Abstract
BACKGROUND:
The coronavirus, which is caused by acute respiratory syndrome, appeared in Wuhan, China, in December 2019 and gradually spread around the world until almost all countries became infected with the coronavirus. In Iran, the outbreak of coronavirus began on February 21, 2020, with the report of infection of two people in the city of Qom. The aim of this study is to evaluate the clinical findings of neonates born to pregnant women with corona disease.
MATERIALS AND METHODS:
During this case study (February 21 to November 30, 2020), out of 88 pregnant mothers who referred to the hospitals of Shahid Beheshti University of Medical Sciences, 44 live neonates were born from 42 pregnant women with COVID-19, who were evaluated for clinical signs by studying their files and reported as a case series, due to limited samples, No statistical analysis of the study was performed.
RESULTS:
In studies of clinical records of hospitalized mothers and infants, among the polymerase chain reactions (PCRs) provided for all infants, one PCR was reported positive 2 days after birth, whereas this infant 10 min after birth, immediately after routine procedures, due to positive mother's PCR was isolated from the operating room. However, all of the infant's clinical symptoms were normal during the 3-day hospital stay for routine postpartum care. Twenty-eight days after birth, the baby was reevaluated for clinical, laboratory, and chest X-ray symptoms, all of which were normal. The PCR of other neonates was negative, and five intubated neonates, two twin, and two single died, and the other neonates were discharged. In evaluating the clinical records of mothers of these infants, the mean age is 30 years, and the average gestational age is 35 weeks, 32 cases of caesarean section, and 10 cases of normal delivery.
CONCLUSION:
We describe epidemiological data, demographics, signs and symptoms on admission, laboratory results, comorbidities, infection COVID-19 in the mothers and neonates, chest radiography and computed tomography findings, treatment received for COVID-19, and clinical maternal, fetal, and neonatal outcomes. Due to the fact that the study population is small consist of 42 mothers with COVID-19 infection, among all PCR samples from infants born to COVID-19 positive mothers, the PCR result of one case was positive, and the rest of was negative. Therefore, vertical transmission of COVID-19 through the placenta to the fetus cannot be confirmed or denied, nor can the COVID-19 confirmed or denied the baby's postnatal complication during pregnancy.
Keywords: Coronavirus, COVID-19, infant, pregnancy
Introduction
Coronaviruses are large groups of viruses that can infect animals and humans and cause respiratory problems. These problems can be as mild as a cold or as severe as pneumonia. COVID-19 is a type of coronavirus that was first identified in Wuhan, Hubei Province, China in late 2019, where it has become widespread and has become a global epidemic. Complications of this new type of corona in patients may include fever, chills, and runny nose. Shortness of breath and more severe cases of this type of infection can cause severe and acute respiratory problems.[1] Due to the coronavirus epidemic, pregnant mothers due to physiological, anatomical, and hormonal changes during pregnancy and the susceptibility of pregnant women to infection various diseases, this study is to find the relationship between coronavirus disease in the mother and complication's Clinically in mother and infant. On the other hand, mechanical and biochemical factors affect gas exchange and pulmonary function during pregnancy and reduce functional residual capacity and residual volume during pregnancy. For this reason, there are concerns about the serious consequences of the coronavirus epidemic for pregnant women[2] and its effect on the infant.[3] In pregnant women with COVID-19, hypertensive disorders and diabetes are common comorbidities is a risk of preterm delivery and maternal death, among the neonates born to mothers with COVID-19, respiratory distress syndrome, and pneumonia are common occurrence, there are reports of still births and neonatal deaths also there is an evidence of vertical transmission of SARS-COV-2 infection in women with COVID-19.[4] Therefore, appropriate prevention and treatment used by the general public may not be effective and appropriate for pregnant women.[5] As a result of the transmission of the disease from an infected mother to the infant, we have examined that, because the incidence of COVID-19 is increasing in pregnant women. According to a case study by Wang et al., in December 2019, on a mother with COVID-19, the clinical data of this infection in infants are very limited, and the vertical transmission of the infection from mother to fetus through the placenta is unclear, so all suspected mothers and their infants should have diagnostic tests for this infection.