Abstract
Collagen type I is the most abundant form of collagen in human tissues, and is composed of two identical α-1 type I chains and an α-2 type I chain organized in a triple helical structure. A previous study has shown that human collagen α-2 type I (hCOL1A2) promotes collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs). However, the biological effects of human collagen α-1 type I (hCOL1A1) on various skin properties have not been investigated. Here, we isolate and identify the hCOL1A1-collagen effective domain (CED) which promotes collagen type I synthesis. Recombinant hCOL1A1-CED effectively induces cell proliferation and collagen biosynthesis in HDFs, as well as increased cell migration and elastin production. Based on these results, hCOL1A1-CED may be explored further for its potential use as a preventative agent against skin aging.
Keywords: Collagen synthesis, Elastin, hCOL1A1, Human dermal fibroblasts
INTRODUCTION
Skin aging is a common dermatological problem and is subject to intrinsic and extrinsic processes (1). Intrinsic skin aging is a physiological change, chronologically influenced by genetic and hormonal factors, whereas skin aging is extrinsically influenced by environment and lifestyle factors, such as chronic light exposure and chemicals (2). These factors incorporate histopathological and immunohistochemical alternations in each skin layer, such as changes in skin appearance after UV exposure (3-5). Furthermore, both intrinsic and extrinsic factors result to decreased levels of elastin and collagen synthesis in fibroblasts and increased melanin production in melanocytes (6-8).
Collagen and elastin are structural proteins in the skin that maintain both elasticity and firmness (9). Interestingly, the fibrous protein collagen constitutes the majority of the skin and contributes to maintain mechanical strength of tissues (10). The collagen molecule is composed of three chains wound together in a tight triple helix. Every third amino acid is glycine that fits perfectly inside the helix. The triple helix is mainly composed of glycine, followed by proline (or hydroxyproline), and then alanine residues (11). Fibroblasts that produce both collagen and the elastin matrix can be induced by glycolic acid, and these distinctive fibroblast cells are observed in the papillary level of the second layer of the dermis (9). Together, collagen has a specific amino acid composition, which is further associated with fibrogenesis.
Collagen type I, the most abundant type of collagen in humans, is a major structural protein dominant in the bone (more than 90% of the organic mass), tendons, ligaments, cornea, and many interstitial connective tissues (11). Different types of collagen vary in the primary amino acid sequences of their polypeptide chains. Structurally, type I collagen comprises two identical α-1 type I chains and an α-2 type I chain organized in a triple helix (11).
Migration of human dermal fibroblasts (HDFs) is essential for skin wound healing and remodeling. The proliferation of HDFs causes their migration into the wound bed, leading to expression of thick actin bundles in myofibroblasts and the synthesis of new extracellular matrix (ECM) (12). Previous studies have identified that growth factors/cytokines such as fibroblast growth factors (FGF), hepatocyte growth factor (HGF), transforming growth factor-β 1 (TGF-β1), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF) are directly or indirectly associated with HDF motility (12-14). The impact of these growth factors on HDFs covers a broad range of biological phenotypes, such as cellular proliferation, regeneration, and metabolism (15-17).
Our previous study has shown that human collagen alpha-2 type I (hCOL1A2) promotes collagen synthesis, elastin production, and wound healing in normal dermal fibroblasts (18). Our analysis has identified that the hCOL1A2-derived peptide promotes the improvement of skin properties compared with the effects of marine collagen. Although human collagen type I is composed of a triple helix of human collagen alpha-1 type I (hCOL1A1) wound together with hCOL1A2 (11), the effects of hCOL1A1 on skin properties have not been investigated. In this study, we found that hCOL1A1-derived proteins promote collagen synthesis, elastin production, and cell migration in HDFs and HaCaT keratinocytes.
