Abstract
Objectives
Isatis indigotica Fort. (I. indigotica) is an herbaceous plant belonging to Cruciferae family. Its leaf (IIL) and root (IIR) are commonly used in traditional Chinese medicines (TCMs) with good clinical efficacies such as clearing away heat and detoxification, cooling blood and reducing swelling. This review aimed to provide a systematic summary on the phytochemistry, pharmacology and clinical applications of I. indigotica.
Key findings
This plant contains alkaloids, organic acids, flavonoids, lignans, nucleosides, amino acids, and steroids. Previous pharmacological researches indicated that I. indigotica possesses promising antivirus, antibacterial, immunoregulatory, anti-inflammation, and cholagogic effects. Importantly, it can inhibit various viruses, such as influenza, hepatitis B, mumps, herpes simplex, cytomegalovirus, and coxsachievirus. Clinically, it is frequently used to treat various viral diseases like viral influenza, parotitis and viral hepatitis. Consequently, I. indigotica may be beneficial for the prevention and treatment of coronavirus disease 2019 (COVID-19).
Summary
This paper reviewed the chemical constituents, pharmacological effects and clinical applications of I. indigotica which may guide further research and application of this plant.
Keywords: traditional Chinese medicine, Isatis indigotica, phytochemistry, pharmacology, clinical application
Introduction
Isatis indigotica Fort., a biennial herb of Isatis genus in Cruciferae, is mainly distributed in Gansu, Shaanxi, Hebei, Shandong, Jiangsu, Zhejiang, Anhui, and Guizhou provinces of China.[1] Owing to the efficacies of heat-clearing and detoxifying, cooling blood and eliminating ecchymoses, antibiosis and anti-inflammation,[2] its root (IIR, Chinese name Ban-lan-gen) and leaf (IIL, Chinese name Da-qing-ye) have been widely used in combination with other Chinese medicines to treat and prevent a variety of diseases such as influenza, parotitis, epidemic encephalitis B, epidemic myelitis, epidemic cerebrospinal meningitis, acute infectious hepatitis and sore throat.[3, 4] In recent years, studies have shown that the indigotin and indirubin, present in I. indigotica, display many important pharmacological activities such as liver protection and anti-microbial, and indirubin also has anti-tumour effects.[5] Furthermore, the leaves have the highest content of indigotin and indirubin followed by stems and roots.[6, 7] Besides alkaloids, there are many other active constituents such as organic acids, flavonoids, lignans, nucleosides, steroids, and amino acids, among which, flavonoids and nucleosides are two main components also present in the leaf.[6] In addition, amino acids, and organic acids, sinigrin and sulfur ingredients are also presented in the roots and display antiviral properties.[8]
Chemical Constituents
Leaf
The fresh leaves contain isatan B, 3-indlymethyglucosinolate, glucobrassicin, neoglucobrassicin, 1-sulpho-3-indolymethy glucosinolate.[9] While the dried leaves contain alkaloids, including indigotin, indirubin,[10] 2,4(1H,3H)-quinazolinedion, 5-hydroxy-2-indolinone, 10H-indolo[3,2-b]quinolone,[11] 4(3H)-quinazolinone, deoxyvascinone, tryptanthrin,[12] Isatisine A.[13] Indigotin and indirubin are fat-soluble compounds displaying poor solubility and are only soluble in chloroform, acetone and other organic solvents. They have a life span of only 24 hours in the dark after which they begin to decompose.[14]
Some of the other components in the leaves are: (1) Organic acids:[15, 16] 3,5-dimethoxy-4-hydroxy benzoin acid, syringic acid, nicotic acid, succinic acid, salicylic acid, anthranilic acid. (2) Flavonoids:[17] isovitexin, 6-β-D-glucopyranosyldiosmetin. (3) Lignans:[18] (-)-lariciresinol, (+)-isolariciresinol. (4) Nucleosides:[19] uridine, adenosine, xanthine, hypoxanthine. (5) Steroids:[20] β-rosasterol, β-sitosterol, γ-sitosterol. (6) Amino acid:[21] L-pyroglutamic acid. (7) Minerals:[22] Iron, titanium, manganese, zinc, copper, cobalt, nickel, selenium, chromium, arsenic, etc. There are also volatile oil components present in folium isatidis.