[6] According to a study by Yu et al. In December 2019 on 7 pregnant women with COVID-19, three of whom were primiparous and four were multiparous; the clinical and gynecological characteristics and outcomes of their infants were studied. In the evaluation, all of these mothers were treated and discharged from the hospital, and all their clinical symptoms improved. The infants born to these mothers had Apgar and normal weight, four infants were cared for at home and a diagnostic test (COVID-19) was not performed. They did not have any clinical signs of high fever or other symptoms during follow-up 28 days after birth. Moreover, three other neonates were cared for in the neonatal ward in hospital and were sent for them a diagnostic nucleic acid test. One of the three neonates was tested positive for nucleic acid, and the other two were negative. After admission, these neonates did not have fever and cough but symptoms of brief shortness of breath was reported doing chest X-ray (CXR) revealed a brief pulmonary infection. These infants were reported healthy 28 days after birth and showed no signs of breathing problems or fever.[7] Currently, there is no clear evidence regarding optimal delivery timing, the safety of vaginal delivery, or whether cesarean delivery prevents vertical transmission at the time of delivery; therefore, route of delivery and delivery timing should be individualized based on obstetrical indications and maternal–fetal status.[8] Under the premise of full evaluation of vaginal delivery conditions and strict protection measures, pregnant women with ordinary type COVID-19 can try vaginal delivery without exacerbation of COVID-19 and without increasing the risk of SARS-CoV-2 infection in neonates.[9] During this study (February 21 to November 30, 2020), out of 88 pregnant mothers who referred to the hospitals of Tehran Shahid Beheshti University of Medical Sciences, 44 live neonates were born from 42 pregnant women with COVID-19, who were evaluated for clinical signs by studying their files. There is currently no definitive information on the epidemiology and clinical features of COVID-19-induced pneumonia and its treatment experience in pregnancy. The aim of this study is to describe the epidemiological features, clinical, laboratory, radiological, and treatment outcomes of neonates born to pregnant women with COVID-19. In this study, maternal and neonatal demographic factors including maternal age, number of pregnancies, gestational age, multiple births, sex, neonatal weight, and duration of maternal coronavirus infection until delivery and clinical signs of mother and fetus including fever, lethargy, headache, tachypnea, tachycardia, respiratory distress, fetal distress, placental abruption, PPROM, preterm labor, and history of underlying diseases in the mother including preeclampsia, hypothyroidism, RH negative and receiving Rhogam, thrombocytopenia, liver disease, diabetes and drug history, infertility and infertility treatment (IVF), three medications for pre- and post-partum coronavirus infection, receiving betamethasone during pregnancy, and any history of surgery in the mother and type of delivery, and any drug treatment in the newborn including resuscitation at birth, receiving surfactant, receiving epinephrine, antibiotic treatment, phototherapy, receiving blood and blood products, oxygen therapy, intubation, as well as diagnostic procedures in mother and infant including polymerase chain reaction (PCR) test, complete blood count (CBC) tests/erythrocyte sedimentation rate (ESR)/C-reactive protein (CRP), and Arterial Blood Gas (ABG), Venous Blood Gas (VBG) cord blood samples, and paraclinical procedures including computed tomography (CT) scan, CXR, and ultrasound.
Materials and Methods
Study sampling
This retrospective study with the participation of 44 live neonates were born from 42 pregnant women with COVID-19, who were evaluated for clinical signs by studying their files and reported as a case series was conducted from February 21 to November 30, 2020. Our Sample size was all pregnant women with COVID-19 who were hospitalized by studying their clinical records, in during this study, That were randomly selected and in terms of clinical features, treatment and neonatal outcomes, epidemiological factors, demographics, clinical signs, laboratory, radiography, delivery method, and maternal medical records were evaluated by studying the clinical records of mothers and infants. Newborns born to mothers with coronavirus immediately after birth they were transferred to the neonatal ward in isolation to evaluation clinical signs and perform a PCR test to confirm or rule out COVID-19 infection. During this study, out of 88 pregnant mothers who referred to the hospitals of Shahid Beheshti University of Medical Sciences in Tehran, 44 live neonates were born to 42 pregnant women with COVID-19 who were evaluated for clinical symptoms and reported. data of 42 pregnant women and their neonates was completed in researcher-made questionnaire after confirming informed consent form.also This retrospective study was approved by the Shahid Beheshti University of Medical Sciences by ethical committee (IR.SBMU.RETECH.REC.1399.663).
