Abstract
The lymphatic vasculature plays important role in regulating fluid homeostasis, intestinal lipid absorption, and immune surveillance in humans. Malfunction of lymphatic vasculature leads to several human diseases. Understanding the fundamental mechanism in lymphatic vascular development not only expand our knowledge, but also provide a new therapeutic insight. Recently, Hippo-YAP/TAZ signaling pathway, a key mechanism of organ size and tissue homeostasis, has emerged as a critical player that regulate lymphatic specification, sprouting, and maturation. In this review, we discuss the mechanistic regulation and pathophysiological significant of Hippo pathway in lymphatic vascular development.
Keywords: Hippo signaling pathway, Lymphatic endothelial cells (LECs), Lymphatic vascular development, PROX1, TAZ, VEGF-C/VEGFR3, YAP
INTRODUCTION
In mammals, there exist complementary vascular networks. Blood vasculature delivers nutrients and oxygen to cells, and the lymphatic vasculature maintains fluid homeostasis by collecting and returning interstitial fluid into the bloodstream. Additionally, lymphatic vasculature regulates lipid absorption and immune response (1, 2). Dysfunction of lymphatic vessels is associated with several human diseases such as lymphedema, Alzheimer’s disease, cancer metastasis, obesity, atherosclerosis, and inflammatory diseases (3-10). A major part of the lymphatic vascular network is established during embryonic stages (2, 11). Three stepwise events regulate the lymphatic vasculature development: specification of lymphatic endothelial cell (LECs) progenitors, differentiation of LECs and formation of lymph sacs, and patterning and maturation of the lymphatic vessels. Different signaling pathways such as VEGFC-VEGFR3, NOTCH, BMP, WNT, PCP, G-protein-coupled receptors (GPCRs), ECM-integrin, and mechanotransduction signaling pathway regulate LEC identity, morphology, and behaviors via their downstream kinases, adaptor molecules, and transcriptional factors (1, 12-18).
The Hippo signaling is an evolutionarily conserved organ size-control mechanism and plays pivotal roles in maintaining tissue homeostasis by regulating cell proliferation, growth, and survival (19-22). In mammals, the central transcription factors of the Hippo pathway are Yes-associated protein (YAP) and its paralogue WW domain-containing transcription regulator (WWTR, hereinafter referred to as TAZ) (23, 24). In general, the phosphorylation status has been generally accepted as the most important regulatory mechanism for determining YAP/TAZ’s subcellular localization and transcriptional activity (25, 26). The Hippo signaling can be tightly controlled by a core kinase cascade consisting of the Ste-20 family of protein kinase MST1/2, the scaffolding protein Salvador (SAV), and large tumor suppressor kinase LATS1/2 (27-32). MOB kinase activator 1A/1B (MOB1A/1B) forms a complex with LATS1/2 kinases. MST1/2 activates MOB1A/1B and LATS1/2 by phosphorylation. The tumor suppressor NF2/Merlin associates with LATS1/2 and accelerates LATS1/2 phosphorylation through the MST1/2–SAV complex. In parallel to MST1/2, MAP4K family kinases can also directly phosphorylate and activate LATS1/2 (25, 33). The wide range of intrinsic or extrinsic signals regulate the Hippo pathway that precedes to the LATS1/2-mediated YAP/TAZ phosphorylation. As a result, YAP/TAZ are located in the cytoplasm through interaction with 14-3-3, and E3 ligase β-TrCP results in the proteasome-dependent YAP/TAZ degradation (34-37). In addition, growth factor signaling or cytoskeleton rearrangement inhibits YAP/TAZ phosphorylation by suppressing the Hippo pathway and enables YAP/TAZ to translocate into the nucleus. Then, YAP/TAZ associates with TEA domain family member 1-4 (TEAD1-4) and binds with several transcription factors SMADs, RUNXs, and p63/p73 to activate the transcriptional program involved in anti-apoptosis and cell proliferation (38, 39). Recently, several studies have identified Hippo-YAP/TAZ signaling components as novel players in lymphatic vascular development by regulating LEC specification, proliferation, and migration. Therefore, in this review, we provide the current findings concerning the Hippo-YAP/TAZ signaling pathway mediated regulation of lymphatic vessel formation and maturation.
YAP/TAZ EXPRESSION IN LYMPHATIC VASCULATURE
It has been reported that YAP/TAZ are dynamically expressed in blood vascular endothelial cells (BECs) during angiogenesis (40-42). Remarkably, TAZ expression has been reported to be higher in several types of BECs compared with YAP (42). YAP/TAZ are highly restricted in the nucleus of BECs between E10.5 to 11.5. However, YAP/TAZ are also found in the cytosol of most of the brain BECs at E14.5 (42). While YAP is mainly located in the cytoplasm in the migrating tip cells, TAZ is localized in the nuclei in the retinal blood vasculature at postnatal stage P5 (40, 41). In addition, YAP is detectable at cell-cell junctions in blood vessels of neonatal mice (43). Moreover, dynamic and differential YAP/TAZ expression patterns are observed during lymphatic vasculature development. In primary human LECs (hLECs), TAZ is expressed at a much higher level compared to YAP at the protein level. However, based on RNA-seq data, YAP expression is extremely high compared to TAZ (44). The mechanisms that regulate the post-transcriptional regulation of YAP/TAZ are still not known in LECs. TAZ is mainly located in the nuclei, but YAP is diffusely distributed in the LECs of the lymphatic plexus (45). In the newly forming lymphatic valve-endothelial cells (LV-ECs), TAZ is mainly localized in the cytoplasmic compartment at E16.5 (44, 45). However, TAZ is predominantly located in the nucleus of mature LV-EC after E17.5 (44, 46). In premature LVs CTGF and ANGPT2, the target genes of YAP/TAZ were not present, but high expression has been reported in mature LVs (44, 46). It has been proposed that YAP/TAZ have both distinct and redundant functions so they can compensate for each other in a context-dependent manner (47, 48). Single deletion of YAP or TAZ in LECs does not lead to any obvious developmental defect (44). Single deletion of TAZ leads to very mild lymphatic valve defect (45). On the contrary, genetic inactivation of both YAP/TAZ in LECs results in dramatic dilation of lymphatic vessels and structural LV deterioration (44, 45).
