Abstract
The fluorescent dye 1,6-diphenyl-1,3,5-hexatriene (DPH) is widely used as a probe of membrane order. We show that DPH also interacts with amyloid fibrils formed by human amylin (h-amylin, also known as islet amyloid polypeptide) in solution and this results in a 100-fold increase in DPH fluorescence for a sample of 20 μh-amylin and 0.25 μΜ DPH. No increase in DPH fluorescence is observed with the non-amyloidogenic rat amylin or with freshly dissolved, non-fibrillar h-amylin. The time course of amyloid formation by amylin was followed by monitoring the fluorescence of added DPH as a function of time and was similar to that monitored by the standard fluorescent probe thioflavin-T. The inclusion of DPH in the buffer did not perturb the time course of amyloid formation under the conditions examined and the time course was independent of the range of DPH concentrations tested (0.25 to 5 μΜ). Maximum final fluorescence intensity is observed at substoichiometric ratios of DPH to amylin. No significant increase in fluorescence was observed during the lag phase of amyloid formation, and the implications for the structure of amylin pre-fibril oligomers are discussed. h-Amylin contains three aromatic residues. A triple aromatic to leucine mutant forms amyloid and DPH binds to the resulting fibrils, indicating that interactions with aromatic side chains are not required for DPH amylin amyloid interactions. DPH may be especially useful for studies on mutant amylins and other polypeptides in which changes in charged residues might complicate interpretation of thioflavin-T fluorescence.
Keywords: Amylin, Diphenylhexatriene, DPH, Islet Amyloid Polypeptide, Amyloid, Fluorescent Dyes
Graphical Abstract
Introduction
Amyloid formation refers to the aggregation of proteins and polypeptides into partially ordered fibril structures rich in cross-β structure. The process of amyloid formation has been implicated in more than 30 different human diseases, and a large number of proteins which do not form amyloid in vivo can be induced to do so in vitro1–3. Prominent examples of proteins involved in disease related amyloids include the Aβ peptide and tau protein of Alzheimer’s disease, alpha- synuclein in Parkinson’s disease and amylin, (also known as islet amyloid polypeptide, IAPP) in type-2 diabetes1–5. Amyloid formation is commonly monitored in vitro using fluorescence-based dye binding assays in which the added dye displays an increase in fluorescence when bound to fibrils. Probably the most widely used fluorescence amyloid probe is the cationic benzylamine-benzothiazole based dye, thioflavin-T (Figure-1). Thioflavin-T undergoes a significant increase in fluorescence upon binding to amyloid fibrils and usually does not exhibit enhanced fluorescence in the presence of pre-amyloid species or monomeric proteins6, 7. There is interest in the discovery and development of new amyloid binding dyes since thioflavin-T, and other dye-based assays of amyloid formation can give both false positives and false negatives8–13. The cationic nature of thioflavin-T also indicates that it may not be optimum for following amyloid formation by polypeptides which have a significant net positive charge14. In some cases, dye binding studies can provide information about the properties of amyloid fibrils or pre amyloid intermediates15–17.
Figure-1:
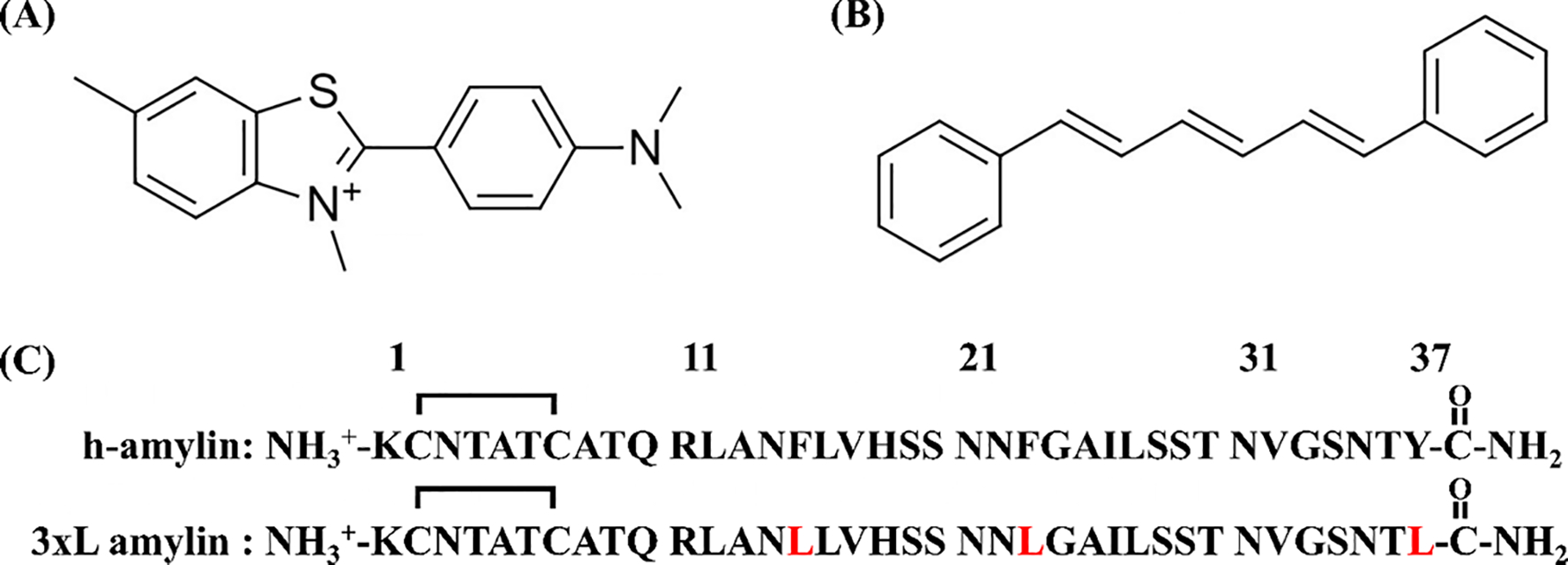
The structure of (A) Thioflavin-T and (B) 1,6 diphenylhexatriene. (C) The primary sequences of h-amylin and the triple aromatic to Leu mutant of h-amylin. The polypeptides have an amidated C-terminus and an intramolecular disulfide between Cys-2 and Cys-7. Residues in the triple mutant which differ from wild type are in red.