Data analysis
Due to limited samples, no statistical analysis of the study was performed. Results of clinical features of pregnant women with COVID-19, information on their newborns and their Pathogen identification results was reported directly.
Results
In evaluation of clinical outcomes of neonates born to mothers with coronavirus (COVID-19) in Shahid Beheshti hospitals, clinical features of prenatal mothers with COVID-19 showed that the symptoms of COVID-19 involvement were present before delivery and the duration of symptoms until delivery varied from 2 days to 3 weeks, and mothers underwent azithromycin, oral oseltamivir, hydroxychloroquine, and ceftriaxone were injected. Drug therapy was started before delivery and continued after delivery. After diagnosis by laboratory methods (CBC, ESR, CRP, and PCR) and paraclinical (CT and CXR), these women showed mild-to- severe symptoms of coronavirus disease, which led to the hospitalization of six patients in the intensive care unit. Among pregnant women with coronavirus disease, 32 had cesarean section and 10 had normal delivery, of which two mothers died and 40 were treated and discharged. Infants born to pregnant women showed the following symptoms: one case of placental abruption, 6 case of fetal distress, one case of Intra Utrine Growth Restrition (IUGR), 7 cases of ruptured amniotic fluid, one case of abnormal amniotic fluid (meconium), five cases of respiratory distress, and one case organ cyanosis, 13 cases of Apgar 8 and below 8, two of which died immediately after birth and three during hospitalization.
Discussion
According to a study by Schwartz in April 2020, which analyzed 38 pregnant women with (COVID-19) and their newborns and methods of transmitting the infection and their pregnancy outcome, the presence of this infection in one of the infants 17 days later from birth and in another infant 36 h after delivery. It was confirmed that, in both cases, there was no direct evidence that the transfer was vertical.[10] According to a February 2020 study by Zhu et al., on 10 infants (one twins) out of nine mothers with COVID-19 factors such as gestational age, the 1st and 5th min Apgar scores, sex, birth weight, singleton or multiple births, AGA (suitable infant For reproductive age), primary symptoms, other secondary symptoms, blood product intake, and its complications were evaluated. Therefore, according to this study, in infants of corona-positive mothers may have symptoms such as: fetal distress/respiratory distress/preterm labor/thrombocytopenia.[11] According to the study of Karimi-Zarchi et al., in April 2020 on three infants born to mothers with COVID-19, there has been limited evidence for vertical transmission (COVID-19) from mothers with fetuses.[12] According to a study by Lu and Shi conceived this review, COVID-19 can result in asymptomatic to severe illness; fortunately, children without underlying diseases appeared to have mild disease. The disease condition of the neonates was also minor. Although this new virus comes out without specific antiviral drugs treatment, neonatologist needs to more virological, epidemiological, and clinical data to treat and manage COVID-19.[13] The first published paediatric studies with data of case series in China described an incidence in children ranging from 0.8% to 2% of the total reported cases, with a milder disease course compared to adults and a predominance of respiratory symptoms.