UPSTREAM SIGNALS REGULATING HIPPO-YAP/TAZ PATHWAY IN LECs
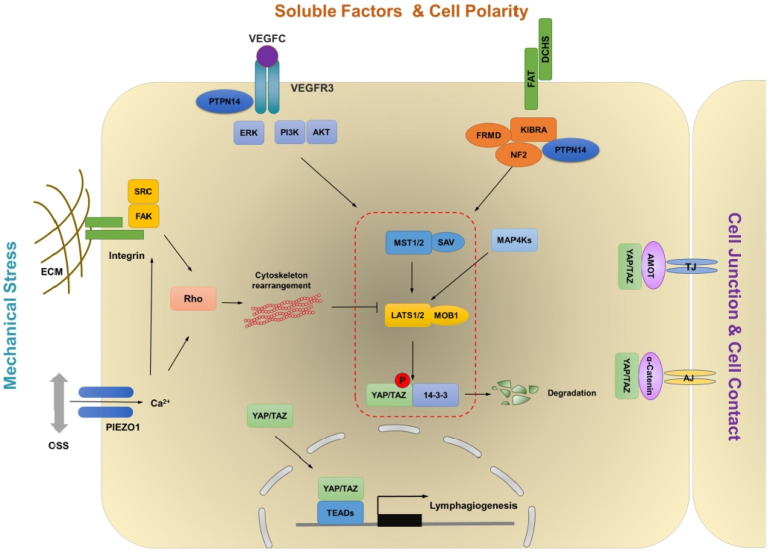
Over 20 years of research has firmly established that YAP/TAZ, the central players of the Hippo pathway, are the molecular determinants for organ size control (19). The multiple signaling pathways, such as mechanical stress, WNT, TGF-β, NOTCH, and VEGF, have been suggested to affect the growth-regulatory abilities of YAP/TAZ and interact with the Hippo pathway to coordinate numerous biological processes, indicating the significance of the signaling network (20). Here, we summarize the details of upstream signals that hold the potential to modulate the Hippo pathway in LECs (Fig. 1).
Fig. 1.
Schematic diagram of the pro-posed model of Hippo-YAP/TAZ signaling pathway in LECs. The Hippo pathway is a kinase cascade involving MST1/2 mediated activation of LATS1/2 and LATS1/2 mediated inactivation of YAP/TAZ through phosphorylation. Multiple stimulations including VEGF-C, mechanical stress, cell density, and cell polarity may suppress upstream kinases to activate YAP/TAZ transcriptional activity.
VEGF-C/VEGFR3 signal
While VEGF, a ligand of VEGFR2 is an essential factor for blood vessel development, VEGF-C is the major ligand that activates VEGFR3 for lymphatic vascular development (1, 49). VEGF-C/VEGFR3 interaction activates PI3K-AKT and PKC-ERK pathways to regulate LECs proliferation, survival, and migration (50). It has been reported that VEGF-C treatment increased phospho-LATS1 and facilitated cytoplasmic YAP whereas VEGFR3 knockdown promoted nuclear localization of YAP, thereby suggesting that VEGF-C activates the Hippo signaling to repress YAP/TAZ in hLECs (45). However, Hogan and colleagues suggested that Vegfc can promote nuclear Yap1 in a zebrafish model (51). In addition, VEGF-C decrease phospho-YAP and phospho-LATS1 in low confluent hLECs in vitro and YAP/TAZ activity is downregulated in Vegfc+/− embryos (44). The activation of VEGF/VEGFR2 signaling induces PI3K-AKT and MEK-ERK signaling pathways that lead to the inhibition of MST1/2 and LATS1/2 in (52). Although Vegfc activates Yap1 in zebrafish via ERK activation, the detailed molecular mechanisms downstream of VEGF-C/VEGFR3 in LECs are still unknown. Interestingly, KRAS/MAPK pathway which is triggered by VEGF-C/VEGFR3 can induce YAP expression during skin cancer progression (53). Overall, the current data suggest that YAP/TAZ are regulated by the VEGF-C/VEGFR3 signaling pathway in a high context- and cell type-specific manner.
Cell polarity and cell-cell contact
Elucidating the critical roles of apical–basal cell polarity in the regulation of the Hippo pathway provides insight to better understand the link between the cellular structural components and growth-regulatory mechanism. At the adherent junction, the FERM domain proteins Merlin (Mer) and Expanded (Ex) have been reported to connect the transmembrane proteins to the cytoskeleton. Mer and Ex genetically and functionally co-operate to mediate activation of LATS1/2 and consequent inhibition of YAP/TAZ (54). Moreover, the atypical cadherin Fat has emerged as an upstream regulator of Ex, which promotes its junctional localization and stability (55). However, the FAT4-DCHS1 signaling is essential for vertebral growth in YAP/TAZ independent manner (56). Mutations in FAT4 have been reported in Hennekam lymphangiectasialymphedema syndrome, features of which include lymphedema, lymphangiectasia, and mental retardation (57). Fat4 inactivation leads to dysmorphic lymphatic valves and impaired polarization of LECs in response to the flow. However, YAP/TAZ target genes are not affected by the loss of Fat4 (58). VANGL2 is also a core PCP component and YAP activity is reduced in the lung airways of Vangl2Lp embryos (59). Looptail embryos, Vangl2 mutants, possess lymphatic valve maturation defect (13). It is still unclear whether the PCP pathway coordinates with the Hippo pathway in the lymphatic vasculature.