RESULTS AND DISCUSSION
Identification of the efficiency of hCOL1A1 domains in collagen type I synthesis
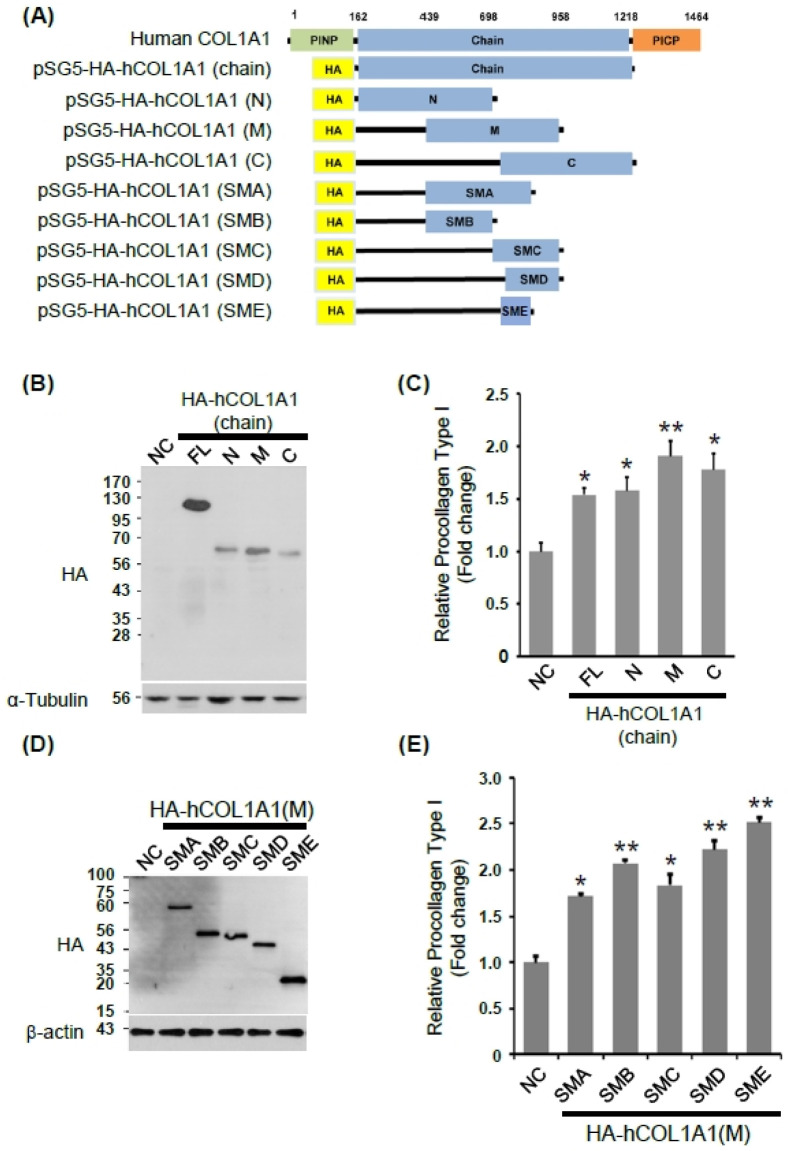
Previous studies have suggested that collagen type I synthesis and fibroblast cell proliferation are required to maintain the strength and elasticity of the skin (19-21). We have previously reported that hCOL1A2-derived proteins promote collagen type I synthesis, elastin production, and fibroblast cell proliferation in normal HDFs (18). To investigate the biological function of hCOL1A1—another major component of human collagen type I, in human skin fibroblasts—we examined the effects of hCOL1A1 domains on collagen type I synthesis in normal HDFs, similar to our previous study (18). Full-length hCOL1A1 and various deletion mutants were generated (Fig. 1A), and Normal HDFs were transfected with plasmids encoding hemagglutinin (HA) (negative control; NC), HA-tagged full-length COL1A1 (FL; chain domain), and the deletion mutants, namely of the N-terminal (N), middle (M), and C-terminal chain (C). The expression of hCOL1A1 FL and deletion mutants was analyzed by immunoblotting (Fig. 1B), and the levels of collagen type I were measured using an hCOL1A1 enzyme-linked immunosorbent assay (ELISA) kit. Interestingly, the M deletion mutant significantly induced collagen type I synthesis compared with the NC or other deletion mutants (Fig. 1C).
Fig. 1.