Roots
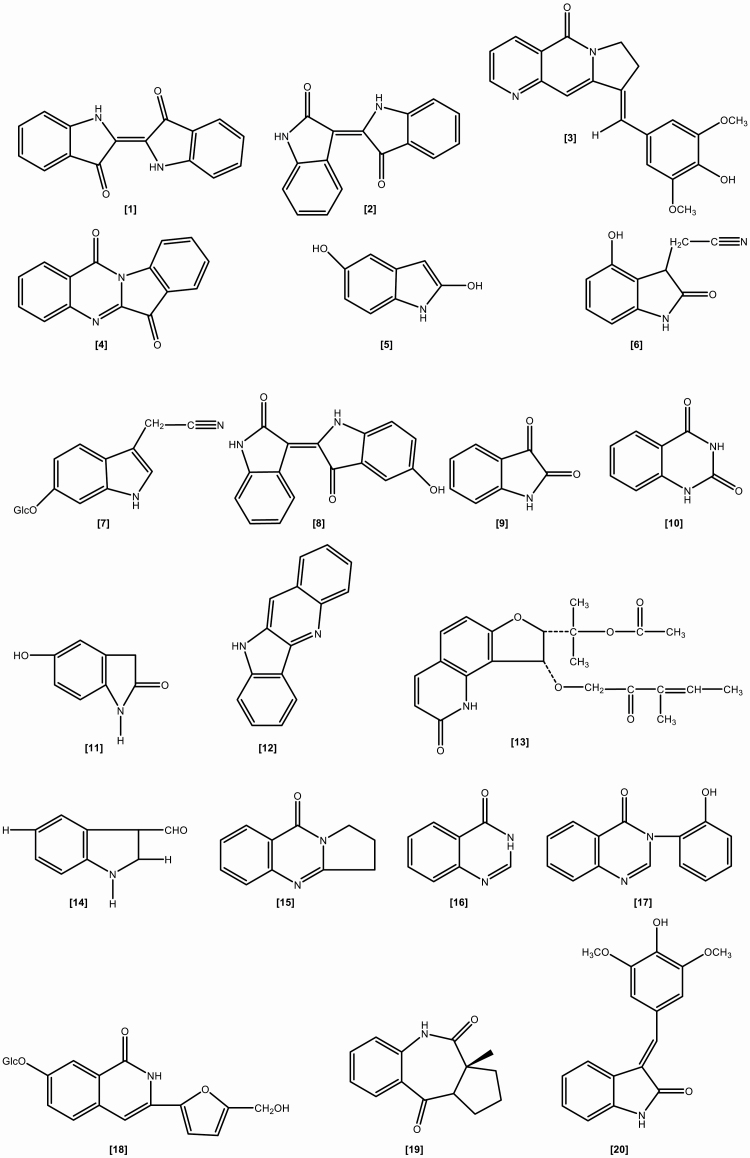
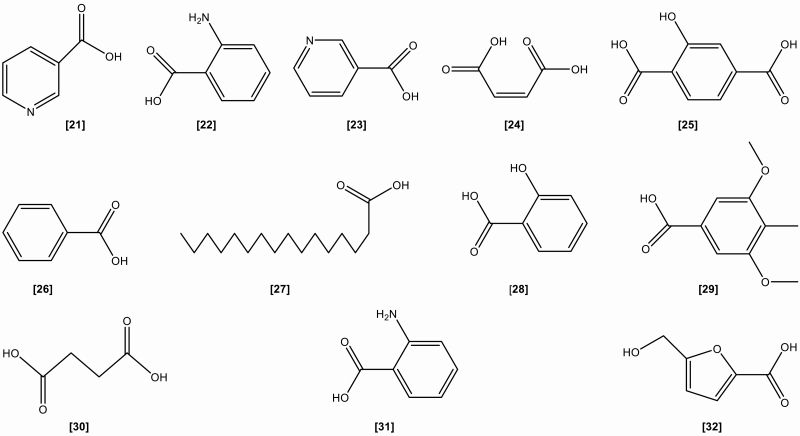
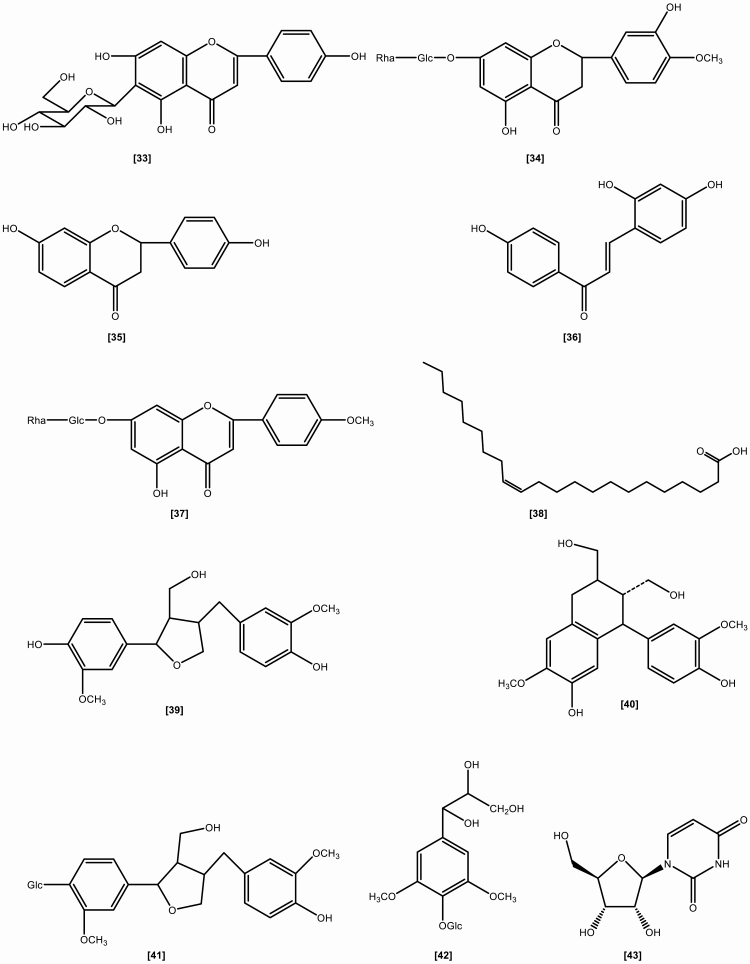
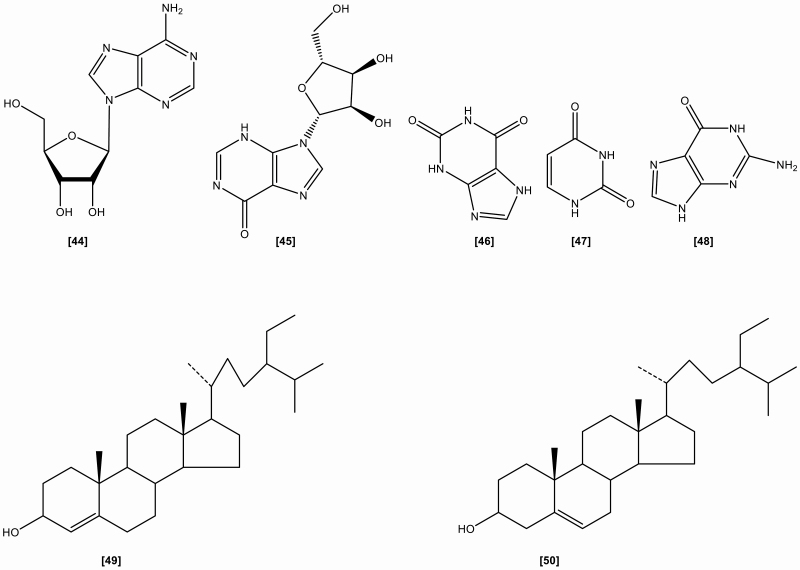
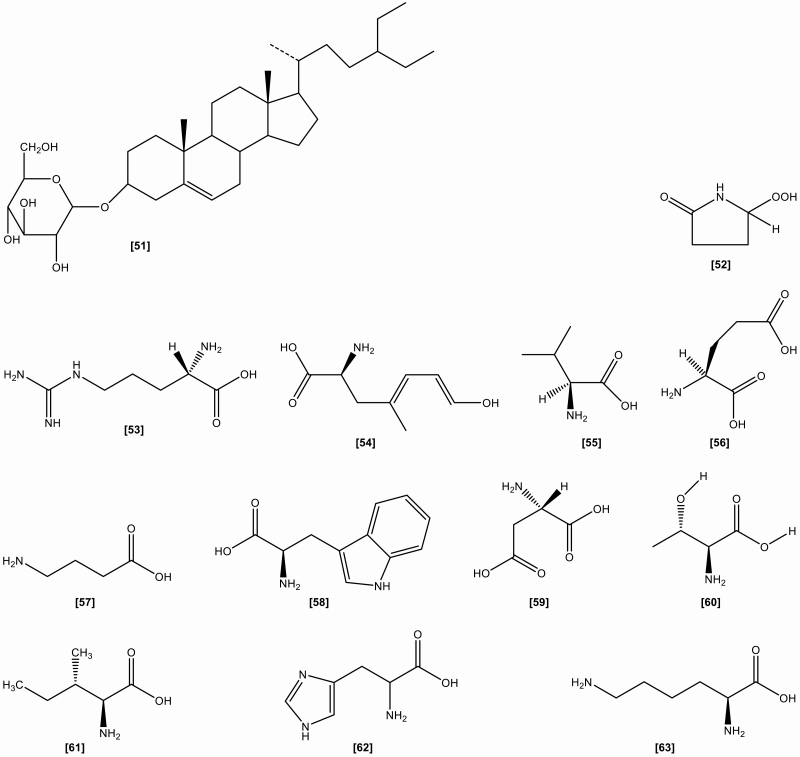
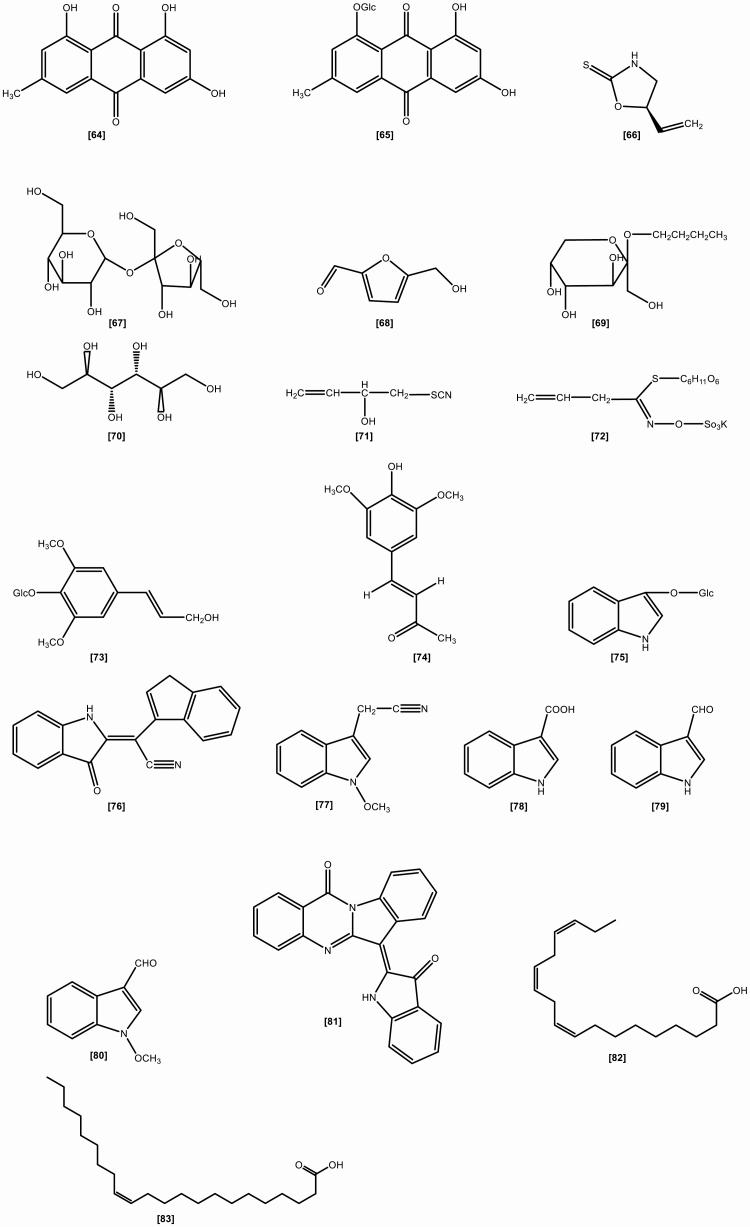
The roots include the following chemical constituents (1) Alkaloids: indigotin, isatin, indirubin,[10] indoxyl-β-glucoside, 2,5-dihydroxy-indole, 2,3-dihydro-4-hydroxy-2-oxo-indole-3-acetonitrile, indole-3-acetonitrile-6-O-β-D-glucopyranoside,[23] hydroxyindirubin, isaindigodione, (E)-3-(3',5'-dimethoxy-4'-hydroxybenzylidene)-2-indolinone, 