[14] To a study by Liu et al., the aim of this study was to investigate the clinical characteristics of neonates born to SARS-CoV-2-infected mothers and increase the current knowledge on the perinatal consequences of COVID-19. Nineteen neonates were admitted to Tongji Hospital from January 31 to February 29, 2020. Their mothers were clinically diagnosed or laboratory confirmed with COVID-19. There are 19 neonates included in the research. Among them, ten mothers were confirmed COVID-19 by positive SARS-CoV-2 RT-PCR in throat swab, and 9 mothers were clinically diagnosed with COVID-19. Delivery occurred in an isolation room, and neonates were immediately separated from the mothers and isolated for at least 14 days. No fetal distress was found. SARS-CoV-2 RT-PCR in throat swab, urine, and faces of all neonates were negative. The delivery should occur in isolation, and neonates should be separated from the infected mothers and caregivers.[15] The risk of vertical and perinatal transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, which causes COVID-19), the most appropriate management, and the neonate's risk of developing COVID-19 during the perinatal period are unknown.[16] Person-to-person transmission of SARS-CoV-2 has been confirmed by epidemiological studies of cases associated with COVID-19.[17] However, a previous study showed that there were no clinical findings suggestive of COVID-19 in 19 neonates born to infected mothers, and all samples including nasopharyngeal and rectal swabs from neonates, amniotic fluid, cord blood, and breastmilk were detected negative for SARS-CoV-2.[11,18] According to a study by Karimi-Zarchi, a total of 31 infected pregnant mothers with COVID-19 were reported. No COVID-19 infection was detected in their neonates or placentas.[12] Therefore, the criterion may need to be revised based on the available data. Currently, no evidences have shown that the SARS-CoV-2 can be transmitted from neonates to other neonates or to caregivers or health-care workers. Importantly, as the possibility of fecal–oral transmission exists, the appropriate education should be offered to parents with respect to hand hygiene and disinfection of children's excreta at home.[19] The most common manifestations of COVID-19 consist of fever, cough, and fatigue or myalgia, sputum production, and headache.[20,21] As COVID-19 virus is still spreading, more infections in pregnant women are likely to be seen. Whether COVID-19 increases, the risk of miscarriage, stillbirth, preterm delivery, fetal tachycardia, and fetal distress is unknown. According to the official website of the Ministry of Health and Medical Education in Mazandaran and Zanjan provinces, Iran, three infants were born from infected pregnant mothers. Among these three cases of COVID-19 infection, there were two mothers who developed acute respiratory distress syndrome after delivery and died. According to the websites, their neonates were negative when tested for COVID-19.[22] Limited information is available on pregnant women with COVID-19, but pregnant women may be more susceptible to viral respiratory infections including COVID-19, due to immunological and physiological changes. Therefore, pregnant women should take routine preventive measures, such as washing their hands frequently and avoiding contact with infected people, to prevent infection.[23] On the other hand, the optimal management strategy to prevent thrombosis in critically-ill patients with COVID-19 remains unknown.[24]
Results of clinical features of pregnant women with COVID-19
Mothers infected with the coronavirus had a mean age of 30 years (17–48) with a gestational age of 25–40 weeks and 1 day. Ten cases of labor pain, one case of vaginal bleeding (placental abruption), nine case of fever, five cases of premature rupture of the amniotic sac, one case of meconium tics, 42 cases of singleton, and two case of twins, 32 cases of cesarean section, and ten cases of normal delivery, one case of history infertility, and the use of assisted reproductive techniques, received four cases of preeclampsia. Two cases of glass addiction, three cases of hypothyroidism, one case of fatty liver, two cases of received blood products, six cases of hospitalization in intensive care unit, two cases of negative blood group with receive Rhogam, one deaths, 41 were treated and discharged, one was a heart problem, and four were intubations [Table 1].
