cogn. Neurosci. 2011;24(5):1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Orekhova E.V., Stroganova T.A., Posikera I.N. Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. Int. J. Psychophysiol. 1999;32(2):151–172. doi: 10.1016/S0167-8760(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Orekhova E.V., Stroganova T.A., Posikera I.N. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clin. Neurophysiol. 2001;112(5):740–749. doi: 10.1016/S1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Orekhova E.V., Stroganova T.A., Posikera I.N., Elam M. EEG theta rhythm in infants and preschool children. Clin. Neurophysiol. 2006;117(5):1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Hildebrandt A., Schmitz F., Herrmann C.S. Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. Neuroimage. 2020;205:116304. doi: 10.1016/j.neuroimage.2019.116304. [DOI] [PubMed] [Google Scholar]
- Paulino C., Flores A., Gomez C. Developmental changes in the EEG rhythms of children and young adults analyzed by means of correlational, brain topography and principal component analysis. J. Psychophysiol. 2011;25:143–158. doi: 10.1027/0269-8803/a000052. [DOI] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Stancák A., Neuper C. Event-related synchronization (ERS) in the alpha band — an electrophysiological correlate of cortical idling: a review. Int. J. Psychophysiol. 1996;24(1):39–46. doi: 10.1016/S0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Podvalny E., Noy N., Harel M., Bickel S., Chechik G., Schroeder C.E. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J. Neurophysiol. 2015;114(1):505–519. doi: 10.1152/jn.00943.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Maunsell J.H.R. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard Clark C., Veltmeyer M.D., Hamilton R.J., Simms E., Paul R., Hermens D., Gordon E. Spontaneous alpha peak frequency predicts working memory performance across the age span. Int. J. Psychophysiol. 2004;53(1):1–9. doi: 10.1016/j.ijpsycho.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Riddle J., Hwang K., Cellier D., Dhanani S., D’Esposito M. Causal evidence for the role of neuronal oscillations in top-down and bottom-up attention. J. Cogn. Neurosci. 2019;31(5):768–779. doi: 10.1162/jocn_a_01376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle J., Ahn S., McPherson T., Girdler S., Frohlich F. Progesterone modulates theta oscillations in the frontal-parietal network. Psychophysiology. 2020:e13632. doi: 10.1111/psyp.13632. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle J., Scimeca J.M., Cellier D., Dhanani S., D’Esposito M. Causal evidence for a role of theta and alpha oscillations in the control of working memory. Curr. Biol. 2020;30(9):1748–1754. doi: 10.1016/j.cub.2020.02.065. e1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle J., Vogelsang D.A., Hwang K., Cellier D., D’Esposito M. Distinct oscillatory dynamics underlie different components of hierarchical cognitive control. J. Neurosci. 2020;40(25):4945–4953. doi: 10.1523/jneurosci.0617-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M.M., Furlong S., Voytek B., Donoghue T., Boettiger C.A., Sheridan M.A. EEG power spectral slope differs by ADHD status and stimulant medication exposure in early childhood. J. Neurophysiol. 2019;122(6):2427–2437. doi: 10.1152/jn.00388.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Martínez E., Ruiz-Martínez F., Paulino C.B., Gómez C.M. Frequency shift in topography of spontaneous brain rhythms from childhood to adulthood. Cogn. Neurodyn. 2017;11(1):23–33. doi: 10.1007/s11571-016-9402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V., Brodbeck V., Michel C., Amedi A., Pascual-Leone A., Thut G. Spontaneous fluctuations in posterior α-band EEG activity reflect variability in excitability of human visual areas. Cereb. Cortex. 2008;18(9):2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F., Uhlhaas P.J. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn. Sci. 2014;18(1):16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Rubenstein J.L.R., Merzenich M.M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann Y.B., Pinsk M.A., Wang L., Li X., Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby J.N., Marshall P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012;37(3):253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Schabus M., Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005;57(2):97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Heise K.F., Gruber W.R., Holz E., Karim A.A. Brain oscillatory substrates of visual short-term memory capacity. Curr. Biol. 2009;19(21):1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Schäfer C.B., Morgan B.R., Ye A.X., Taylor M.J., Doesburg S.M. Oscillations, networks, and their development: MEG connectivity changes with age. Hum. Brain Mapp. 2014;35(10):5249–5261. doi: 10.1002/hbm.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaworonkow N., Voytek B. Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. bioRxiv. 2020 doi: 10.1101/2020.08.18.256016. 2020.2008.2018.256016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaworonkow N., Voytek B. Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Dev. Cogn. Neurosci. 2020:100895. doi: 10.1016/j.dcn.2020.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz S.J., Santesso D.L., Jetha M.K. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Seitzman B.A., Gratton C., Laumann T.O., Gordon E.M., Adeyemo B., Dworetsky A. Trait-like variants in human functional brain networks. Proc. Natl. Acad. Sci. 2019;116(45):22851–22861. doi: 10.1073/pnas.1902932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L.-R., Habela C.W., Stafstrom C.E. Pediatric epilepsy mechanisms: expanding the paradigm of excitation/inhibition imbalance. Children. 2019;6(2) doi: 10.3390/children6020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.R. The electroencephalogram during normal infancy and childhood: I. Rhythmic activities present in the neonate and their subsequent development. Pedagog. Semin. J. Genet. Psychol. 1938;53(2):431–453. doi: 10.1080/08856559.1938.10533820. [DOI] [Google Scholar]