The Hippo-YAP/TAZ pathway regulates several cellular processes in response to cell-cell contact. Cell-adherent molecules are the regulator of the Hippo pathway. In high cell density, the Hippo pathway is activated and LATS1/2 kinase activity is increased, thereby leading to YAP/TAZ phosphorylation (36). YAP activity could be regulated by VE-cadherin-mediated cell-cell contact in blood endothelial cells via PI3K-AKT (43, 60). We observed that YAP/TAZ activity was down-regulated in high cell density in in vitro hLECs, thereby indicating conservation of contact inhibition in LECs. During LV maturation, LV-ECs have discontinuous and low-density cell-cell junctions (13) and LV-ECs have high YAP/TAZ activity (44). Deletion of VE-cadherin from LECs leads to the up-regulation of YAP/TAZ activity in the dermal and mesenteric LECs (61). However, YAP/TAZ activity is downregulated in the intestinal LECs (61, 62).
Tyrosine phosphatase PTPN14 which is associated with Choanal Atresia-Lymphedema interacts with VEGFR3 and inhibits its downstream signaling cascade (63). PTPN14 also has an interesting relationship with the Hippo signaling pathway; it interacts with the Kibra and induced LATS1 activation to negatively regulate oncogenic YAP activity (64, 65). In addition, PTPN14 protein level has been reported to be elevated in response to an increase in cell density; the protein regulates nucleus-to-cytoplasm translocation of YAP in MCF10A cells (66).
Physical signal
Accumulating evidence has suggested YAP/TAZ as the central mechanosensor and mechanotransducer in response to several kinds of mechanical stresses including shear stress, stiffness, and cell geometry. These physical signals regulate the localization and activities of YAP/TAZ to coordinate complex organ architectures (21, 67, 68). BECs are constantly exposed to mechanical forces generated by blood flow which affects cell proliferation and morphogenesis. Laminar shear stress (LSS) inactivates YAP/TAZ, whereas oscillatory shear stress (OSS) stimulates YAP/TAZ activity in BECs (69, 70). However, a following report suggested that even LSS can transiently activate YAP in BECs (71), and flow patterns could control the localization of YAP (72). Lymph flow generates shear stress in lymphatic vessels; the stress has been reported as critical for lymphatic vascular development (14, 73). LSS can enhance the proliferation and sprouting of LECs through ORAI1 mediated calcium influx and inhibition of NOTCH1 (73). LSS also enhances VEGF-C signaling through unknown mechanisms (74). Ca2+ entry through the ORAI channel can inhibit YAP/TAZ human glioblastoma cell lines (75). In addition, OSS is critical for lymphatic valve formation (12, 46). Shear stress sensing molecules such as PIEZO1 and VE-cadherin regulate lymphatic valve development (61, 62, 76, 77). PIEZO1 activation elicits transient Ca2+ influx and positively regulates nuclear localization of YAP in neural stem cells and osteoblasts (78-80). OSS increases YAP/TAZ activity and promotes nuclear localization of YAP/TAZ in in vitro cultured hLECs (46).
Physical changes induced by extracellular stiffness induce cytoskeleton rearrangement through actin remodeling, which controls the Hippo pathway in response to the activity of Integrin, Rho-GTPase, or FAK-SRC (67, 68, 81, 82). In cultured hLECs, soft matrix inhibits YAP/TAZ but promotes nuclear accumulation of GATA2 (83). In human mammary epithelial cells stretched by fluid pressure, YAP/TAZ can be activated thereby resulting in the entry of cells into the proliferative S phase (84). Migrating LECs are mechanically stretched by interstitial fluid pressure thereby resulting in the swelling of the interstitium (85). Integrin β1, a key component of ECM stiffness dependent YAP/TAZ activation, is necessary for inducing response to mechanical stretch to enhance VEGF-C/VEGFR3 signaling during LECs migration (85).
G-protein-coupled receptors (GPCRs) signaling
Yu and colleagues have accomplished a conceptual development in the regulation of the Hippo-YAP/TAZ pathway and G-protein-coupled receptors (GPCRs), the largest group of membrane receptors. GPCRs function as a critical upstream regulator in the Hippo pathway and relay the extracellular signal to Hippo signaling components (86). Depending on the type of ligands, GPCRs activate different types of heterotrimeric G-protein, thereby causing differential regulation of the Hippo signaling. Lysophosphatidic acid (LPA) or sphingosine 1-phosphate (S1P) promotes YAP/TAZ activation through G12/13-dependent LATS1/2 inhibition while epinephrine or glucagon suppresses YAP/TAZ activation by Gs signaling (86). Adrenomedullin (AM) and its receptor complex, the G protein-coupled receptor CLR (calcitonin receptor-like receptor; Calcrl) and Ramp2, play critical roles in lymphatic development during embryogenesis and maintenance of normal lymphatic function in adults (15, 87). LPA, a positive regulator of YAP/TAZ activity, is essential for lymphatic vascular development (88, 89). PROX1 and LYVE1 expression are induced by LPA stimulation in BECs (90). Also, S1P promotes lymphangiogenesis by activating S1P receptor 1 (S1PR1) which couples stringently to the Gi protein (91) and S1PR1 signaling is active in mature and quiescent lymphatic vessels during development (74). Taken together, several studies suggest that GPCRs signaling pathway regulates LEC proliferation and migration, and determines lymphatic vessel integrity and permeability. Therefore, it will be worthy to explore the potential cross-talk between GPCR and Hippo-YAP/TAZ signaling pathway.
WNT signaling
The WNT/β-catenin signaling is a critical regulator that is involved in embryo development and tissue homeostasis. Abnormal regulation of WNT signaling causes diverse human diseases, including cancer and neurodegenerative disorders (92, 93). In the canonical WNT pathway, β-catenin is a major transcription factor activating WNT-responsive target gene expression. Without WNT stimulation, β-catenin is sequestered and phosphorylated by destruction complex containing Axin, APC, and GSK3β, followed by β-TrCP-mediated proteasomal degradation in the cytosol. In response to the WNT stimulus, accumulated β-catenin translocates into the nucleus and ultimately activates WNT transcriptional program (94). Interestingly, growing evidence has suggested that WNT and Hippo pathways integrate and converge in the multiple layers of signaling pathways to respond to physiological inputs or alterations (95). Indeed, TAZ is known to functionally mediate WNT signaling (96). Also, YAP/TAZ can be seized by β-catenin destruction complex via physical interaction with Axin (97). In addition to the canonical WNT pathway, noncanonical WNT ligands regulate YAP/TAZ activation via Gα12/13-Rho-LATS signaling (98). Both canonical and non-canonical WNT signaling are critical for lymphatic vascular development (12, 99, 100). It will be interesting to elucidate whether cross-talk between WNT-YAP/TAZ signaling is involved in this process.