Table 1.
Mothers information
| Case | Age | First symptom | Delivery mode | Outcome |
|---|---|---|---|---|
| 1 | 35 | Cough, tachypnea | C/S | Cured |
| 2 | 32 | Cough, tachypnea | C/S | Cured |
| 3 | 23 | Cough, tachypnea | C/S | Cured |
| 4 | 32 | Cough, tachypnea | C/S | Cured |
| 5 | 26 | Cough, tachypnea | C/S | Cured |
| 6 | 20 | Cough, tachypnea | NVD | Cured |
| 7 | 41 | Cough, tachypnea | C/S | Died |
| 8 | 32 | Cough, tachypnea | NVD | Cured |
| 9 | 35 | Cough, tachypnea | C/S | Cured |
| 10 | 20 | Fever, tachypnea, cough | C/S | Cured |
| 11 | 29 | Cough, fever | NVD | Cured |
| 12 | 36 | Cough, fever | C/S | Cured |
| 13 | 26 | Skin itching, hyperbilirubinemia | C/S | Cured |
| 14 | 48 | Cough, fever | C/S | Died |
| 15 | 23 | Cough, tachypnea, fever | C/S | Cured |
| 16 | 32 | Cough, tachypnea, dyspnea | C/S | Cured |
| 17 | 20 | Fever, tachypnea, cough | NVD | Cured |
| 18 | 31 | Cough, body pain | C/S | Cured |
| 19 | 30 | No | C/S | Cured |
| 20 | 30 | Cough, tachypnea, headach | C/S | Cured |
| 21 | 34 | Fever, pain body | C/S | Cured |
| 22 | 37 | Cough, pain body | NVD | Cured |
| 23 | 24 | No | NVD | Cured |
| 24 | 24 | Cough/tachypnea/dyspnea | C/S | Cured |
| 25 | 39 | No | C/S | Cured |
| 26 | 33 | No | C/S | Cured |
| 27 | 34 | Cough | NVD | Cured |
| 28 | 37 | Hyperbilirubinemia, skin itching, runny nos | C/S | Cured |
| 29 | 22 | Cough, fever | C/S | Cured |
| 30 | 22 | Cough, fever | NVD | Cured |
| 31 | 37 | Cough, fever | C/S | Cured |
| 32 | 32 | Cough, tachypnea, dyspnea | C/S | Cured |
| 33 | 32 | No | C/S | Cured |
| 34 | 25 | No | C/S | Cured |
| 35 | 26 | No | C/S | Cured |
| 36 | 24 | Cough, tachypnea, vomit, nausea, epigastric pain | C/S | Cured |
| 37 | 20 | Dyspnea, shiver, sweating | C/S | Cured |
| 38 | 39 | Tachypnea | C/S | Cured |
| 39 | 17 | Labor pain | NVD | Cured |
| 40 | 37 | No | C/S | Cured |
| 41 | 21 | BPP=4/8 | NVD | Cured |
| 42 | 39 | No | C/S | Cured |
BPP=Biophysical profile, NVD=Normal vaginal delivery, C/S=Cesarean Section
Information on newborns born to mothers with COVID-19
Among the infants born to mothers with coronavirus disease, 26 were term and 18 were premature, 26 were male, 18 were female, 2 was twin, 40 were single, 31 had an Apgar score of 9/10, and two had 1/0, seven 8/10, one case 2/10, and 3 case 7/10. Thirty-nine cases normalization saturation and five cases abnormal, ten cases of respiratory problems, and three cases of low BS, one case of intolerance to nutrition, from radiographs were performed, and two were abnormal, one case received positive PCR result, three cases received blood products, two cases of cyanotic organs at birth, 22 cases were admitted to NICU due to preterm and unstable clinical condition after birth, three cases received surfactant, five cases were intubated immediately after birth, six cases received adjacent oxygen, result of ABG VBG of umbilical cord of five case were abnormal infants, seven infants died, eight cases underwent phototherapy, 39 cases were treated and discharged [Table 2].
Table 2.
Neonates information
| Case | Gestational age | Birth weight (g) | Pregnancy | Apgar Score 1-min | Apgar Score 5-min | First symptom | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 37 weeks | 2020 | Singleton | 9 | 10 | No | Cured |
| 2 | 39 weeks | 3500 | Singleton | 9 | 10 | No | Cured |
| 3 | 38 weeks | 3300 | Singleton | 9 | 10 | RDS/grunting | Cured |
| 4 | 36 weeks | 3200 | Singleton | 9 | 10 | No | Cured |
| 5 | 33 weeks | 2100 | Singleton | 9 | 10 | No | Cured |
| 6 | 36 weeks | 2415 | Singleton | 9 | 10 | No | Cured |
| 7 | 28 weeks, 1 day | 870 | Singleton | 1 | 0 | RDS | Died |
| 8 | 36 weeks, 6 days | 2640 | Singleton | 9 | 10 | Grunting | Cured |
