Abstract
BACKGROUND:
Since the advent of coronavirus disease 2019 (COVID-19) infection, there is debate whether pregnancy outcome in COVID-19 is more severe as compared to general population. Pregnant population is particularly susceptible to viral infections due to altered immune response. H1N1 infection and Zika virus infection led to unfavorable maternal and fetal outcomes. SARS during pregnancy has been linked previously with high risk of spontaneous abortions, preterm births and intrauterine growth restriction. The effects of this novel virus need to be studied.
MATERIALS AND METHODS:
This is a single-center descriptive prospective observational study of 65 pregnant women with reverse transcriptase–polymerase chain reaction confirmed COVID-19 infection, regardless of gestational age at diagnosis, admitted from April 15 to June 30, 2020, at the COVID hospital in SN Medical college a tertiary center of Agra in North India. Maternal and perinatal outcomes were studied. Data were analyzed using the SPSS software for windows. Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as numbers and percentages.
RESULTS:
Majority 88.4% of the women were asymptomatic. Rest had mild illness only. Majority 94.23% were third-trimester pregnancies; preterm birth was not reported in any singleton pregnancy. Majority 85% were delivered by cesarean section done for obstetric indications. Maternal outcome of all patients was favourable, and only two women who had moderate pneumonia recovered. Maternal mortality was reported in only 1 case. All neonates were negative for COVID-19. Neonatal outcome was favorable.
CONCLUSION:
COVID-19 in pregnancy led to mild symptoms only. Infection in the third trimester did not led to adverse obstetric outcome including preterm labor and premature membrane rupture. SARSCoV2 infection in pregnancy did not increase the risk of maternal mortality. Vertical transmission of COVID-19 was not found in neonates. The maternal, neonatal, and perinatal outcomes of COVID-19 patients infected in late pregnancy were favorable.
Keywords: Coronavirus disease 2019 in pregnancy, maternal and fetal outcomes, perinatal outcome, severe acute respiratory syndrome coronavirus 2, vertical transmission of coronavirus disease 2019
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is one of the most catastrophic events in the recent history of human beings. Two β-coronaviruses have caused mortality of 10% for severe acute respiratory syndrome coronavirus (SARS-CoV) and 37% for Middle East respiratory syndrome CoV (MERS-CoV).[1,2,3] SARS-COV-2 belongs to the same β-CoV subgroup, and it has genome similarity with SARS-CoV and MERS-CoV.[4]
Based on the present epidemiological information, the disease incubation period varies from 1 to 14 days, usually 3–7 days.[5] It is also contagious during the latency period. Disease symptoms range from moderate to severe, including fever, cough, shortness of breath, and pneumonia. In severe cases, neurological, gastrointestinal, hepatic, and respiratory complications may occur.[6]
Pregnancy can compromise the immune system and potentially SARS-CoV-2 infection can increase the risk of pneumonia in pregnant women in comparison to nonpregnant women. H1N1 infection led to unfavorable maternal and fetal outcomes. No clear scientific evidence has been provided on the vertical transmission of SARS-CoV-2 (infection in utero from an infected mother to her infant before birth). Whether pregnant women would have worse outcomes compared with nonpregnant women of similar ages when infected with SARS-CoV-2 is a major concern. The existing data are still insufficient. SARS-CoV-2 is a newly identified virus, and it remains unknown whether viral shedding occurs during delivery. Thus, the need for the present study arises so that we have a better understanding of SARS-Cov2 and its implications for maternal and perinatal health. This study was conducted to highlight our management of COVID-19 patients which would add to the global data collected regarding this pandemic and help formulate better guidelines to manage this confusing situation.
We aimed to study the clinical characteristics and maternal, fetal, and perinatal outcomes of COVID-19 infection in pregnancy and the vertical transmission potential of SARS-COV-2 infection.
Materials and Methods
Study design
This was a single-center descriptive prospective observational study. Duration of study – April 15 to June 30, 2020.
Place of study – COVID hospital in S. N. Medical College, Agra.
Patient inclusion criteria
We enrolled all reverse transcriptase–polymerase chain reaction (RTPCR)-positive pregnant women regardless of gestational age.
Exclusion criteria
Patients who had negative RT-PCR report were excluded from the study.
Ethical clearance was taken by the Institutional Ethical Committee of our medical college. Written informed consent was taken from all patients. Clinical characteristics, pregnancy and neonatal outcomes of all COVID RT-PCR-positive pregnant women were analysed.