Identification of hCOL1A1 domain for the synthesis of collagen type I. (A) Schematic representing full-length hCOL1A1 and its various deletion mutants. (B and C) Human dermal fibroblasts (HDFs) were transfected with plasmids encoding hemagglutinin (HA), as the negative control (NC), HA-tagged full length hCOL1A2 (FL), and each of the deletion mutants (N-terminal chain [N], middle chain [M], or C-terminal chain [C]). Forty-eight hours after transfection, cells were collected and cell lysates were analyzed by immunoblotting with anti-HA and anti-tubulin antibodies (B). The amount of collagen type I in transfected HDF culture media was measured with an enzyme-linked immunosorbent assay (ELISA) kit (C). (D and E) HDF cells were transfected with plasmids encoding HA (NC) and each of the indicated mutants (small middle A [SMA], small middle B [SMB], small middle C [SMC], small middle D [SMD], or small middle E [SME]). Forty-eight hours after transfection, cells were collected and cell lysates were analyzed by immunoblotting with anti-HA and anti-tubulin antibodies (D). The amount of collagen type I was measured with an ELISA kit in a transfected HDF-cultured media (E). Results are presented as mean ± SD of three independent experiments. Student’s t-test was used for statistical analyses (*P < 0.05, **P < 0.005).
In order to narrow down the protein region in the M-domain critical for collagen type I synthesis, we further constructed several deletion mutants inside the M-domain. HDFs were transfected with plasmids encoding HA (NC) and the deletion mutants, namely small middle A (SMA), small middle B (SMB), small middle C (SMC), small middle D (SMD), and small middle E (SME). Cells were analyzed after 48 h of transfection using immunoblotting (Fig. 1D) and the amount of collagen type I was measured with an hCOL1A1 ELISA kit. The SME deletion mutant significantly increased collagen type I synthesis compared with the other deletion mutants; however, the other mutants (SMA, SMB, SMC, or SMD) were also capable of increasing collagen type I synthesis (Fig. 1E). Thus, the C-terminal region spanning amino acid residues 743-880 of SMA and overlapping with both SMC and SMD is the functional and effective region of hCOL1A1 that affects collagen type I synthesis. Then, the SME was further designated as hCOL1A1-collagen effective domain (hCOL1A1-CED).
Isolation and identification of recombinant hCOL1A1-CED protein
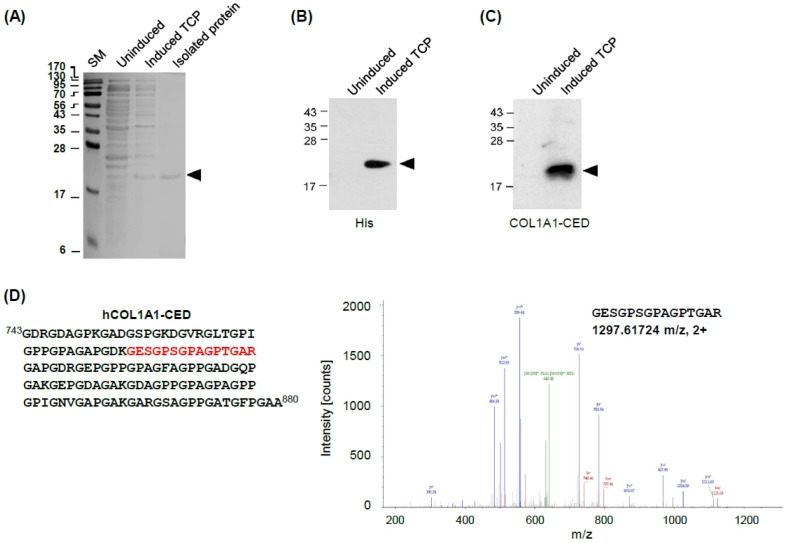
Next we cloned a histidine (His)-tagged hCOL1A1-CED into the pET-28a (His-tag) vector. Recombinant proteins were pro-duced by the Escherichia coli Rosetta2 (DE3) strain and purified as previously represented (22). To ensure the molecular composition ratio, purified hCOL1A1-CED was isolated and visualized as a single band of approximately 21 kDa by SDS-PAGE, as also shown the uninduced or induced TCP (total cell proteins) (Fig. 2A). Then, hCOL1A1-CED was confirmed using an anti-His antibody (Fig. 2B) as well as an anti-COL1A1 (CED-specific) antibody (Fig. 2C).