NOTCH signaling
NOTCH signaling plays a central role in various biological processes and is activated by direct cell-cell communication between the NOTCH receptors and their ligands including Jagged and Delta-like. After the binding, the NOTCH receptor can be cleaved sequentially and converted into a NOTCH intracellular domain (NICD) that acts as a transcription factor to activate the NOTCH-responsive target gene (101, 102). YAP regulates expression of NOTCH receptors and their ligand Jagged1. In turn, NICD augments YAP/TAZ protein stability and creates a positive loop for tumor development (103, 104). NOTCH inhibits lymphatic development by repressing PROX1 expression (105). Genetic inactivation of Notch1 in LECs of mouse embryos leads to enlarge lymph sac and increase LEC populations (16, 106). However, inactivating the DLL4/NOTCH signaling using blocking antibodies leads to decline of lymphatic vessel density (107). Likewise, Dll4+/− mice have reduced lymphatic vascular density (74). The role of NOTCH in LECs is still not fully resolved. The molecular mechanism by which NOTCH pathway controls PROX1 is a key step that remains to be identified. Certainly, further work is necessitated to determine whether the NOTCH-NICD-YAP/TAZ positive feedback loop operates in the lymphatic vasculature.
HIPPO-YAP/TAZ PATHWAY IN LYMPHATIC VASCULAR DEVELOPMENT
Role of Hippo-YAP/TAZ signaling in the specification of lymphatic endothelial cell progenitors
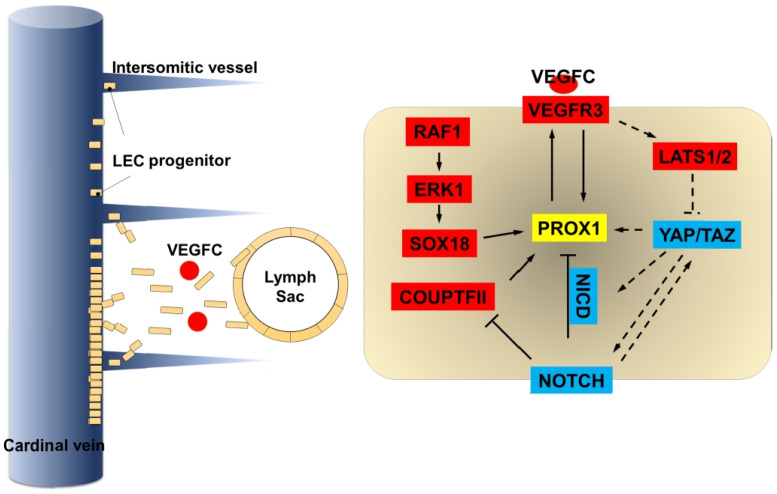
Most of the embryonic LEC progenitors derive from the cardinal veins (108-110). In mouse embryos, around E9.5, a unique group of venous endothelial cells starts to express PROX1 and becomes LEC progenitors. The homeobox transcription factor PROX1 not only controls LEC cell-fate determination but also maintains their identity (49, 109, 111, 112). It has been known that COUP-TFII (111) and SOX18 (113) are required to activate PROX1 expression by binding directly to the PROX1 promoter. On the other hand, NOTCH signaling inhibits PROX1 expression during LEC cell-fate specification (16, 106). A Positive feedback loop between PROX1-VEGFR3 is necessary for controlling the LEC specification and for preserving LEC identity (114, 115) (Fig. 2). Koh and colleagues for the first time reported the presence of YAP/TAZ in the cytoplasm of most of the LEC progenitors (45). Activation of YAP/TAZ in cultured hLECs leads to down-regulation of PROX1 while knocking down of YAP/TAZ increases PROX1 expression (45). Hyperactivation of YAP/TAZ using Prox1-CreERT2;Lats1f/f;Lats2f/f mouse demonstrated a reduction in the number of Prox1+ LECs in cardinal vein thereby indicating that Hippo signaling activates PROX1 expression and promotes LECs specification (45). However, Yap1 localization has been reported to be dynamically altered in parachordal LECs in zebrafish (51). Interestingly, yap1 mutants show the normal specification of lymphatic progenitors and yap1 is not necessary for specification in the zebrafish model (51).
Fig. 2.
Schematic representation of the molecular mechanisms controlling lymphatic specification and sprouting. PROX1 is a key transcription factor that drives lymphatic cell fate specification. Lymphatic endothelial progenitor cells expressing PROX1 arise from the cardinal veins (CV) and intersomitic veins. After specification, LECs migrate out from the CV in a VEGF-C/VEGFR3 dependent manner and form lymph sac. Hippo-YAP/TAZ signaling regulates PROX1 expression during LEC specification and sprouting.
Role of Hippo-YAP/TAZ signaling in lymphatic endothelial cell migration
In mouse embryos, around E10.5, LEC progenitors start to migrate out from the cardinal vein into mesenchyme as loosely connected spindle-shaped LECs. These LECs form lumenized lymphatic structure such as the lymph sac, the peripheral longitudinal lymphatic vessel, and the primordial thoracic duct around E11.5 (116, 117). Sprouting process of LECs is governed by VEGF-C/VEGFR3 in both mice and zebrafish (118-120) (Fig. 2). It appears that VEGF-C/VEGFR3 signaling represses YAP/TAZ activity via LATS1 phosphorylation in vitro (45). Hyperactivation of YAP/TAZ at E10.5 reduced Prox1 expression thereby leading to a decrease in lymph sac size (45). On the other hand, yap1 mutant showed abnormal cellular sprouting in zebrafish (51). Hogan and colleagues suggested that Vegfc promotes nuclear Yap1 with subsequent regulation of LECs proliferation (51). During migration, LECs are exposed to a soft ECM environment, thereby leading to a decrease in YAP/TAZ activity and activation of GATA2-dependent VEGFR3 expression (83). However, migrating LECs are mechanically stretched because of high interstitial fluid pressure (85). Integrin β1, a key component of ECM stiffness dependent YAP/TAZ activation, is necessary for responding to mechanical stretch to enhance VEGF-C/VEGFR-3 signaling during LECs migration (85).