| 9 | 34 weeks, 6 days | 4050 | Singleton | 9 | 10 | RDS/acrocyanosis | Cured |
| 10 | 39 weeks, 6 days | 3300 | Singleton | 9 | 10 | No | Cured |
| 11 | 40 weeks, 1 days | 3500 | Singleton | 9 | 10 | No | Cured |
| 12 | 39 weeks | 1070 | Singleton | 9 | 10 | No | Cured |
| 13 | 27 weeks, 6 days | 1210 | Singleton | 8 | 10 | RDS/shortness of breath | Died |
| 14 | 28 weeks, 2 days | 1300 | Twin | 2 | 10 | RDS/shortness of breath | Died |
| 15 | 37 weeks, 5 days | 3165 | Twin | 7 | 10 | RDS/shortness of breath | Died |
| 16 | 37 weeks | 3000 | Singleton | 8 | 10 | Acrocyanosis | Cured |
| 17 | 40 weeks | 3000 | Singleton | 9 | 10 | No | Cured |
| 18 | 36 weeks, 6 days | 2000 | Singleton | 8 | 10 | No | Cured |
| 19 | 36 weeks, 6 days | 3720 | Singleton | 9 | 10 | No | Cured |
| 20 | 34 weeks | 2100 | Singleton | 9 | 10 | No | Cured |
| 21 | 34 weeks | 2415 | Twin | 8 | 9 | RDS/grunting | Cured |
| 22 | 32 weeks | 2200 | Twin | 8 | 9 | RDS/grunting | Cured |
| 23 | 40 weeks, 6 days | 4300 | Singleton | 9 | 10 | No | Cured |
| 24 | 40 weeks | 3150 | Singleton | 9 | 10 | No | Cured |
| 25 | 41 weeks | 3300 | Singleton | 9 | 10 | No | Cured |
| 26 | 40 weeks | 3200 | Singleton | 9 | 10 | No | Cured |
| 27 | 32 weeks | 2100 | Singleton | 9 | 10 | RDS | Cured |
| 28 | 33 weeks | 1650 | Singleton | 9 | 10 | No | Cured |
| 29 | 32 weeks | 2900 | Singleton | 9 | 10 | No | Cured |
| 30 | 37 weeks | 650 | Singleton | 9 | 10 | No | Cured |
| 31 | 25 weeks | 3275 | Singleton | 1 | 0 | No | Died |
| 32 | 37 weeks | 3235 | Singleton | 9 | 10 | No | Cured |
| 33 | 34 weeks | 2500 | Singleton | 9 | 10 | No | Cured |
| 34 | 38 weeks | 2600 | Singleton | 8 | 10 | No | Cured |
| 35 | 40 weeks | 3600 | Singleton | 9 | 10 | No | Cured |
| 36 | 32 weeks | 2340 | Singleton | 9 | 10 | No | Cured |
| 37 | 32 weeks, 3 days | 2500 | Singleton | 8 | 9 | RDS/grunting | Cured |
| 38 | 38 weeks, 4 days | 2950 | Singleton | 9 | 10 | No | Cured |
| 39 | 32 weeks | 1525 | Singleton | 7 | 10 | No | Cured |
| 40 | 39 weeks | 3345 | Singleton | 9 | 10 | No | Cured |
| 41 | 38 weeks, 5 days | 33350 | Singleton | 9 | 10 | No | Cured |
| 42 | 40 weeks, 4 days | 3500 | Singleton | 7 | 9 | No | Cured |
| 43 | 37 weeks, 5 days | 3355 | Singleton | 9 | 10 | No | Cured |
| 44 | 33 weeks | 2100 | Singleton | 9 | 10 | No | Cured |
RDS=Respiratory distress syndrome
Pathogen identification results
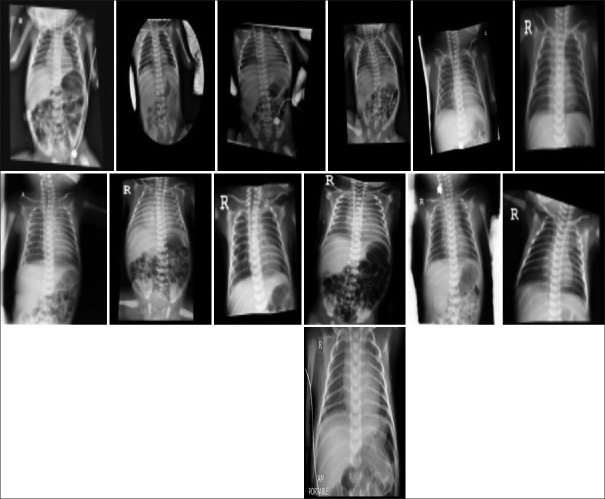
All patients admitted as suspected coronavirus, results of PCR and CT showed COVID-19 infection. The results of the infection were severe in the mother one of twins and two cases of singleton (one case of placental abruption); finally, the mother of twins and another mother died, and the other mother was treated and discharged. Moreover, finally (one of the twins died 2 days and the other 5 days after birth due to preterm birth and lung bleeding) and the other newborn, which was the result of emergency cesarean section due to breech and placental abruption, immediately 1 minute after birth in the operating room died with Apgar 1/0, and the mother suffered a cardiac arrest and intubation due to severe infection and unstable blood pressure during cesarean section was transferred to ICU after cesarean section and less than 12 h after delivery due to cardiac arrest and severe infection died. According to the PCR sample of infants, a positive case has been reported, and this infant was admitted to the isolated ward 10 min after birth to perform the necessary examinations and was discharged in good general condition 3 days after birth and did not receive any viral treatment. CXR was not performed for the infant at the time of admission. On examination and CXR performed 28 days after birth, all clinical signs of neonatal and normal CXR were reported, from other infants normal CXR were reported [Figure 1 and Table 3].