The fluorescence of 1,6 diphenyl 1,3,5 hexatriene (DPH, Figure-1) is weak in water, but increases significantly in a hydrophobic environment18, 19. The dye is widely used as a probe of membrane order, an assay that relies on changes in its fluorescence anisotropy 19–22. It can also be used to detect the formation of detergent micelles or detect the level of lipid vesicles by the enhancement of its fluorescence intensity in the presence of a hydrophobic aggregate23, 24. Here we demonstrate that the fluorescence of DPH is also enhanced in the presence of amyloid fibrils formed by human amylin (h-amylin, also known as islet amyloid polypeptide or IAPP). The probe can be used to accurately follow the kinetics of h-amylin amyloid formation, but shows no significant fluorescence enhancement in the presence of the non-amyloidogenic variant of amylin derived from rats and mice or in the presence of freshly dissolved non fibril h-amylin.
Islet amyloid formation by h-amylin contributes to pancreatic β-cell dysfunction and death in type 2 diabetes (T2D), and in islet transplants5, 25–34. h-amylin is a cationic 37 residue neuro pancreatic polypeptide which has a conserved amidated C-terminus and a conserved disulfide bridge between residues 2 and 7 (Figure-1). The polypeptide lacks acidic residues and has a net charge at physiological pH of between +2 and +4, depending on the pKa of the N-terminus and His-18. h-amylin is one of the most amyloidogenic naturally occurring sequences known and forms amyloid in vitro more rapidly than the Aβ peptide of Alzheimer’s disease 29. The sequence of h-amylin is displayed in figure-1 together with the structures of DPH and thioflavin-T. There are limited investigations of the use of DPH to follow amyloid formation. Prior studies have shown that DPH can bind to aggregates of Aβ and α-synuclein, but its ability to monitor amylin formation has not been tested and its suitability as a dye for in situ kinetics assays has not been investigated in depth35.
Results and Discussion
DPH exhibits enhanced fluorescence in the presence of h-amylin amyloid fibrils, but not in the presence of non-amyloidogenic forms of h-amylin or rat amylin.
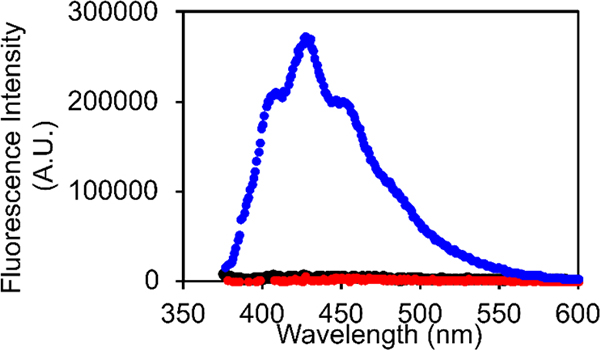
We formed amyloid fibrils of h-amylin by incubating the polypeptide in buffer at pH 7.4 with 100 mM NaCl, conditions chosen to mimic those used in prior studies of amylin amyloid formation. Fibril formation was verified by thioflavin-T assays and by transmission electron microscopy (TEM). DPH exhibited a significant increase in fluorescence with an emission maximum of 427 nm when added to the pre-formed h-amylin fibrils. We examined potential effects of the added NaCl by recording fluorescence emission spectra of DPH plus h-amylin fibrils in the presence of 0, 100 and 200 mM added salt. No change in the shape or intensity of the spectrum was detected. In contrast to the result with h-amylin, no significant DPH fluorescence was detected in the presence of freshly dissolved, non-fibril h-amylin (Figure-2). Not all variants of amylin form amyloid, and mouse/rat amylin is not amyloidogenic in vitro under standard conditions, nor do rats or mice develop islet amyloid or diabetes4, 27. DPH showed no increase in fluorescence in the presence of rat amylin (Figure-2).
Figure-2:
Fluorescence emission spectra of DPH in the presence of h-amylin fibrils (blue), freshly dissolved non-fibril h-amylin (black), and rat amylin (red). The black and red curves overlap. Experiments were conducted with 0.25 μM DPH, at pH 7.4 25 °C in 20 mM tris 100 mM NaCl buffer. The concentration of rat and human amylin were 20 μM. A TEM image of the amyloid fibrils formed by h-amylin is displayed in figure-3.
DPH fluorescence accurately reports on the kinetics of h-amylin formation
Fluorescence based dye binding assays are widely used to study the kinetics of amyloid formation and to detect amyloid fibrils6, 7. They are most convenient when the dye is added at the start of an assay and its fluorescence continuously monitored. However, dyes are extrinsic probes, and the possibility always exists that they could perturb the kinetics of the process they are designed to monitor. Dyes are still useful in this case, but a kinetic experiment will then need to be conducted by removing aliquots of a reaction mixture at different times points, adding dye, and measuring the fluorescence signal. This is much more time consuming than in situ experiments and does not lead itself to high throughput experiments. Consequently, it is important to test if a dye perturbs the time course of amyloid formation.