A laboratory confirmed case was defined as positive by RT-PCR of nasopharyngeal and oral swabs. Neonatal nasopharyngeal swab was collected after 36–48 h of birth and was tested for infection with SARS-CoV-2.
Viral clearance was confirmed by serial RT-PCR, from nasopharyngeal swab, with at least two consecutive negative results taken 24 h apart considered cleared.
Virus clearance time was taken from the date of RT-PCR positive and ending on the date of the first negative RT-PCR. Patients were initially discharged after 2 consecutive RT-PCRs were negative (sampling interval 24 h) as per our discharge policy. Afterward, our discharge criteria were revised; patients if clinically stable could be discharged after single RT-PCR was negative.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences software for windows version 21.0 Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as numbers and percentages.
Results
From April 15, 2020, to June 30, 2020, total 6411 women who came for antenatal checkups or with labor pains and delivery plans were screened by triage for COVID-19 (novel CoV 2019) according to ICMR guidelines at that time. We tagged patients into two categories: red tag and green tag. Criteria for red tag were stay in hot spot of COVID-19 area, close contact of COVID positive patient, and history of fever, cough, sore throat, or dyspnea (not explainable by other disorders). All red tag patients (185) were tested, out of which 52 pregnant women were laboratory confirmed for COVID-19 and were admitted in isolation ward as per our institutional policy. We admitted confirmed cases of COVID-19 from other private centers; also, many of them were found positive in universal screening done at their centers, so asymptomatic patients were also tested.
All cases had body mass index in the normal range (24.5 ± 1.3 kg/m2). At presentation, the mean age of pregnant women was 27.2 years and the mean gestational age was 37.3 weeks. We also had referred cases of cesarean section done outside our institute and being transferred to our center once they became COVID-19 positive according to our state policy of treating COVID-19 patients at institutional isolation hospital only. One patient was in the first trimester, one in the second trimester (16 weeks pregnancy), and 96.92% (63/65) were in the third trimester. Two of our patients are 32–34-week [Figure 1] preterm ongoing pregnancies (they delivered health babies vaginally at term on follow-up done telephonically) which were discharged after negative RT-PCR: one intrauterine (conceived with in vitro fertilization [IVF]) and twin pregnancy landed in missed abortion (first twin was missed abortion at 6 weeks and second twin had crown–rump length of 12 weeks with no cardiac activity for which her evacuation was done. There was a hemodynamically stable ruptured tubal ectopic pregnancy, for which her exploratory laparotomy and salpingectomy were done. There was only one case of second-trimester (16 weeks) asymptomatic pregnancy and she was discharged after her COVID RT-PCR became negative. Seventy-five percent of the women (49/65) were multiparous and 24.61% (16/65) were primigravida.
Figure 1.
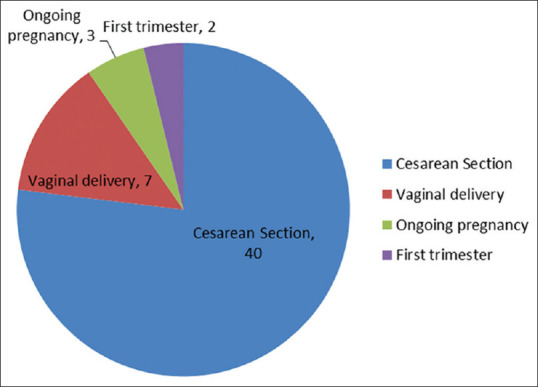
Outcome of COVID-19-positive pregnancy
All were singleton pregnancies except one case of twin pregnancy. During the study period, 65 pregnant women participated, out of which 57 (87.69%) women were asymptomatic and 6/65 (9.23%) had mild symptoms of upper respiratory tract infection for 3–4 days before admission and 2 cases developed left lower zone pneumonia after the 3rd day of delivery and were shifted to intensive care unit (ICU) but did not require mechanical ventilation. During the course of their hospital stay, none of the patients which were asymptomatic developed symptoms. There was only 1 (1.53%) mortality reported in a vaginally delivered woman who expired 5 days after her discharge, though she was stable at the time of discharge [Table 1].
Table 1.