Figure 1.
Chest X-ray 13 neonates
Table 3.
Pathogen identification results
| Case | Specimen | Result (mothers) | Result (neonates) | CT (mothers) | CXR (neonates) |
|---|---|---|---|---|---|
| 1 | Throat swab | (+) | (+) | (+) | None |
| 2 | Throat swab | (+) | (_) | (+) | None |
| 3 | Throat swab | (+) | (_) | (+) | None |
| 4 | Throat swab | (+) | (_) | (+) | None |
| 5 | Throat swab | (+) | (_) | (+) | None |
| 6 | Throat swab | (+) | (_) | (+) | (_) |
| 7 | Throat swab | (+) | (_) | (+) | (_) |
| 8 | Throat swab | (+) | (_) | (+) | None |
| 9 | Throat swab | (+) | (_) | (+) | None |
| 10 | Throat swab | (+) | (_) | (+) | None |
| 11 | Throat swab | (+) | (_) | (+) | None |
| 12 | Throat swab | (+) | (_) | (+) | None |
| 13 | Throat swab | (+) | (_) | (+) | None |
| (Twins mother) 14 | Throat swab | (+) | (_) | (+) | (_) |
| (Twins mother) 15 | Throat swab | (+) | (_) | (+) | (_) |
| 16 | Throat swab | (+) | (_) | (_) | (_) |
| 18 | Throat swab | (+) | (_) | (_) | (_) |
| 19 | Throat swab | (+) | (_) | (+) | None |
| 20 | Throat swab | (+) | (_) | (+) | None |
| (Twins mother) 21 | Throat swab | (+) | (_) | (_) | None |
| (Twins mother) 22 | Throat swab | (+) | (_) | (+) | None |
| 23 | Throat swab | (+) | (_) | (+) | None |
| 24 | Throat swab | (+) | (_) | (+) | (_) |
| 25 | Throat swab | (+) | (_) | (_) | (_) |
| 26 | Throat swab | (+) | (_) | (+) | (_) |
| 27 | Throat swab | (+) | (_) | (_) | (_) |
| 28 | Throat swab | (+) | (_) | (_) | (_) |
| 29 | Throat swab | (+) | (_) | (_) | (_) |
| 30 | Throat swab | (+) | (_) | (+) | (_) |
| 31 | Throat swab | (+) | (_) | (_) | None |
| 32 | Throat swab | (+) | (_) | (+) | None |
| 33 | Throat swab | (+) | (_) | (+) | None |
| 34 | Throat swab | (+) | (_) | (+) | (_) |
| 35 | Throat swab | (+) | (_) | (+) | (_) |
| 36 | Throat swab | (+) | (_) | (+) | (_) |
| 37 | Throat swab | (+) | (_) | (+) | (_) |
| 38 | Throat swab | (+) | (_) | (+) | (_) |
| 39 | Throat swab | (+) | (_) | (+) | (_) |
| 40 | Throat swab | (+) | (_) | (+) | (_) |
| 41 | Throat swab | (+) | (_) | (_) | (_) |
| 42 | Throat swab | (+) | (_) | (+) | (_) |
| 43 | Throat swab | (+) | (_) | (+) | (_) |
| 44 | Throat swab | (+) | (_) | (+) | (_) |
CT=Computed tomography, CXR=Chest X-ray,(_) Normal result,(+) Abnormal result
Conclusion
We describe epidemiological data, demographics, signs and symptoms on admission, laboratory results, comorbidities, infection COVID-19 in the mothers and neonates, chest radiography and CT findings, treatment received for COVID-19, and clinical maternal, and fetal and neonatal outcomes. Due to the fact that the study population is small consist of 42 mothers with COVID-19 infection, among all PCRs samples from infants born to COVID-19-positive mothers, the PCR result of one case was positive, and the rest of was negative. Therefore, vertical transmission of COVID-19 through the placenta to the fetus cannot be confirmed or denied, nor can the virus corona confirmed or denied the baby's postnatal complication during pregnancy.