Amyloid formation by h-amylin displays the typical kinetic progress curve observed for amyloidogenic proteins; a lag time in which few if any detectable amyloid fibrils are formed, followed by a growth phase which results in increased material deposited as fibrils, and increased dye fluorescence. The system reaches a plateau phase in which fibrils and any remaining soluble polypeptide is in equilibrium. This leads to a sigmoidal curve of fluorescence vs time. Thioflavin-T has previously been showed to not perturb the kinetics of amyloid formation by h-amylin under the conditions of our experiments5, 17, 33. Thus, we conducted side by side kinetic experiments with h-amylin to test if in situ experiments with DPH accurately report on h-amylin amyloid formation. One set of experiments was run with added thioflavin-T as the reporter dye and another set used DPH as the probe. Initial experiments indicated that DPH had a tendency to bind to the standard plastic 96 well plates used in our plate reader fluorescence assays. We did not attempt to find optimum plastic plates for the DPH assays, but instead used a quartz plate for the proof-of-principle studies reported here. The time courses detected by DPH and thioflavin-T are similar and give the same value of T50 (9 h) the time required to reach 50% of the final intensity in an assay (Figure-3). This indicates that DPH is as reliable at monitoring h-amylin amyloid formation as thioflavin-T. A 100-fold increase in DPH fluorescence was observed when a 20 μM sample of h-amylin formed amyloid fibrils in the presence of 0.25 μM DPH dye, and an even larger enhancement is observed for higher DPH concentrations (Fugure-4). For comparison, 20 μM thioflavin-T lead to a 100-fold fluorescence when a 20 μM sample of h-amylin formed amyloid fibrils.
Figure-3:
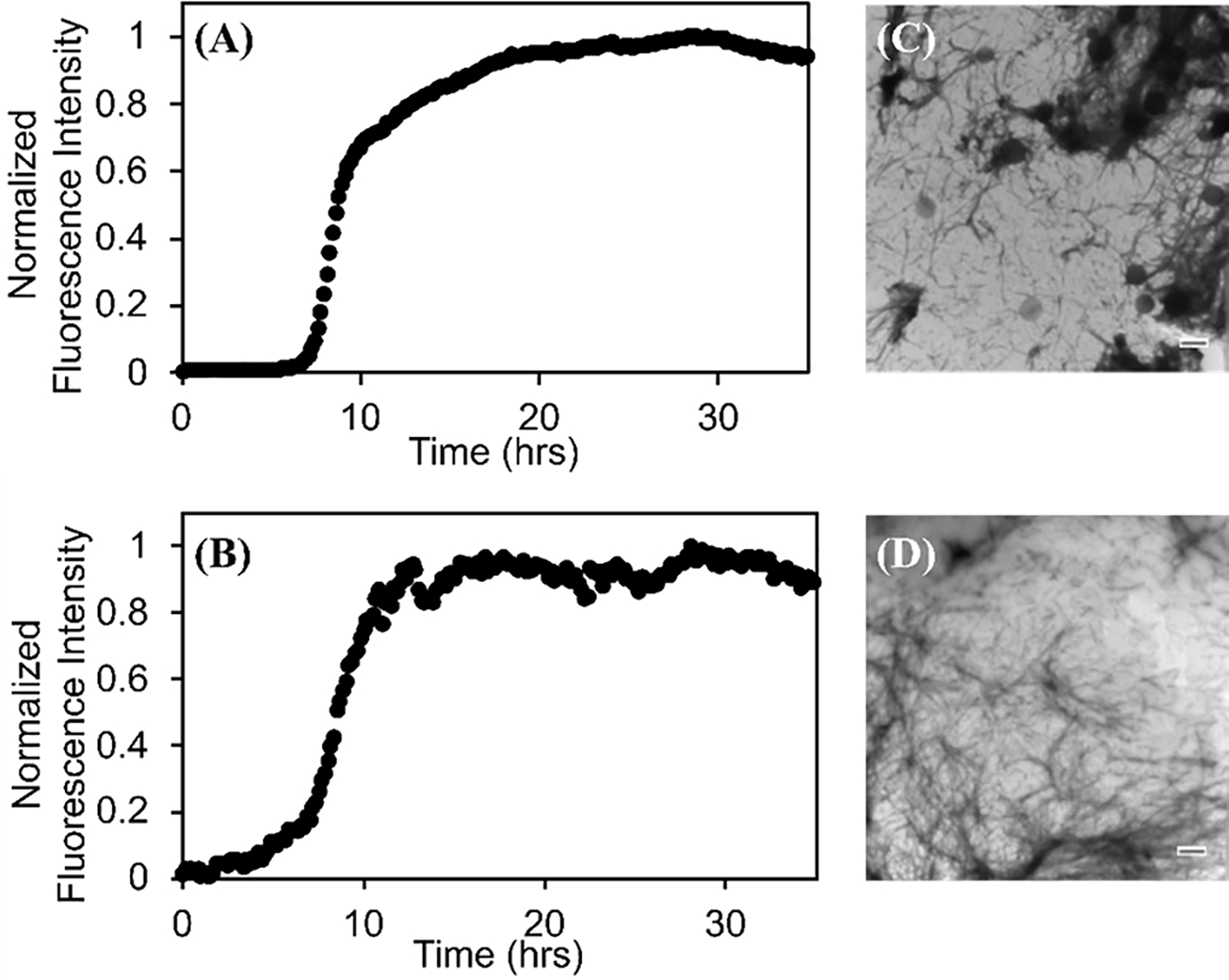
The kinetics of h-amylin amyloid formation followed by (A) a thioflavin-T assay with a 40 μM thioflavin-T concentration and (B) a DPH assay with a 0.25 μM DPH concentration. The signal to noise of the DPH experimental can be enhanced significantly by increase the DPH concentration. TEM images of samples collected at the end of the (C) thioflavin-T assay and the (D) DPH assay. Experiments were conducted at 25 °C, pH 7.4 in 20 mM tris 100 mM buffer. The concentration of h-amylin was 20 μM in both experiments and the concentration of the dyes were 40 μM for thioflavin-T and 0.25 μM for DPH.
We further tested the use of DPH in in situ studies by conducting a set of experiments in which the concentration of added DPH spanned a 20-fold range, from 0.25 μΜ to 5 μΜ for a fixed concentration of h-amylin (40 μΜ). If DPH modulated the time course of amyloid formation, then one would expect more pronounced effects as the concentration of the dye increased, and the measured T50 values could depend on the dye concentration. No detectable DPH concentration dependent effects were observed, providing additional evidence that the dye does not alter the time course of amyloid formation (Supporting Figure-1).
We next examined the effect of varying DPH concentration on the final fluorescence observed in the plateau region after amyloid formation is complete. The final DPH fluorescence intensity appeared to saturate above 2 μΜ DPH for 20 μΜ h-amylin under these conditions (Figure-4).