Fig. 2.
Identification of purified hCOL1A1-CED. (A) Coomassie Blue staining of uninduced (without IPTG), induced TCP (total cell protein), or isolated hCOL1A1-CED showed a prominent band; arrows indicate that hCOL1A1-CED was identified using (B) anti-His and (C) anti-hCOL1A1 antibodies by western blot analysis. (D) Identification of hCOL1A1-CED protein in the gel band of isolated hCOL1A1-CED by liquid chromatography-mass spectrometry. The panels show the representative MS/MS spectrum for the identified peptides of GESGPSGPAGPTGAR (1297.61724 m/z, 2+).
To further confirm the sequences of purified recombinant hCOL1A1-CED, we analyzed the peptides from in-gel digestion with trypsin using liquid chromatography-mass spectrometry (LC/MS), showing a representative MS/MS spectrum for the identified peptides originating from hCOL1A1 (781GESGPSGPAGPTGAR796, 1297.61724 m/z) in Fig. 2D and Supplementary Table 1.
Effects of recombinant hCOL1A1-CED protein on cell proliferation and collagen biosynthesis
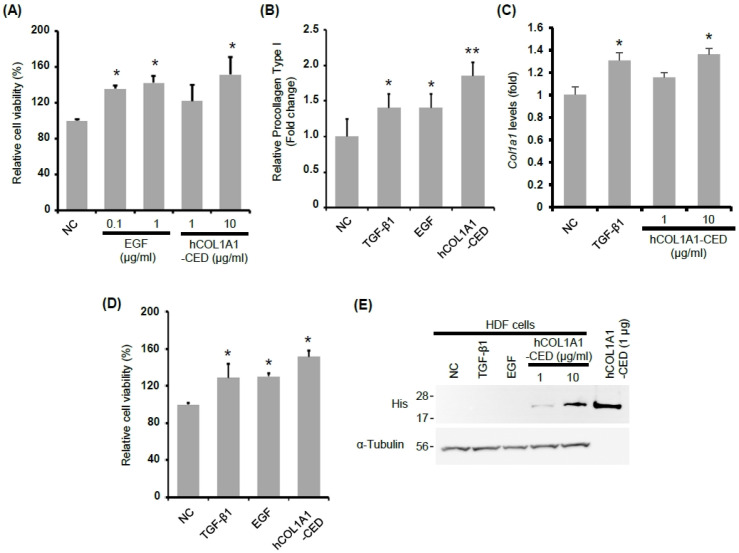
We compared the effects of hCOL1A1-CED and EGF on HDF cell proliferation, as these have previously been reported to promote cell proliferation (8, 18, 23). As shown in Fig. 3A, both hCOL1A1-CED protein (10 μg/ml) and EGF (1 μg/ml) showed similar HDF cell proliferation (Fig. 3A). TGF-β1 has been reported to significantly increase collagen type I synthesis and proliferation in both embryonic pulmonary fibroblasts and HDFs in a crowded cell culture system (8, 24). Therefore, to further investigate the effects of hCOL1A1-CED, we compared its effect with that of TGF-β1 or EGF on collagen synthesis. We determined the amount of collagen type I secreted in the culture media of HDFs stimulated with TGF-β1 (10 ng/ml), EGF (1 μg/ml), or hCOL1A1-CED (10 μg/ml). We found that hCOL1A1-CED (10 μg/ml), TGF-β1 (10 ng/ml) and EGF (1 μg/ml) showed similar increases in collagen type I synthesis induction, endogenous Col1a1 gene expression and fibroblast cell proliferation (Fig. 3B-D).