Financial support and sponsorship
The present study was funded by Preventative Gynecology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors wish to express their thanks to all the mothers who participated in the present research. This study was approved by the Shahid Beheshti University of Medical Sciences by ethical committee (IR.SBMU.RETECH.REC.1399.663).
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International journal of antimicrobial agents. 2020 Mar 1;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon LC, Yang H, Lee JC, Copel JA, Leung TY, Zhang Y, et al. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: Information for healthcare professionals. Ultrasound Obstet Gynecol. 2020;55:700–8. doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assiri A, Abedi GR, Al Masri M, Bin Saeed A, Gerber SI, Watson JT. Middle east respiratory syndrome coronavirus infection during pregnancy: A report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63:951–3. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajbhiye RK, Modi DN, Mahale SD. Pregnancy outcomes, newborn complications and maternal-fetal transmission of SARS-CoV-2 in women with COVID-19: A systematic review of 441 cases. [Last accessed on 2020 Jan 01];MedRxiv. [Google Scholar]
- 5.Malik A, El Masry KM, Ravi M, Sayed F. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis. 2016;22:515–7. doi: 10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, et al. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin Infect Dis. 2020;71:853–7. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–64. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Yang H, Cao Y, Cheng W, Duan T, Fan C, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. 2020;149:130–6. doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao J, He X, Gong Q, Yang L, Zhou C, Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynecol Obstet. 2020;150:53–7. doi: 10.1002/ijgo.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Archives of pathology & laboratory medicine. 2020 Jul;144(7):799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: A review. Fetal Pediatr Pathol. 2020;39:246–50. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Q, Shi Y. Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. J Med Virol. 2020;92:564–7. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centro de Coordinación de Alertas y Emergencias Sanitarias. Dirección General de Salud Pública. Ministerio de Sanidad. Información científico-técnica. Enfermedad por coronavirus, COVID-19. Actualización; 4 de abril. 2020. [Last accessed on 2020 Apr 04]. Disponible from: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/20200404_ITCoronavirus.pdf .
- 15.Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14:193–8. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: An observation cohort study. Lancet Child Adolesc Health. 2020;4:721–7. doi: 10.1016/S2352-4642(20)30235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao TT, Yan K, Wang LS, Zhou WH. What can we learn from neonates with COVID-19? World J Pediatr. 2020;16:280–3. doi: 10.1007/s12519-020-00376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma K, Chen T, Han MF, Guo W, Ning Q. Management and clinical thinking of Coronavirus Disease 2019. Zhonghua Gan Zang Bing Za Zhi. 2020;28:E002. doi: 10.3760/cma.j.issn.1007-3418.2020.0002. [DOI] [PubMed] [Google Scholar]
- 21.Zhu ZB, Zhong CK, Zhang KX, Dong C, Peng H, Xu T, et al. Epidemic trend of COVID-19 in Chinese mainland. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54:620–4. doi: 10.3760/cma.j.cn112150-20200222-00163. [DOI] [PubMed] [Google Scholar]
- 22.Tasnim Agency. Birth of a Neonate from Infected Mother COVID-19 in Babol City; March 3. 2020. [Last accessed on 2020 Mar 04]. Available from: https://www.tasnimnews.com/fa/news/1398/12/14/2216407/
- 23.Irani M, Pakfetrat A, Mask MK. Novel coronavirus disease 2019 and perinatal outcomes. J Educ Health Promot. 2020;9:78. doi: 10.4103/jehp.jehp_189_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikdeli B, Talasaz AH, Rashidi F, Sharif-Kashani B, Farrokhpour M, Bakhshandeh H, et al. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: Rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb Res. 2020;196:382–94. doi: 10.1016/j.thromres.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]