- Sohal V.S., Rubenstein J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry. 2019;24(9):1248–1257. doi: 10.1038/s41380-019-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal V.S., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen R.J.M., van’t Klooster B.J., van der Molen M.W., van Leeuwen H.M.P., Licht R. Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biol. Psychol. 1997;44(3):187–209. doi: 10.1016/S0301-0511(96)05218-0. [DOI] [PubMed] [Google Scholar]
- Sonkusare S., Breakspear M., Guo C. Naturalistic stimuli in neuroscience: critically acclaimed. Trends Cogn. Sci. 2019;23(8):699–714. doi: 10.1016/j.tics.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Stroganova T.A., Orekhova E.V. EEG and infant states. Infant EEG and event-related potentials. 2007;251:280. [Google Scholar]
- Stroganova T.A., V. Orekhova E., Posikera I.N. Externally and internally controlled attention in infants: an EEG study. Int. J. Psychophysiol. 1998;30(3):339–351. doi: 10.1016/S0167-8760(98)00026-9. [DOI] [PubMed] [Google Scholar]
- Stroganova T.A., Orekhova E.V., Posikera I.N. EEG alpha rhythm in infants. Clin. Neurophysiol. 1999;110(6):997–1012. doi: 10.1016/S1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Tatti R., Haley M.S., Swanson O.K., Tselha T., Maffei A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol. Psychiatry. 2017;81(10):821–831. doi: 10.1016/j.biopsych.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I., Jones O.P., Mars R.B., Smith S., Behrens T., Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science. 2016;352(6282):216–220. doi: 10.1126/science.aad8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team R.C. 2020. R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- Tran T., Rolle C., Gazzaley A., Voytek B. Linked sources of neural noise contribute to age-related cognitive decline. J. Cogn. Neurosci. 2020:1–110. doi: 10.1162/jocn_a_01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröndle M., Popov T., Langer N. Decomposing the role of alpha oscillations during brain maturation. bioRxiv. 2020 doi: 10.1101/2020.11.06.370882. 2020.2011.2006.370882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Front. Syst. Neurosci. 2010;4(21) doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr. Bull. 2011;37(3):514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Singer W., Haenschel C., Sireteanu R., Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Rodriguez E., Rotarska-Jagiela A., Singer W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Unger K., Ackerman L., Chatham C.H., Amso D., Badre D. Working memory gating mechanisms explain developmental change in rule-guided behavior. Cognition. 2016;155:8–22. doi: 10.1016/j.cognition.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher M., Stemmler M., Olami Z. Dynamic pattern formation leads to $\frac{1}{f}$ noise in neural populations. Phys. Rev. Lett. 1995;74(2):326–329. doi: 10.1103/PhysRevLett.74.326. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vanderwal T., Kelly C., Eilbott J., Mayes L.C., Castellanos F.X. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;122:222–232. doi: 10.1016/j.neuroimage.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewouw M.M., Dunkley B.T., Lerch J.P., Anagnostou E., Taylor M.J. Characterizing inscapes and resting-state in MEG: effects in typical and atypical development. Neuroimage. 2021;225:117524. doi: 10.1016/j.neuroimage.2020.117524. [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Knight R.T. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry. 2015;77(12):1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Kramer M.A., Case J., Lepage K.Q., Tempesta Z.R., Knight R.T., Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 2015;35(38):13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. Ieee Trans. Audio Electroacoust. 1967;15(2):70–73. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
- Whedon M., Perry N.B., Bell M.A. Relations between frontal EEG maturation and inhibitory control in preschool in the prediction of children’s early academic skills. Brain Cogn. 2020;146:105636. doi: 10.1016/j.bandc.2020.105636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: J. Econom. Soc. 1980:817–838. [Google Scholar]
- Whitford T.J., Rennie C.J., Grieve S.M., Clark C.R., Gordon E., Williams L.M. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007;28(3):228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys B.E., Jankowski K.F., Shook D., Rosenberger L.R., Barnes K.A., Berl M.M. The fMRI success rate of children and adolescents: Typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum. Brain Mapp. 2009;30(10):3426–3435. doi: 10.1002/hbm.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O., Fenno L.E., Prigge M., Schneider F., Davidson T.J., O’Shea D.J. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J., Kolev V. Alpha response system in children: changes with age. Int. J. Psychophysiol. 1997;26(1):411–430. doi: 10.1016/S0167-8760(97)00779-4. [DOI] [PubMed] [Google Scholar]
- Zelinski E.M., Kennison R.F., Watts A., Lewis K.L. 2009. Convergence Between Cross-Sectional and Longitudinal Studies: Cohort Matters. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.