Fig. 3.
hCOL1A1-CED stimulates cell proliferation and collagen synthesis in HDFs. (A) HDF cells were treated with EGF (0.1 and 1 μg/ml) and hCOL1A1-CED (1 and 10 μg/ml) for 48 h. Cell proliferation, induced by EGF and hCOL1A1-CED, was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. (B and C) HDF cells were treated with EGF (1 μg/ml; 166.7 nM), hCOL1A1-CED (10 μg/ml; 840.9 nM), or TGF-β1 (10 ng/ml; 781.3 pM) for 48 h. The amount of collagen type I in the HDF culture media and mRNA levels of Col1a1 in HDF cells were measured by enzyme-linked immunosorbent assay (B) or qRT-PCR (C), respectively. Cell proliferation was determined by MTT assay (D). (E) HDF cells were treated with EGF (1 μg/ml), TGF-β1 (10 ng/ml), or hCOL1A1-CED (1 and 10 μg/ml), for 48 h. Cells were collected 48 h post-treatment and cell lysates were analyzed by immunoblotting with anti-His (upper panel) or anti-α-tubulin antibodies, respectively. Results are presented as the mean ± standard deviation of three independent experiments. Student’s t-test was used for statistical analyses (*P < 0.05, **P < 0.005).
Furthermore, hCOL1A1-CED was detectable in HDF cell lysates even after the culture medium treated with hCOL1A1-CED was washed out (Fig. 3E), suggesting that hCOL1A1-CED is bioactive, similar to other hydrolyzed collagen peptides, and can be absorbed and utilized by HDFs. Together, these results demonstrate that hCOL1A1-CED significantly increases collagen type I synthesis and fibroblast proliferation.
Effects of recombinant hCOL1A1-CED protein on migration and elastin production
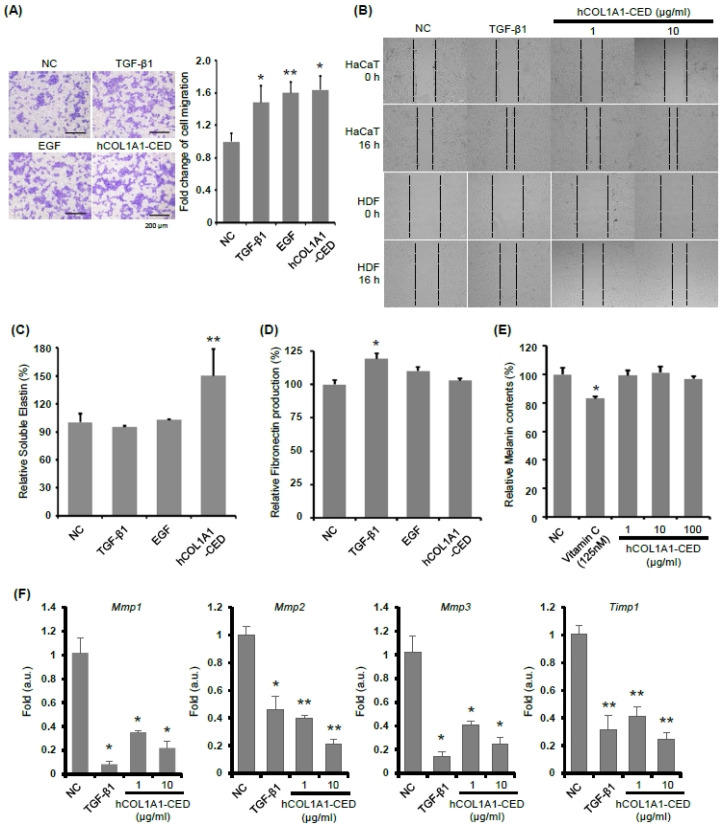
Previous studies have determined that the abundant amino acid residues in collagen peptides support cellular growth and proliferation (25). We further investigated the effects of hCOL 1A1-CED on cell adhesion and growth using the human keratinocyte cell line HaCaT, which is an in vitro model of skin wound healing (26). We tested the effects of TGF-β1, EGF, or hCOL1A1-CED in the transwell migration assay using HaCaT cell monolayers and the scratch-wound healing assay using HaCaT and HDF cells, and as shown in Fig. 4A, B, hCOL1A1-CED treatment significantly increased cell migration, compared with the NC.