Role of Hippo-YAP/TAZ signaling in dermal lymphatic vascular development
The first lymphatic vessels reach the skin from the jugular lymph sac around E12.5. Then, arising superficial lymphatic vessels on the lateral side of the embryo actively move towards until they reach the dorsal midline around E15.5-E16.5. (108, 121, 122). They show honeycomb-like structure in the plexus region and have actively sprouting tips in the migratory front region, reflecting a dynamic process in lymphatic vascular patterning. The molecular mechanisms regulating formation of the dermal lymphatic vasculature remain incompletely understood. However, PROX1, VEGFC, FOXC2, GATA2, and NRP2 are recognized to be necessary for the dermal lymphatic development (3, 118, 123-125). Koh and colleagues demonstrated that around E16.5, YAP/TAZ are very less expressed in the tip LECs but TAZ are nucleo-cytoplasmically located in LV-ECs in the plexus (45). Conditional deletion of YAP/TAZ in LECs from E11.5 causes enlarged, ballooned, and mispatterned lymphatic vessels with no lymphatic valves. Genetical inactivation of LATS1/2 blocks lymphatic sprouting and leads to the formation of dysmorphic lymphatic vessels (45). Lyve1-Cre;Yapf/f;Tazf/f embryos have defective lymphatic vasculature with dilated vessels, fewer branch points, and migration defect around E18.5 (44). Therefore, Hippo-YAP/TAZ signaling pathway is unquestionably essential for dermal lymphatic vascular development.
Role of Hippo-YAP/TAZ signaling in lymphovenous and lymphatic valve development
Lymph returns to the blood circulatory system particularly through four lymphovenous valves (LVVs) (126, 127). They start forming around E12 at the junction of the jugular and subclavian veins (128, 129). The development of LVV starts with the formation of two distinct cell populations. LECs from the lymph sacs and LVV-forming endothelial cells (LVV-ECs) from the veins interact to build the LVVs. LVV-ECs quickly aggregate again and invaginate into the vein to create valve leaflets around E12.5. Then, LVVs experience gradual maturation by assembling mural cells to the gap between the LVV-ECs between E14.5 to E16.5. The expression of PROX1, GATA2, and FOXC2 are increased in LVV-ECs and strong expression of VEGFR3 is remained in the LECs that create LVVs as well (128). YAP/TAZ and CTGF are almost absent in LVV-ECs between E12.0 to E14.5 but enriched around E16.5 (44). Consistent with the expression data, Lyve1-Cre;Yapf/f;Tazf/f embryos lack any obvious morphologic defects at E14.5 in LVVs. However, mutants with the complete absence of LVVs at E17.5 indicate that YAP/TAZ activity progressively augments during LVV maturation and YAP/TAZ are necessary for preserving LVV-ECs. We also found that YAP/TAZ positively control PROX1 expression in LVV-ECs (44).
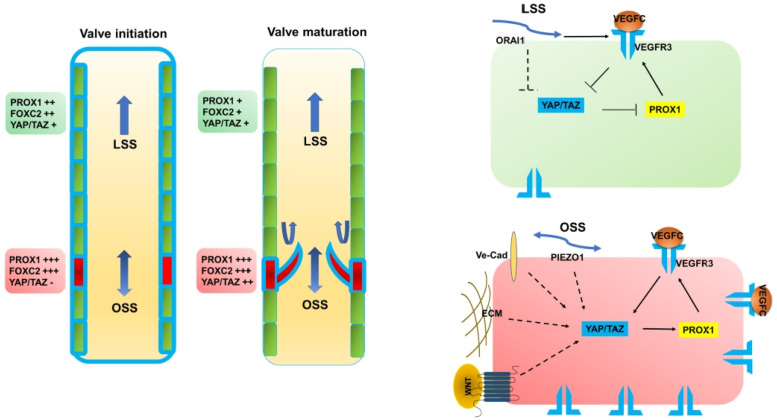
Skin and mesentery lymphatic valves (LVs) start developing around E15.5-E16.5 (18, 130). Differentiation of PROX1high, FOXC2high, and GATA2high LV-ECs is the opening stage of LV development (Fig. 3). OSS generated by lymph flow is one of the most critical factors for FOXC2 and GATA2 expression (14, 131) along with activation of NFATc1 (14) and Wnt/β-catenin signaling (12, 100). The highly elongated LV-ECs line up along the wall of lymphatic vessels at E16.5, and then they aligned perpendicular to lymph flow at E17.5. ECM molecules such as collagen IV, laminin-α5, fibronectin (FN)-EIIIA, and EMILIN1 are accumulated in between the LV-EC layers around E17.5 (14, 18, 130, 132). Next, the LV-EC layers stretch along the direction of the lymph flow to produce mature LV leaflets after E18.5 (129, 133). At E16.5, TAZ is mainly localized in the cytoplasm (45) in LV-Ecs; however, at the maturation stage, TAZ appears to be located in the nucleus (44, 46). E18.5 of Lyve1-Cre;Yapf/f;Tazf/f embryos show dilated lymphatic vessel with no LVs and immature phenotype with strong expression of LYVE1, VEGFR3, and PROX1 (44). While deletion of YAP/TAZ after birth using tamoxifen delivery system from P1 to P7 leads to a reduction in the LV number with high expression of FOXC2 and PROX1, hyperactivation of YAP/TAZ causes a decrease in LV number with low expression of PROX1 and Integrin-α9 (45). Therefore, a balance in YAP/TAZ activity is important for maintaining the lymphatic valve.
Fig. 3.