Figure-4:
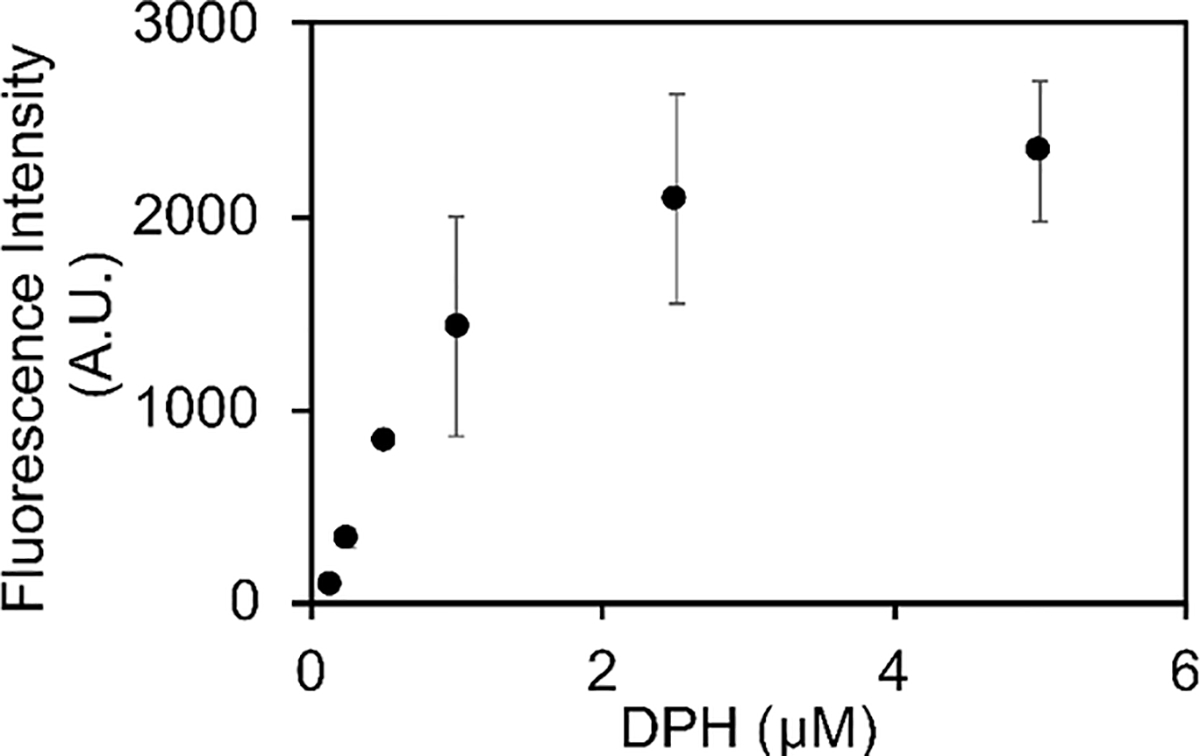
A plot of the final intensity observed in a set of DPH assays. Assays were conducted in a 96 well quartz plate using a plate reader at 25 °C, pH 7.4 in 20 mM tris 100 mM buffer. The concentration of h-amylin was 20 μM.
π- π interactions are not required for DPH h-amylin fibril interactions.
h-amylin contains three aromatic residues, two Phe residues at positions 15 and 23 and a strongly conserved Tyr at the C-terminus (Figure-1, Figure-5). Given the highly conjugated structure of DPH, we wondered if the aromatic residues in h-amylin play a critical role in binding DPH via π- π interactions. There are several structural models of the h-amylin fibrils formed in vitro. One based on solid state NMR studies, one on crystallographic studies of small steric zipper-forming peptides derived from amylin, and three cryo-EM structures34, 36–39. The different structures differ in their details and this may be due to the fact that the fibrils were formed under different conditions, but all contain extensive cross β-sheet structure, and the fibril is made up of two stacks of h-amylin monomers (Figure-5).
Figure-5:
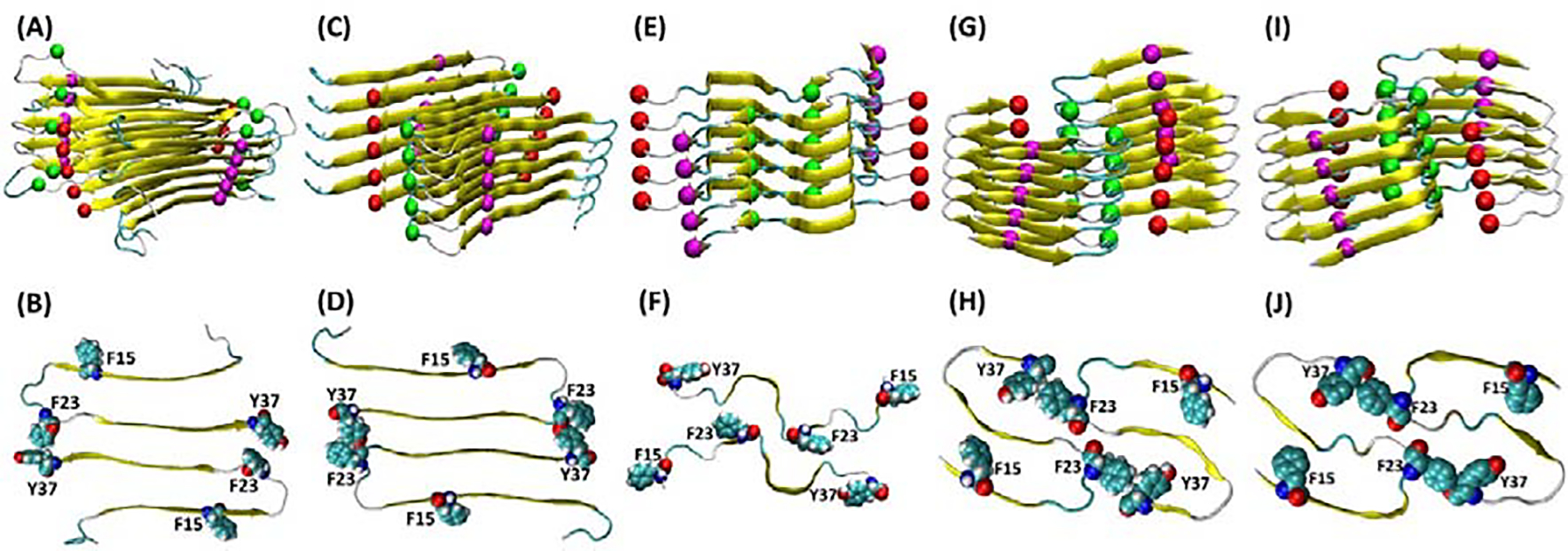
Structural models of the h-amylin amyloid fibril with the location of the aromatic residues shown. The top row displays ribbon diagrams showing the arrangement of individual polypeptides in the fibril and spheres showing the Cα atoms of Phe-15 (magenta), Phe-23 (green) and Tyr-37 (red). The bottom row shows a top-down view and displays a cross section of the fibril with the aromatic residues shown in space-filling format. (A, B) structure based on solid state NMR studies. (C, D) structural model based on x-ray diffraction studies of small steric zipper peptides. (E, F) Cryo-EM structure of a h-amylin fusion determined at pH 8.0 (pdb code 6VW2). (G, H) Cryo-EM structure determined at pH 6.0 (pdb code 6Y1A). (I, J) Cryo-EM structure determined at pH 6.8 (pdb code 6ZRF).