Perinatal outcomes
| Outcome parameter | Total births (n=48), n (%) |
|---|---|
| Fetal complications | |
| Intrauterine Growth restriction (IUGR) | 1 (2.1) |
| Thick meconium stained liquor | 7 (14.9) |
| Still born | 1 (2.1) |
| Mean Apgar score (1 min) | 8±1 |
| Mean Apgar score (5 min) | 9±1 |
| Mean birth weight (g) | 2800±386 |
| SARS-CoV-2 infection in neonate tested by RT-PCR 36-48 hours after delivery | 0 |
| Perinatal complications | |
| Premature delivery | 1 (2.1) |
| Low birth weight (<2500 g) | 3 (6.3) |
| Live birth | 47 (97.9) |
| Severe neonatal asphyxia | 2 (4.1) |
| Neonatal intensive care unit admission | 2 (4.2) |
| Neonatal death | 2 (4.2) |
Majority of the pregnant women in this study had favorable maternal and neonatal outcomes. COVID status in the third trimester does not alter the course of pregnancy nor it leads to adverse maternal and neonatal outcomes.
However, whether vertical transmission occurs during early first trimester is still not known. Vertical transmission cannot be ruled out as per a study by Fornari.[7] In our study, women affected by COVID-19 were mostly term pregnancies, and fetal growth is not likely to be affected in this time period.
Maternal outcome
The mean days for RTPCR to become negative was 9.2 days. The mean duration of stay in hospital was 9.5 days. The mean admission to delivery duration was 1.4 days.
Mother-to-child transmission of coronavirus disease 2019
None of the women had preexisting chronic diseases except hypothyroidism in 2 cases. Two women had gestational diabetes, preeclampsia was found in 3, and oligohydramnios in 5 cases. Hemolysis, Elevated liver enzymes and low platelets syndrome (HELLP) syndrome with jaundice was reported in one case of preeclampsia with platelet count (80,000/μl).
Only 1 neonate turned out to be COVID-19 positive after 48 h of delivery because the baby was not separated from the mother because mothers’ COVID status was not known at the time of delivery. Infection in the third trimester was less likely to be associated with neonatal complications and adverse outcomes. Other comorbid conditions were gestational diabetes (GDM) in 9%, preeclampsia in 6.25%, and hypothyroidism in 1.5% cases.
Out of 65 patients, 55 (84.61%) were term pregnancies; preterm birth was not reported in any singleton pregnancy. One twin pregnancy had preterm labor at 35.4 weeks. Majority 48/60 (80%) cases were delivered by cesarean section done for obstetric indications though an early decision was taken considering their COVID status [Figure 2]. Meconium staining of liquor was present in early labor in few cases. Out of 48 cases of cesarean reported in our study, 10 cases of cesarean section were done outside our hospital and were transferred to our hospital after they were diagnosed with COVID-19 (their data were collected retrospectively) and 12/60 (20%) were delivered vaginally. All of them had spontaneous labor. Premature rupture of membranes (PROM) was reported in one case.
Figure 2.
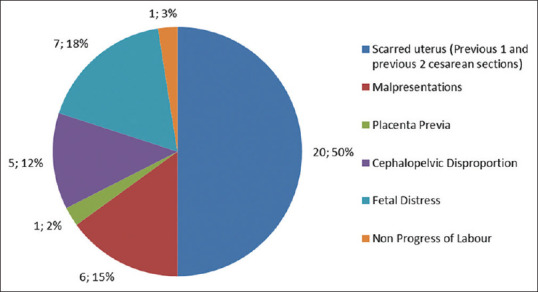
Indications of cesarean section
Perinatal outcome
Perinatal outcomes are shown in [Table 2]. Fetal distress and passage of thick meconium were present in 11.6% (7/60) of cases. All infants had good Apgar scores except 2 neonates and were shifted to neonatal ICU (NICU). One was stillborn with transverse lie with fetal distress in labor.
Postpartum hemorrhage was reported in 2 cases, one patient of vaginal delivery was managed by intrauterine balloon tamponade by condom catheter (removed after 24 h), whereas other case was managed by B-lynch sutures at the time of cesarean section done in unicornuate uterus for dystocic labor. It was not significant.
All 59/60 neonates (including one twins) tested negative for RT-PCR. In five cases amniotic fluid which was collected at the time of cesarean was sent for RTPCR and was found to be negative for COVID-19. In single placenta studied by us, one swab was taken from amniotic surface of placenta and second between amnion and chorion and it was RTPCR negative for CoV.
Discussion
Due to physiological changes in the cardiovascular and respiratory systems (increased heart rate, stroke volume, oxygen consumption, and decreased lung capacity), there is an increased risk for pregnant women to develop severe respiratory disease.