Fig. 4.
hCOL1A1-derived protein increases the migration efficiency and elastin production, but not fibronectin production. (A) HaCaT cells were treated with TGF-β1 (10 ng/ml), EGF (1 μg/ml), or hCOL1A1-CED (10 μg/ml) and then subjected to transwell migration assays. Migration was monitored for up to 20 h. The same fields were photographed immediately after treatment and after 20 h, and images were superimposed using Adobe Photoshop. Areas were measured using Image J. A representative result is shown. (B) In vitro wound healing assay in HaCaT (top) or HDF (bottom) cells with TGFβ-1 (10 ng/ml) or hCOL1A1-CED at the indicated concen-tration. The cells were photographed immediately after wounding and 16 hours later, and the pictures were superimposed. A representative result is shown. (C and D) HDF cells were treated with TGF-β1 (10 ng/ml), EGF (1 μg/ml), and hCOL1A1-CED (10 μg/ml) for 48 h. The amount of elastin (C) and fibronectin (D) in the HDF culture media was measured using enzyme-linked immunosorbent assay. (E and F) Melanin contents in B16F10 melanoma cells (E) or expression of indicated genes in HDF cells (F) after treatment with TGFβ-1 or hCOL1A1. Results are presented as the mean ± standard deviation of three independent experiments. Student’s t-test was used for statistical analyses (*P < 0.05, **P < 0.005).
Previous studies have suggested that the skin aging is induced by intrinsic and extrinsic pathway, which are both accompanied with histopathological and immunohistochemical alternations (1). Intrinsic pathway of skin aging is a process of both the qualitative and quantitative changes, including diminished or defective collagen and elastin production in the dermis (27, 28). To evaluate the effect of hCOL1A1-CED on elastin synthesis, we analyzed elastin levels in the culture media of HDFs treated with TGF-β1 (10 ng/ml), EGF (1 μg/ml), or hCOL1A1-CED (10 μg/ml). Elastin synthesis significantly increased in the hCOL1A1-CED-treated group, compared with the TGF-β1 (10 ng/ml) and EGF (1 μg/ml)-treated groups and the NC group, whereas fibronectin synthesis was not affected by hCOL1A1-CED treatment (Fig. 4C, D), likely representing that the lack of binding affinity to fibronectin (29) does not speculate on fibronectin synthesis.
Next, to test whether hCOL1A1-CED is associated with skin aging, we further measured melanin synthesis. We observed that melanin synthesis was not altered by treatment with hCOL1A1-CED in B16F10 melanoma cells, whereas vitamin C treatment significantly reduced melanin synthesis (Fig. 4E). Additionally, the gene expression of matrix metalloproteinase 1 (Mmp1), Mmp2, Mmp3, or tissue inhibitor of metalloproteinase 1 (Timp1) was significantly downregulated by hCOL1A1-CED treatment in HDF cells (Fig. 4F), which is consistent with previous results that these changes are associated with aging and collagen degradation (30). Together, these results demonstrate that hCOL1A1-CED induces cell migration and elastin synthesis, but not fibronectin synthesis.
In conclusion, hCOL1A1-derived CED enhances collagen type I and elastin synthesis, cell proliferation as well as cell migration; both of these proteins are known to be effective anti-skin aging agents. Although the hCOL1A1-derived CED could induce anti-wrinkle effects, such as those facilitated by the migration and proliferation of fibroblasts, we should remain cognizant of its potential side effects when used as a cosmeceutical ingredient.
MATERIALS AND METHODS
(The detailed methods are described in the “Supplementary Materials and Methods”.)