Each model contains several aromatic residues within the cross-β core; this leads to a quasi-infinite arrangement of stacked aromatic sidechains. The location of each of the aromatic residues in each model are displayed in figure-5. Analysis of the solvent exposed surface area reveals different levels of exposure of the aromatic residues in the different models. In two of the three cryo-EM based models, all three aromatics are largely buried. The % exposed solvent accessible surface area (% SASA) is less than 4% for all residues in both of the stacks in 6Y1A and 6ZRF. In 6VW2, F15 and Y37 have 53 to 84 % and 40 to 53% SASA respectively. The quoted range reflects for the values in the two different stacks that make up the fibril structure. In the model based on x-ray studies of steric zipper peptides, F15 and F23 exhibit 38 to 60 % and 37 to 59% SASA, respectively. In the model based on solid state NMR studies F23 is largely buried (% SASA from 10 to 17%), but the % SASA of F15 and Y37 range from 47 to 74% and 40 to 74%.
We tested whether the aromatic residues are required for DPH binding to amylin amyloid fibrils. A triple aromatic to Leu mutant of h-amylin (F15L, F23L, Y37L, amylin, denoted as 3xL-amylin) has been shown to form amyloid, albeit it at a slower rate than wild type5, 17, 33. There is a significant increase in DPH fluorescence when the dye is added to 3xL amylin fibrils, but not when it is added to freshly dissolved non fibril 3xL amylin (Figure-6, supporting Figure-S2). The intensity at the fluorescence emission maximum is somewhat less for the 3xL amylin variant than for the wild type. This could arise because the binding of DPH is weaker, its quantum yield when bound is less, or because fewer fibrils are formed by the mutant. The key point is that these experiments demonstrate that aromatic residues are not required for DPH to interact with amylin amyloid fibrils.
Figure-6:
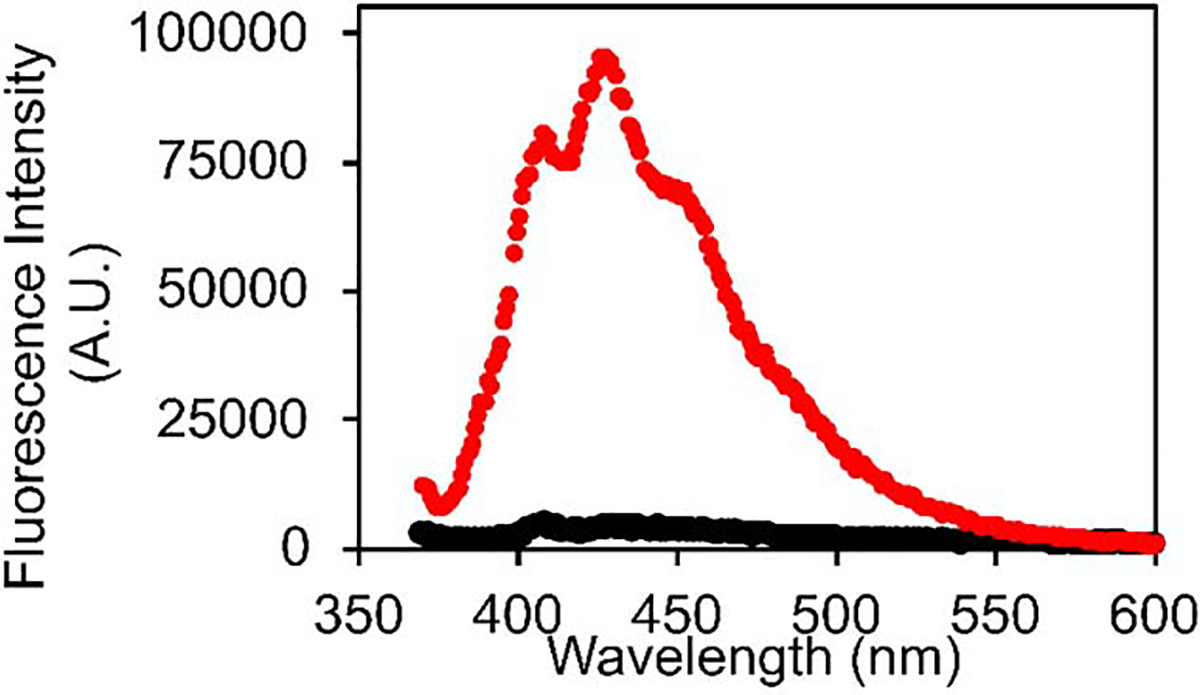
Fluorescence emission spectra of DPH in the presence of: 3xL-amylin fibrils (red), and freshly dissolved non-fibril 3xL-amylin (black). Experiments were conducted with 20 μM peptide, 0.25 μM DPH, at pH 7.4, 25 °C in 20 mM tris 100 mM NaCl buffer.