A proposed mechanism of Hippo-YAP/TAZ signaling pathway function in lymphatic valve (LV) formation. LVs develop in a step-wise manner. LV-ECs are specified in red and LECs are in green. Blue lines indicate VEGFR3. Briefly, during the initiation stage, OSS promotes high PROX1 and FOXC2 expression in LV-ECs. During the valve maturation stage, LV-ECs orient perpendicular to the flow and migrate into the vessel lumen. Then, LV-ECs elongate to form a bi-layered leaflet with a thick extracellular matrix (ECM). YAP/TAZ activities are gradually increased during the maturation stage, thereby indicating that Hippo YAP/TAZ signaling pathway is regulated by a multitude of signaling mechanisms for LV development.
CONCLUDING REMARKS
In summary, although our understanding of lymphatic vessel functions under physiological or pathological conditions has improved in the past decade, many questions remain unclear. Therefore, it has been considered that the identification of lymphangiogenic modulators and a clear understanding of the involved signaling pathways will provide opportunities to develop therapeutic targets for lymphatic diseases. Among the factors, PROX1 and VEGF-C/VEGFR3 signaling are the most critical regulators of lymphatic vascular development. The Hippo-YAP/TAZ signaling pathway has been established as a key mechanism of regulation of organ size and tissue homeostasis. Recent studies reveal that YAP/TAZ are the vital molecules of the PROX1/VEGFR3 feedback loop in LEC specification, migration, and LV maturation. Based on the current Hippo signaling pathway, it has been hypothesized that MAP4K family kinases acting in parallel to the MST1/2-SAV1 complex can phosphorylate LATS1/2 to inactivate YAP/TAZ in a context-dependent manner. We generated SAV1 conditional knockout mice using two different Cre lines (Lyve1-Cre;Sav1f/f and Tie2-Cre;Sav1f/f); the mutants showed no obvious phenotypes in the lymphatic vasculature. Interestingly, Czech and colleagues showed that mice constitutively lacking Map4k4 displayed LVs defect and lymphatic flow disorder with increased Prox1 expression (134). Besides VEGF-C and mechanical stress such as EMC stiffness and shear force, multiple signaling pathways are able to control the development of lymphatic vasculature. It must be elucidated whether YAP/TAZ could be regulated by these signaling pathways in a functionally related manner. Moreover, Hippo pathway molecules have been considered as therapeutic targets in several human diseases. Therefore, it is hypothesized that targeting those molecules will provide new therapeutic strategies to cure lymphatic disease in the future.
ACKNOWLEDGEMENTS
We sincerely apologize as we are unable to cite multiple key research papers due to space limitations. We thank Dr. R. Sathish Srinivasan for his insightful comments. This work is supported by the grant from the National Research Foundation of Korea (2020R1F1A1060680) to B. Cha (2020R1C1C100705011), S. Moon, and (2021R1A2C4001704) W. Kim.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 3.Harvey NL, inivasan RS, Sr, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich LC, Seidel CD, Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis. 2014;17:359–371. doi: 10.1007/s10456-013-9406-1. [DOI] [PubMed] [Google Scholar]
- 5.Lim K-C, Hosoya T, Brandt W, et al. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J Clin Invest. 2012;122:3705–3717. doi: 10.1172/JCI61619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martel C, Li W, Fulp B, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 8.Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiig H, Schröder A, Neuhofer W, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho YC, inivasan RS., Sr Lymphatic vasculature in energy homeostasis and obesity. Front Physiol. 2020;11:3. doi: 10.3389/fphys.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaahtomeri K, Karaman S, Mäkinen T, Alitalo K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev. 2017;31:1615–1634. doi: 10.1101/gad.303776.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha B, Geng X, Mahamud MR, et al. Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev. 2016;30:1454–1469. doi: 10.1101/gad.282400.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatin F, Taddei A, Weston A, et al. Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Dev Cell. 2013;26:31–44. doi: 10.1016/j.devcel.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabine A, Agalarov Y, Hajjami HM-E, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 15.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 16.Murtomaki A, Uh MK, Choi YK, et al. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development. 2013;140:2365–2376. doi: 10.1242/dev.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunworth WP, Cardona-Costa J, Bozkulak EC, et al. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ Res. 2014;114:56–66. doi: 10.1161/CIRCRESAHA.114.302452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazigou E, Xie S, Chen C, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim W, Jho EH. The history and regulatory mechanism of the Hippo pathway. BMB Rep. 2018;51:106–118. doi: 10.5483/BMBRep.2018.51.3.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra JR, Irvine KD. The Hippo signaling network and its biological functions. Annu Rev Genet. 2018;52:65–87. doi: 10.1146/annurev-genet-120417-031621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma S, Meng Z, Chen R, Guan K-L. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 22.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20:888–899. doi: 10.1038/s41556-018-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 25.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice RW, Woods ODF, Nol M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 28.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 29.Tapon N, Harvey KF, Bell DW, et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 30.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/S0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 31.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/S0092-8674(03)00549-X. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev Cell. 2019;50:264–282. doi: 10.1016/j.devcel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, Lv X, Liu C, et al. The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem. 2012;287:26245–26253. doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Kim YH, Kim J, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127:3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakabe M, Fan J, Odaka Y, et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc Natl Acad Sci U S A. 2017;114:10918–10923. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Valls AF, Schermann G, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell. 2017;42:462–478.e7. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Giampietro C, Disanza A, Bravi L, et al. The actin-binding protein EPS8 binds VE-cadherin and modulates YAP localization and signaling. J Cell Biol. 2015;211:1177–1192. doi: 10.1083/jcb.201501089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cha B, Ho YC, Geng X, et al. YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling. Development. 2020;147:dev195453. doi: 10.1242/dev.195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho H, Kim J, Ahn JH, et al. YAP and TAZ negatively regulate Prox1 during developmental and pathologic lymphangiogenesis. Circ Res. 2019;124:225–242. doi: 10.1161/CIRCRESAHA.118.313707. [DOI] [PubMed] [Google Scholar]