Supplementary Materials
ACKNOWLEDGEMENTS
This work was supported by an INHA UNIVERSITY Research Grant to K.K., research funds for newly appointed professors of Jeonbuk National University to S.B.L., and National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) to K.K. (2020R1C1C1004015) and S.B.L. (2020R1C1C1014281).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immuno-histochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 2.Mancini M, Lena AM, Saintigny G, et al. MicroRNAs in human skin ageing. Ageing Res Rev. 2014;17:9–15. doi: 10.1016/j.arr.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308–319. doi: 10.4161/derm.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31:886–889. discussion 889. doi: 10.1111/j.1524-4725.2005.31736. [DOI] [PubMed] [Google Scholar]
- 5.Petit L, Pierard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169–181. doi: 10.1046/j.1467-2494.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Kang S, Kwon H, Moon H, Park MC. Dual functional bioactive-peptide, AIMP1-derived peptide (AdP), for anti-aging. J Cosmet Dermatol. 2019;18:251–257. doi: 10.1111/jocd.12671. [DOI] [PubMed] [Google Scholar]
- 8.Edgar S, Hopley B, Genovese L, Sibilla S, Laight D, Shute J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci Rep. 2018;8:10474. doi: 10.1038/s41598-018-28492-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krutmann J. [Skin aging/anti-aging strategies] Hautarzt. 2011;62:576. doi: 10.1007/s00105-011-2131-z. [DOI] [PubMed] [Google Scholar]
- 10.Gelse K, Poschl E, Aigner T. Collagens - structure, function, and biosynthesis. Advanced Drug Delivery Reviews. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 13.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–129. doi: 10.1016/S1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 14.Park JS, Kim JY, Cho JY, Kang JS, Yu YH. Epidermal growth factor (EGF) antagonizes transforming growth factor (TGF)-beta1-induced collagen lattice contraction by human skin fibroblasts. Biol Pharm Bull. 2000;23:1517–1520. doi: 10.1248/bpb.23.1517. [DOI] [PubMed] [Google Scholar]
- 15.Van Cauter E, Plat L. Physiology of growth hormone secretion during sleep. J Pediatr. 1996;128:S32–37. doi: 10.1016/S0022-3476(96)70008-2. [DOI] [PubMed] [Google Scholar]
- 16.Bartke A. Growth hormone and aging: a challenging controversy. Clin Interv Aging. 2008;3:659–665. doi: 10.2147/CIA.S3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SJ, Ha GH, Seo WY, Kim CK, Kim K, Lee SB. Human collagen alpha-2 type I stimulates collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs) BMB Rep. 2020;53:539–544. doi: 10.5483/BMBRep.2020.53.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trojanowska M, LeRoy EC, Eckes B, Krieg T. Pathogenesis of fibrosis: type 1 collagen and the skin. J Mol Med (Berl) 1998;76:266–274. doi: 10.1007/s001090050216. [DOI] [PubMed] [Google Scholar]
- 20.Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol. 1996;74:737–744. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- 21.Majora M, Wittkampf T, Schuermann B, et al. Functional consequences of mitochondrial DNA deletions in human skin fibroblasts: increased contractile strength in collagen lattices is due to oxidative stress-induced lysyl oxidase activity. Am J Pathol. 2009;175:1019–1029. doi: 10.2353/ajpath.2009.080832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo WY, Kim JH, Baek DS, et al. Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci Rep. 2017;7:15946. doi: 10.1038/s41598-017-16290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andl CD, Mizushima T, Nakagawa H, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal Keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 24.Chen CZ, Peng YX, Wang ZB, et al. The Scar-in-a-Jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br J Pharmacol. 2009;158:1196–1209. doi: 10.1111/j.1476-5381.2009.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z, Yang P, Zhou CX, Li SD, Hong PZ. Marine collagen peptides from the skin of nile tilapia (oreo-chromis niloticus): characterization and wound healing evaluation. Mar Drugs. 2017;15:102. doi: 10.3390/md15040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WE. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316:1271–1281. doi: 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Quan T, Shao Y, He T, Voorhees JJ, Fisher GJ. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 2010;130:415–424. doi: 10.1038/jid.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seite S, Zucchi H, Septier D, Igondjo-Tchen S, Senni K, Godeau G. Elastin changes during chrono-logical and photo-ageing: the important role of lysozyme. J Eur Acad Dermatol Venereol. 2006;20:980–987. doi: 10.1111/j.1468-3083.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 29.Erat MC, Slatter DA, Lowe ED, et al. Identification and structural analysis of type I collagen sites in complex with fibronectin fragments. Proc Natl Aca Sci U S A. 2009;106:4195–4200. doi: 10.1073/pnas.0812516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freitas-Rodriguez S, Folgueras AR, Lopez-Otin C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim Biophys Acta Mole Cell Res. 2017;1864:2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.