Conclusions
The features of the fibril that lead to DPH binding are not known, but the cross β-structure leads to long exposed faces with grooves on the surface of the fibril. Thioflavin-T is believed to bind in these groves and DPH may as well. The studies reported here show that DPH accurately reports on the kinetics of amyloid formation by amylin and demonstrates that interactions between aromatic residues and DPH are not required for DPH binding. The dye may be used at sub-stoichiometric concentrations, indeed some of the experiments reported here involved an 80-fold excess of h-amylin to DPH. It should be noted that maximum sensitivity observed here was when the dye to peptide ratio was approximately 1:8 or greater (h- amylin in excess) for a 20 μM sample of h-amylin (Figure-4). DPH may be especially useful for studies on mutant amylins and other polypeptides in which changes in charged residues might complicate interpretation of thioflavin-T fluorescence.
Dye binding studies have been used to probe the development of hydrophobic surfaces/ clusters in pre-fibril oligomers. In particular, some amyloidogenic proteins have been reported to bind dyes such as ANS, bis-ANS, and Nile Red in the lag phase and ANS binding has been suggested to be a general property of oligomers formed by a wide range of proteins15, 16. Thus, it is interesting to note that no significant enhancement of DPH fluorescence was observed in the lag phase of h-amylin amyloid formation. This is consistent with previous studies which have shown that h-amylin lag phase species do not bind ANS or Nile Red17 and argues that h-amylin oligomers do not present large well-developed solvent exposed hydrophobic surfaces.
Materials and Methods
Peptide Synthesis and Purification
h-amylin and the triple aromatic to Leu mutant were prepared by solid phase peptide synthesis on a 0.01 mmol scale with 9-fluorenylmethyloxycarbonyl (Fmoc) protected amino acids. The synthesis was conducted using a CEM Liberty microwave peptide synthesizer. Pseudoproline dipeptide (oxazolidine) derivatives of Leu-Ser were used as previously described to improve the yield40, 41. 5-(4’-Fmoc-aminomethyl-3’,5’-dimethoxyphenol) valeric acid resin was employed. This resin provides an amidated C-terminus upon cleavage. Coupling reactions were carried out for 2 min at 90 °C except for Cys and His which were coupled at 55 °C to minimize the risk of racemization. Peptides were cleaved using a cocktail made of 92.5% trifluoroacetic acid (TFA), 2.5% water, 2.5% Triisopropyl silane (TIPS), and 2.5% 3,6-dioxa-1,8-octanedithiol (DODT). Cleaved crude peptides were dissolved in 20% acetic acid (v/v) and lyophilized prior to the formation of the disulfide bond. The intramolecular disulfide bond was formed by dissolving the dry peptide in 100 % dimethyl sulfoxide (DMSO) at a concentration of 10 mg/mL and incubating at room temperature for 3 days with stirring42. Both polypeptides were purified via reverse-phase high pressure liquid chromatography (HPLC) with a C18 preparatory column (Higgins Analytical) using a binary gradient (buffer A =100% H2O and 0.045% HCl and buffer B = 80% Acetonitrile, 20% H2O, and 0.045% HCl). HCl was used as a counter ion instead of TFA since TFA can affect amylin kinetic assays. Residual scavengers were removed after the first round of HPLC purification using a 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) extraction procedure. Peptides, after the first HPLC purification were dissolved in neat HFIP and incubated at room temperature for 4 h, then filtered with 0.45 μM GHP membrane filter, and purified via RP-HPLC. Masses of purified peptides were confirmed using matrix assisted laser desorption time-of-flight mass spectrometry; h-amylin expected: 3903.3, observed: 3902.7; 3xL-amylin expected: 3785.3 observed: 3785.9.
Preparation of Peptide Stock Solutions and Sample Preparation
Stock solutions were prepared by dissolving the purified peptides into neat HFIP to a concentration of 1.6 mM (determined by lyophilizing a 20 μL aliquot of the HFIP stock for at least 12 h, dissolving in 120 μL of 20 mM Tris buffer (pH 7.4) and measuring the absorbance at 280 nm for h-amylin). An extinction coefficient of 1615 M−1cm−1 at 280 nm was used for h-amylin. The concentration of the triple Leu mutant was determined using the BCA assay with BSA solutions used as the standard.
DPH Fluorescence Emission Spectra
Fluorescence emission spectra were recorded in a 1 cm pathlength cell on a Horiba Quanta Master Spectrofluorimeter (Horiba Scientific, Edison, NJ). The excitation wavelength was 358 nm, and the excitation and emission slits were 4 nm. Spectra were recorded over the range 370 to 600 nm at 25 °C.
DPH and Thioflavin-T Fluorescence Based Kinetic Assays
Aliquots of the HFIP stock solutions were lyophilized for at least 12 h and reconstituted in buffer at pH 7.4 for kinetic assays. The final concentrations of the peptides and dye (DPH or thioflavin-T) were 20 μM and 0.25 μM, respectively. A quartz plate (Hellma, item number: HL730009-B-44) was used for the kinetic assays. Wells at the edge of the plate were not used but were filled with buffer. Fluorescence assays were conducted using a Molecular Devices SpectraMax Gemini EM microplate reader. Fluorescence was measured from the bottom of the wells at 10 min intervals without agitation. The excitation and emission wavelengths for the DPH studies were 358 and 427 nm, respectively. The values for the thioflavin-T experiments were 450 nm (excitation) and 485 nm (emission). Three parallel samples were tested for each experiment. The quoted uncertainties are the estimated standard deviations. All fluorescence assays were performed at 25 °C.