- 46.Sabine A, Bovay E, Demir CS, et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest. 2015;125:3861–3877. doi: 10.1172/JCI80454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plouffe SW, Lin KC, Moore JL, 3rd, et al. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J Biol Chem. 2018;293:11230–11240. doi: 10.1074/jbc.RA118.002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun C, Mello VD, Mohamed A, et al. Common and distinctive functions of the Hippo effectors Taz and Yap in skeletal muscle stem cell function. Stem Cells. 2017;35:1958–1972. doi: 10.1002/stem.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escobedo N, Oliver G. Lymphangiogenesis: origin, specification, and cell fate determination. Annu Rev Cell Dev Biol. 2016;32:677–691. doi: 10.1146/annurev-cellbio-111315-124944. [DOI] [PubMed] [Google Scholar]
- 50.Bui K, Hong YK. Ras pathways on Prox1 and lymphangiogenesis: insights for therapeutics. Front Cardiovasc Med. 2020;7:597374. doi: 10.3389/fcvm.2020.597374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimm L, Nakajima H, Chaudhury S, et al. Yap1 promotes sprouting and proliferation of lymphatic progenitors downstream of Vegfc in the zebrafish trunk. Elife. 2019;8:e42881. doi: 10.7554/eLife.42881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azad T, Rensburg HJJ, Lightbody ED, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Commun. 2018;9:1061. doi: 10.1038/s41467-018-03278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh YW, Cheng CC, Yang ST, et al. Targeting the VEGF-C/VEGFR3 axis suppresses Slug-mediated cancer metastasis and stemness via inhibition of KRAS/YAP1 signaling. Oncotarget. 2017;8:5603–5618. doi: 10.18632/oncotarget.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamaratoglu F, Willecke M, Kango-Singh M, et al. The tumour-suppressor genes NF2/Merlin and expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 55.Willecke M, Hamaratoglu F, Kango-Singh M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Kuta A, Mao Y, Martin T, et al. Fat4-Dchs1 signalling controls cell proliferation in developing vertebrae. Development. 2016;143:2367–2375. doi: 10.1242/dev.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alders M, Al-Gazali L, Cordeiro I, et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum Genet. 2014;133:1161–1167. doi: 10.1007/s00439-014-1456-y. [DOI] [PubMed] [Google Scholar]
- 58.Betterman KL, Sutton DL, Secker GA, et al. Atypical cadherin FAT4 orchestrates lymphatic endothelial cell polarity in response to flow. J Clin Invest. 2020;130:3315–3328. doi: 10.1172/JCI99027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheong SS, Akram KM, Matellan C, et al. The planar polarity component VANGL2 is a key regulator of mechanosignaling. Front Cell Dev Biol. 2020;8:577201. doi: 10.3389/fcell.2020.577201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi HJ, Zhang H, Park H, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 61.Hägerling R, Hoppe E, Dierkes C, et al. Distinct roles of VE-cadherin for development and maintenance of specific lymph vessel beds. EMBO J. 2018;37:e98271. doi: 10.15252/embj.201798271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Cha B, Motawe ZY, inivasan RS, Sr, Scallan JP. VE-Cadherin is required for lymphatic valve formation and maintenance. Cell Rep. 2019;28:2397–2412.e4. doi: 10.1016/j.celrep.2019.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Au AC, Hernandez PA, Lieber E, et al. Protein tyrosine phosphatase PTPN14 is a regulator of lymphatic function and choanal development in humans. Am J Hum Genet. 2010;87:436–444. doi: 10.1016/j.ajhg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Yang N, Figel SA, et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32:1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson KE, Li YW, Yang N, Shen H, Orillion AR, Zhang J. PTPN14 forms a complex with Kibra and LATS1 proteins and negatively regulates the YAP oncogenic function. J Biol Chem. 2014;289:23693–23700. doi: 10.1074/jbc.M113.534701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Huang J, Wang X, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 68.Panciera T, Azzolin L, Cordenonsi M, et al. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang KC, Yeh YT, Nguyen P, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A. 2016;113:11525–11530. doi: 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Luo JY, Li B, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 71.Nakajima H, Yamamoto K, Agarwala S, et al. Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev Cell. 2017;40:523–536.e6. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Nakajima H, Mochizuki N. Flow pattern-dependent endothelial cell responses through transcriptional regulation. Cell Cycle. 2017;16:1893–1901. doi: 10.1080/15384101.2017.1364324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi D, Park E, Jung E, et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J Clin Invest. 2017;127:1225–1240. doi: 10.1172/JCI87442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geng X, Yanagida K, Akwii RG, et al. S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight. 2020;5:e137652. doi: 10.1101/2020.02.27.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z, Wei Y, Zhang L, et al. Induction of store-operated calcium entry (SOCE) suppresses glioblastoma growth by inhibiting the Hippo pathway transcriptional coactivators YAP/TAZ. Oncogene. 2019;38:120–139. doi: 10.1038/s41388-018-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi D, Park E, Jung E, et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. 2019;4:e125068. doi: 10.1172/jci.insight.125068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nonomura K, Lukacs V, Sweet DT, et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci U S A. 2018;115:12817–12822. doi: 10.1073/pnas.1817070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pathak MM, Nourse JL, Tran T, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou T, Gao B, Fan Y, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-β-catenin. Elife. 2020;9:e52779. doi: 10.7554/eLife.52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 2020;11:282. doi: 10.1038/s41467-019-14146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215:445–456. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rausch V, Hansen CG. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48. doi: 10.1016/j.tcb.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Frye M, Taddei A, Dierkes C, et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun. 2018;9:1511. doi: 10.1038/s41467-018-03959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 85.Planas-Paz L, Strilić B, Goedecke A, Breier G, Fässler R, Lammert E. Mechanoinduction of lymph vessel expansion. EMBO J. 2012;31:788–804. doi: 10.1038/emboj.2011.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoopes SL, Willcockson HH, and Caron KM. Characteristics of multi-organ lymphangiectasia resulting from temporal deletion of calcitonin receptor-like receptor in adult mice. PLoS One. 2008;7:e45261. doi: 10.1371/journal.pone.0045261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee SJ, Chan TH, Chen TC, Liao BK, Hwang PP, Lee H. LPA1 is essential for lymphatic vessel development in zebrafish. FASEB J. 2008;22:3706–3715. doi: 10.1096/fj.08-106088. [DOI] [PubMed] [Google Scholar]