Immunological changes during pregnancy that allow a mother to tolerate an antigenically distinct fetus, cause an increased risk of severe infections, as was the situation in 1918 for Spanish influenza and the H1N1 2009 influenza virus.
Our pregnant women were mostly asymptomatic as in a study by Sutton et al.[8] where 88% of the women were asymptomatic, also in review of 441 pregnancies, women had mostly mild disease and were asymptomatic on initial presentation in more than half cases and were diagnosed with COVID-19 after they were admitted for induction of labor.[9]
Chen et al. also concluded that there is no evidence that pregnant women with COVID-19 are more prone to develop severe pneumonia, in comparison to nonpregnant patients.[10,11] In our study also, maternal outcome was good with 2 cases of moderate pneumonia and only one women required ventilator support. In a study published in the American Journal of Obstetrics and Gynecology by Sentilhes et al.,[12] 24% of the women required oxygen support with nasal cannula. Preterm birth was medically indicated for COVID-19 in 24% (14.3% – very preterm deliveries). In a study by Schwartz of 38 pregnancies, there were no maternal deaths.[13]
In a systematic review by Gajbhiye et al.,[9] fever (54%), cough (35%), myalgia (17%), dyspnea (12%), and diarrhea (4%) were the most common symptoms. Cesarean section was the mode of delivery in 80% cases like our study, done for either fetal distress, or was an empirical decision.[9] In our study, cesarean was done for obstetrical indications along with fear of worsening of COVID [Figure 2]; also, ours is a tertiary care center where referred cases are entertained.
GDM was associated in 9% in systematic review of 441 cases and preterm labor in 26%, though preterm birth in our case was only one case of twin pregnancy, fetal distress (8% vs. 11.5% in our study), PROM (9% vs. 2% in our study), maternal mortality 3%, still births (1.6%) and neonatal death 1%, and respiratory distress syndrome in 8%.[9]
Mother-to-child transmission of coronavirus disease 2019
In a study by Schwartz et al. of 38 pregnancies, there were no confirmed cases of intrauterine vertical transmission of SARS-CoV-2 from mothers to their fetuses. All neonatal specimens tested, including placentas, were negative by RT-PCR.[12,13]
In a study by Chen et al.,[10] amniotic fluid, cord blood and neonatal throat swabs, and breast milk at birth were negative for COVID-2019, suggesting that there were no intrauterine fetal infections due to the COVID-19,[13] but the possibility of intrauterine vertical transmission during the early trimesters cannot be ruled out. Infection acquired in the third trimester may not lead to adverse maternal and neonatal outcomes. Chen et al.[10] reported favourable outcome in all of the nine patients in the third trimester. Similar to their study the duration between infection and delivery was short in our study also.
In addition to intrauterine vertical transmission, intrapartum and contacted transmission also could be possible. However, one study found that the expression of the angiotensin-converting enzyme 2 (surface receptor of sensitive cells for 2019-nCoV) was very low in maternal-fetal interface; this might explain no intrauterine vertical transmission. Few cases of vertical transmission have been reported till date. These results were contradictory as reported by Gajbhiye et al.[9] where vertical transmission rate of SARS-CoV-2 was estimated to be 11% (7% of the neonates were positive by RT-PCR and 3% by antibody testing). Moreover, they concluded evidence of vertical transmission of SARS-CoV-2 infection in women with COVID-19.[9] We need more data so that we can reach to a conclusion.
Sun and Yeh[14] found placentas negative for SARS-CoV-2 RT-PCR, but fibrin deposition inside and in proximity to villi and local syncytial nodule along with massive placental infarction in one placenta was reported. No pathological placental changes due to SARS-CoV-2 were found in the three placentas.
In a study of Wuhan,[15] the researchers found no association with pregnancy outcomes such as severity of disease or virus clearance time. Patients became SARS-CoV-2 negative by RT-PCR in average 9 days whereas severe pneumonia in 7%, leading to ICU admission and moderate pneumonia in 86%.[13]
However, whether vertical transmission occurs during early first trimester is still not known. Vertical transmission cannot be ruled out as per a study by Fornari.[7] In our study, women affected by COVID-19 were mostly term pregnancies, and fetal growth is not likely to be affected in this time period. One case of spontaneous miscarriage reported might be because of her previous history of infertility and conception by IVF.