Transmission Electron Microscopy
Samples for TEM were prepared from the samples at the end of the kinetics experiments. 15 μL of peptide solution was blotted on a carbon-coated Formvar 300-mesh copper grid for 1 min and then negatively stained with 2% (w/v) uranyl acetate for 1 min. A FEI Bio TwinG2 transmission electron microscope was used to record TEM images (Life Science Microscopy Center at Stony Brook University).
Calculation of Solvent Accessible Surface Area of the Aromatic Residues
The values of the SASA were calculated for each aromatic residue using the coordinate files with hydrogens included. The “measure SASA” command in Visual Dynamics Software (VMD)43 was used with a probe radius of 1.4 Å for the calculations. Values are reported as % relative to an extended tripeptide of the same sequence for F15 and F23 and relative to a C-terminal capped Thr-Tyr for the c-terminal tyrosine (Y37). Tripeptides and the capped dipeptide were generated using TLEAP in Amber2044.
Supplementary Material
Acknowledgments
We thank Dr. Rehana Akter and Ms. Daeun Noh for helpful discussions concerning amyloid specific dyes. This work was supported by NIH grants GM 078114 and GM 122493 and by NSF grant MCB-1330259.
Abbreviations:
- DODT
3,6-dioxa-1,8-octanedithiol
- DPH
1,6-diphenyl-1,3,5-hexatriene
- Fmoc
9-fluorenylmethyloxycarbonyl
- h
hours
- h-amylin
human amylin
- HFIP
1,1,1,3,3,3-hexafluoro-2-propanol
- HPLC
high performance liquid chromatography
- IAPP
islet amyloid polypeptide
- min
minutes
- SASA
solvent accessible surface area
- T2D
type-2 diabetes
- T50
the time required to reach 50% of the final maximal fluorescence changes in a kinetic experiment
- TEM
transmission electron microscopy
- TFA
trifluoroacetic acid
- TIPS
Triisopropyl silane
- 3xL-amylin
a triple F15L, F23L, Y37L mutant of h-amylin
Footnotes
Conflicts of Interest
The authors declare no competing financial interest.
Associated Content
Supporting Information
One figure showing kinetic curves for a set of DPH monitored amyloid assays with a range of DPH concentrations. One figure showing TEM images of the amyloid fibrils formed by 3xL amylin in the presence of thioflavin-T and DPH.
References
- 1.Sipe JD, Amyloidosis. Crit Rev Clin Lab Sci 1994, 31, 325–54. [DOI] [PubMed] [Google Scholar]
- 2.Riek R; Eisenberg DS, The activities of amyloids from a structural perspective. Nature 2016, 539, 227–235. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F; Dobson CM, Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu Rev Biochem 2017, 86, 27–68. [DOI] [PubMed] [Google Scholar]
- 4.Akter R; Cao P; Noor H; Ridgway Z; Tu LH; Wang H; Wong AG; Zhang X; Abedini A; Schmidt AM; Raleigh DP, Islet Amyloid Polypeptide: Structure, Function, and Pathophysiology. J Diabetes Res 2016, 2016, 2798269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abedini A; Schmidt AM, Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett 2013, 587, 1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeVine H 3rd, Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 1993, 2, 404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeVine H 3rd, Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 1999, 309, 274–84. [DOI] [PubMed] [Google Scholar]
- 8.Cloe AL; Orgel JP; Sachleben JR; Tycko R; Meredith SC, The Japanese mutant Aβ (ΔE22-Aβ(1–39)) forms fibrils instantaneously, with low-thioflavin T fluorescence: seeding of wild-type Aβ(1–40) into atypical fibrils by ΔE22-Aβ(1–39). Biochemistry 2011, 50, 2026–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe LS; Calabrese MF; Nath A; Blaho DV; Miranker AD; Xiong Y, Protein-induced photophysical changes to the amyloid indicator dye thioflavin T. Proc Natl Acad Sci U S A 2010, 107, 16863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong AG; Wu C; Hannaberry E; Watson MD; Shea JE; Raleigh DP, Analysis of the Amyloidogenic Potential of Pufferfish (Takifugu rubripes) Islet Amyloid Polypeptide Highlights the Limitations of Thioflavin-T Assays and the Difficulties in Defining Amyloidogenicity. Biochemistry 2016, 55, 510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabani M; Melki R, The Yarrowia lipolytica orthologs of Sup35p assemble into thioflavin T-negative amyloid fibrils. Biochem Biophys Res Commun 2020, 529, 533–539. [DOI] [PubMed] [Google Scholar]
- 12.Meng F; Marek P; Potter KJ; Verchere CB; Raleigh DP, Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril thioflavin-T interactions: implications for mechanistic studies of beta-cell death. Biochemistry 2008, 47, 6016–24. [DOI] [PubMed] [Google Scholar]
- 13.Akter R; Zhyvoloup A; Zheng B; Bhatia SR; Raleigh DP, The triphenylmethane dye brilliant blue G is only moderately effective at inhibiting amyloid formation by human amylin or at disaggregating amylin amyloid fibrils, but interferes with amyloid assays; Implications for inhibitor design. PLoS One 2019, 14, e0219130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arad E; Green H; Jelinek R; Rapaport H, Revisiting thioflavin T (ThT) fluorescence as a marker of protein fibrillation - The prominent role of electrostatic interactions. J Colloid Interface Sci 2020, 573, 87–95. [DOI] [PubMed] [Google Scholar]
- 15.Bolognesi B; Kumita JR; Barros TP; Esbjorner EK; Luheshi LM; Crowther DC; Wilson MR; Dobson CM; Favrin G; Yerbury JJ, ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol 2010, 5, 735–40. [DOI] [PubMed] [Google Scholar]
- 16.Mannini B; Mulvihill E; Sgromo C; Cascella R; Khodarahmi R; Ramazzotti M; Dobson CM; Cecchi C; Chiti F, Toxicity of protein oligomers is rationalized by a function combining size and surface hydrophobicity. ACS Chem Biol 2014, 9, 2309–17. [DOI] [PubMed] [Google Scholar]