- 89.Sumida H, Noguchi K, Kihara Y, et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060–5070. doi: 10.1182/blood-2010-03-272443. [DOI] [PubMed] [Google Scholar]
- 90.Lin CI, Chen CN, Huang MT, et al. Lysophosphatidic acid up-regulates vascular endothelial growth factor-C and lymphatic marker expressions in human endothelial cells. Cell Mol Life Sci. 2008;65:2740–2751. doi: 10.1007/s00018-008-8314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoon CM, Hong BS, Moon HG, et al. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008;112:1129–1138. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 93.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 94.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 95.Kim M, Jho EH. Cross-talk between Wnt/beta-catenin and Hippo signaling pathways: a brief review. BMB Rep. 2014;47:540–545. doi: 10.5483/BMBRep.2014.47.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azzolin L, Zanconato F, Bresolin S, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 97.Azzolin L, Panciera T, Soligo S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 98.Park HW, Kim YC, Yu B, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lutze G, Haarmann A, Toukam JAD, Buttler K, Wilting J, Becker J. Non-canonical WNT-signaling controls differentiation of lymphatics and extension lymphangiogenesis via RAC and JNK signaling. Sci Rep. 2019;9:4739. doi: 10.1038/s41598-019-41299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cha B, Geng X, Mahamud MR, et al. Complementary Wnt sources regulate lymphatic vascular development via PROX1-dependent Wnt/β-catenin signaling. Cell Rep. 2018;25:571–584.e5. doi: 10.1016/j.celrep.2018.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majumder S, Crabtree JS, Golde TE, Minter LM, Osborne BA, Miele L. Targeting Notch in oncology: the path forward. Nat Rev Drug Discov. 2021;20:125–144. doi: 10.1038/s41573-020-00091-3. [DOI] [PubMed] [Google Scholar]
- 102.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 103.Tschaharganeh DF, Chen X, Latzko P, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137–152. doi: 10.1172/JCI88486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang J, Yoo J, Lee S, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–150. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fatima A, Culver A, Culver F, et al. Murine Notch1 is required for lymphatic vascular morphogenesis during development. Dev Dyn. 2014;243:957–964. doi: 10.1002/dvdy.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niessen K, Zhang G, Ridgway JB, et al. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood. 2011;118:1989–1997. doi: 10.1182/blood-2010-11-319129. [DOI] [PubMed] [Google Scholar]
- 108.inivasan RS, Sr, Dillard ME, Lagutin OV, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/S0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 110.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 111.inivasan RS, Sr, Geng X, Yang Y, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 113.François M, Caprini A, Hosking B, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 114.inivasan RS, Sr, Escobedo N, Yang Y, et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 2014;28:2175–2187. doi: 10.1101/gad.216226.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koltowska K, Lagendijk AK, Pichol-Thievend C, et al. Vegfc regulates bipotential precursor division and Prox1 expression to promote lymphatic identity in zebrafish. Cell Rep. 2015;13:1828–1841. doi: 10.1016/j.celrep.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 116.Yang Y, García-Verdugo JM, Soriano-Navarro M, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–2348. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hägerling R, Pollmann C, Andreas M, et al. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32:629–644. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 119.Semo J, Nicenboim J, Yaniv K. Development of the lymphatic system: new questions and paradigms. Development. 2016;143:924–935. doi: 10.1242/dev.132431. [DOI] [PubMed] [Google Scholar]
- 120.Chen H, Griffin C, Xia L, inivasan RS., Sr Molecular and cellular mechanisms of lymphatic vascular maturation. Microvasc Res. 2014;96:16–22. doi: 10.1016/j.mvr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinez-Corral I, Ulvmar MH, Stanczuk L, et al. Nonvenous origin of dermal lymphatic vasculature. Circ Res. 2015;116:1649–1654. doi: 10.1161/CIRCRESAHA.116.306170. [DOI] [PubMed] [Google Scholar]
- 122.James JM, Nalbandian A, Mukouyama YS. TGFβ signaling is required for sprouting lymphangiogenesis during lymphatic network development in the skin. Development. 2013;140:3903–3914. doi: 10.1242/dev.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petrova TV, Karpanen T, Norrmén C, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 124.Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 125.Mahamud MR, Geng X, Ho YC, et al. GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126. Development. 2019;146:dev184218. doi: 10.1242/dev.184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.inivasan RS, Sr, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 128.Geng X, Cha B, Mahamud MR, et al. Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev Biol. 2016;409:218–233. doi: 10.1016/j.ydbio.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Geng X, Cha B, Mahamud MR, inivasan RS., Sr Intraluminal valves: development, function and disease. Dis Model Mech. 2017;10:1273–1287. doi: 10.1242/dmm.030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Norrmén C, Ivanov KI, Cheng J, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kazenwadel J, Betterman KL, Chong CE, et al. GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest. 2015;125:2979–2994. doi: 10.1172/JCI78888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Danussi C, Belluz LDB, Pivetta E, et al. EMILIN1/α9β1 integrin interaction is crucial in lymphatic valve formation and maintenance. Mol Cell Biol. 2013;33:4381–4394. doi: 10.1128/MCB.00872-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bazigou E, Wilson JT, Moore JE., Jr Primary and secondary lymphatic valve development: molecular, functional and mechanical insights. Microvasc Res. 2014;96:38–45. doi: 10.1016/j.mvr.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roth Flach RJ, Guo CA, Danai LV, et al. Endothelial mitogen-activated protein kinase kinase kinase kinase 4 is critical for lymphatic vascular development and function. Mol Cell Biol. 2016;36:1740–1749. doi: 10.1128/MCB.01121-15. [DOI] [PMC free article] [PubMed] [Google Scholar]