Only 8% of the pregnant women with COVID-19 required ICU care with mechanical ventilation;[15] of these, 10 women were kept on extracorporeal membrane oxygenation.
In a study by Yan et al., 27.3% (6/22) of the women had preterm PPROM. Researchers did not find an increased risk of spontaneous abortion and spontaneous preterm birth. Of the 116 cases, there were 8 cases (6.9%) of severe pneumonia but no maternal deaths.[16]
According to a study by Liu et al., all cases of COVID-19 pneumonia were mild type and they found that pregnancy and childbirth did not alter the course of symptoms.[17]
Strengths of the study
This study is reassuring and is one of the early studies of perinatal outcome of COVID-19 in pregnancy. This study will help healthcare providers of the possible pregnancy outcomes in women infected with SARSCoV2.
Weaknesses of the study
Confirmatory conclusions cannot be drawn from the study due to the small sample size. Future studies should compare the outcomes with a control group of pregnant women without COVID infection.
Conclusion
Pregnant women are a challenge during COVID-19 pandemic. They have multiple encounters with health-care workers. Universal screening of them has several benefits: risk of asymptomatic transmission to health-care workers (HCWs) and other pregnant women will be reduced, patient can be isolated early, proper protection of HCWs can be done by personal protective equipment, and baby can be isolated early from mother and taken care of. There is a high rate of asymptomatic COVID-19 infection. There is no evidence that SARS-CoV-2 undergoes intrauterine or transplacental transmission from infected pregnant women to their fetuses. Compared with SARS and MERS, COVID-19 appears less lethal, acknowledging the limited number of cases reported to date. Pregnancy did not aggravate the severity of COVID-19. Majority of the pregnant women in this study had favorable maternal and neonatal outcomes.
What this study has added to our understanding
Majority of the pregnant women are asymptomatic or having only mild disease. COVID status in the third trimester does not alter the course of pregnancy nor leads to adverse maternal and neonatal outcomes. Vertical transmission of COVID-19 was not reported in our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to all the health workers and researchers around the world, working hard to control COVID-19. In addition, we would like to thank the Principal Sanjay Kala, Head of the Department, Dr Richa singh, Dr Shikha Singh, Dr Nidhi Gupta and residents, S. N. Medical College, for their cooperation.
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Sik CC, Pak C Ng, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–7. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Zhao Xiang, Li Juan, Niu Peihua, Yang Bo, Wu H, onglong, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani M, Pakfetrat Ali, Mask Mahin Kiyani. Novel coronavirus disease 2019 and perinatal outcomes. J Educ Health Promot. 2020;9:78. doi: 10.4103/jehp.jehp_189_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazemi-Karyani A, Safari-Faramani R, Amini S, Ramezani-Doroh V, Berenjian F, Dizaj MY, et al. World one-hundred days after COVID-19 outbreak: Incidence, case fatality rate, and trend. J Educ Health Promot. 2020;9:199. doi: 10.4103/jehp.jehp_483_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornari F. Vertical transmission of COVID-19-A systematic review. J Pediatr Perinatol Child Health. 2020;4:7–13. [Google Scholar]
- 8.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–4. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajbhiye R, Modi D, Mahale S. Pregnancy outcomes, Newborn complications and Maternal-Fetal Transmission of SARS-CoV-2 in women with COVID-19: A systematic review of 441 cases. Preprint 15April 2020 medRxiv 2020PPR: PPR15125. Doi: 10.1101/2020.04.11.200623576. [Google Scholar]
- 10.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382:e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sentilhes L, De Marcillac F, Jouffrieau C, Kuhn P, Thuet V, Hansmann Y, et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020;223:914.e1–15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144(7):799–805. doi: 10.5858/arpa.2020-0901-SA. Doi: 105858/arpa 2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 14.Sun B, Yeh J. Mild and asymptomatic Covid-19 infections: Implications for maternal, fetal, and reproductive health? Front Reprod Health. 2020;2:1. doi: 10.3389/frph.2020.00001. doi: https://doi.org/10.3389/frph0.2020.00001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiancheng X, Jian S, Lingling P, Lei H, Xiaogan J, Weihua L, et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. 2020;95:376–83. doi: 10.1016/j.ijid.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Guo J, Fan C, Juan J, Yu X, Li J, et al. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1–14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L. Women with coronavirus disease (COVID-19) pneumonia: A preliminary analysis. Am J Roentgenol. 2020;215:127–32. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]