- 17.Abedini A; Plesner A; Cao P; Ridgway Z; Zhang J; Tu LH; Middleton CT; Chao B; Sartori DJ; Meng F; Wang H; Wong AG; Zanni MT; Verchere CB; Raleigh DP; Schmidt AM, Time-resolved studies define the nature of toxic IAPP intermediates, providing insight for anti-amyloidosis therapeutics. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birks JB; Tripathi GNR; Lumb MD, The fluorescence of all-trans diphenyl polyenes. Chemical Physics 1978, 33, 185–194. [Google Scholar]
- 19.Dale RE; Chen LA; Brand L, Rotational relaxation of the “microviscosity” probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem 1977, 252, 7500–10. [PubMed] [Google Scholar]
- 20.Lentz BR, Membrane “fluidity” as detected by diphenylhexatriene probes. Chemistry and Physics of Lipids 1989, 50, 171–190. [Google Scholar]
- 21.Kaiser RD; London E, Location of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 1999, 38, 2610. [DOI] [PubMed] [Google Scholar]
- 22.Lentz BR, Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem Phys Lipids 1993, 64, 99–116. [DOI] [PubMed] [Google Scholar]
- 23.London E; Feligenson GW, A convenient and sensitive fluorescence assay for phospholipid vesicles using diphenylhexatriene. Anal Biochem 1978, 88, 203–11. [DOI] [PubMed] [Google Scholar]
- 24.Chattopadhyay A; London E, Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal Biochem 1984, 139, 408–12. [DOI] [PubMed] [Google Scholar]
- 25.Westermark P; Wernstedt C; Wilander E; Hayden DW; O’Brien TD; Johnson KH, Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A 1987, 84, 3881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper GJ; Willis AC; Clark A; Turner RC; Sim RB; Reid KB, Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A 1987, 84, 8628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westermark P; Andersson A; Westermark GT, Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 2011, 91, 795–826. [DOI] [PubMed] [Google Scholar]
- 28.Montane J; Klimek-Abercrombie A; Potter KJ; Westwell-Roper C; Bruce Verchere C, Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab 2012, 14 Suppl 3, 68–77. [DOI] [PubMed] [Google Scholar]
- 29.Cao P; Abedini A; Raleigh DP, Aggregation of islet amyloid polypeptide: from physical chemistry to cell biology. Curr Opin Struct Biol 2013, 23, 82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abedini A; Derk J; Schmidt AM, The receptor for advanced glycation endproducts is a mediator of toxicity by IAPP and other proteotoxic aggregates: Establishing and exploiting common ground for novel amyloidosis therapies. Protein Sci 2018, 27, 1166–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westermark GT; Westermark P; Berne C; Korsgren O, Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med 2008, 359, 977–9. [DOI] [PubMed] [Google Scholar]
- 32.Potter KJ; Abedini A; Marek P; Klimek AM; Butterworth S; Driscoll M; Baker R; Nilsson MR; Warnock GL; Oberholzer J; Bertera S; Trucco M; Korbutt GS; Fraser PE; Raleigh DP; Verchere CB, Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A 2010, 107, 4305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu LH; Raleigh DP, Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry 2013, 52, 333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luca S; Yau WM; Leapman R; Tycko R, Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry 2007, 46, 13505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makwana PK; Jethva PN; Roy I, Coumarin 6 and 1,6-diphenyl-1,3,5-hexatriene (DPH) as fluorescent probes to monitor protein aggregation. Analyst 2011, 136, 2161–7. [DOI] [PubMed] [Google Scholar]
- 36.Wiltzius JJ; Sievers SA; Sawaya MR; Cascio D; Popov D; Riekel C; Eisenberg D, Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin). Protein Sci 2008, 17, 1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Röder C; Kupreichyk T; Gremer L; Schäfer LU; Pothula KR; Ravelli RBG; Willbold D; Hoyer W; Schröder GF, Cryo-EM structure of islet amyloid polypeptide fibrils reveals similarities with amyloid-β fibrils. Nat Struct Mol Biol 2020, 27, 660–667. [DOI] [PubMed] [Google Scholar]
- 38.Gallardo R; Iadanza MG; Xu Y; Heath GR; Foster R; Radford SE; Ranson NA, Fibril structures of diabetes-related amylin variants reveal a basis for surface-templated assembly. Nat Struct Mol Biol 2020, 27, 1048–1056. [DOI] [PubMed] [Google Scholar]
- 39.Cao Q; Boyer DR; Sawaya MR; Ge P; Eisenberg DS, Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat Struct Mol Biol 2020, 27, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abedini A; Raleigh DP, Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation-prone polypeptide. Org Lett 2005, 7, 693–6. [DOI] [PubMed] [Google Scholar]
- 41.Marek P; Woys AM; Sutton K; Zanni MT; Raleigh DP, Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids. Org Lett 2010, 12, 4848–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abedini A; Singh G; Raleigh DP, Recovery and purification of highly aggregation-prone disulfide-containing peptides: application to islet amyloid polypeptide. Anal Biochem 2006, 351, 181–6. [DOI] [PubMed] [Google Scholar]
- 43.Humphrey W; Dalke A; Schulten K, VMD: Visual molecular dynamics. Journal of Molecular Graphics 1996, 14, 33–38. [DOI] [PubMed] [Google Scholar]
- 44.Case DA, Ben-Shalom KB,IY, Brozell SR, Cerutti DS, Cheatham TE III, Cruzeiro VWD, Darden TA, Duke RE, Giambasu G, Gilson MK, Gohlke H, Goetz AW, Harris R, Izadi S, Izmailov SA, Kasavajhala K, Kovalenko A, Krasny R, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Man V, Merz KM, Miao Y, Mikhailovskii O, Monard G, Nguyen H, Onufriev A, Pan F, Pantano S, Qi R, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shen J, Simmerling C, Skrynnikov NR, Smith J, Swails J, Walker RC, Wang J, Wilson L, Wolf RM, Wu X, Xiong Y, Xue Y, York DM and Kollman PA, AMBER 2020. University of California, San Francisco 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.