3-formyl-indole, deosyvasicinone, isaindigotone, tryptanthrin,[24] 3-(2'-carboxyphenyl)-4(3H)-quinazolinone, 4(3H)- quinazolinone, 3-(2'-hydroxyphenyl)-4(3H)-quinazolinone, isaindig otidione, Isatan A,[25] 3-[2'-(5'-hydroxymethyl)furyl]-1(2H)-isoquinolinone-7-O-β-D-glucoside, 2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzodiazepine-5,11(10H,11aH)-dione.[26] (2) Flavonoids:[27–29] neohesperidin, liquiritigenin, isoliquiritigenin, isovitexin, linarin, eupatorin. (3) Lignans:[18] (-)-lariciresinol, lariciresinol-4-O-β-D-glucopyranoside, lariciresinol-4,4'-di-O-β-D-glucopyranoside, 4-(1,2,3-trihydroxypropyl)-2, 6-dimethoxyphenyl-1-O-β-D-glucopyranoside, syringin, (+)-isolariciresinol. (4) Organic acids:[30] 3-pyridinecarboxylic acid, maleic acid, 2-hydroxy-1,4-benzenedicarboxylic acid, benzoic acid, salicylic acid, syringic acid, palmitic acid, succinic acid, 2-amino benzoic acid, 5-hydroxymethyl furoic acid. (5) Anthraquinones:[31] emodin, emodin-8-O-β-D-glucoside. (6) Steroids:[32] β-sitosterol, daucosterol, γ-sitosterol. (7) Sinigrins:[33] 3-indolylmethyl gluosinolate, neoglucobrassicin, 1-sulpho-3-indolylmethylgluosinolate. (8) Sulfur compounds:[34] epigoitrin, 1-thiocyano-2-hydroxy-3-butene. (9) Amino acids:[35] praline, arginine, tyrosine, valine, glutamic acid, γ-aminobutyric acid, leucine, tryptophan, aspartic acid, L-threonine, β-hydroxyalanine, glycine, isoleucine, phenylalanine, histidine, lysine. (10) Nucleotides:[36] uridine, hypoxanthine, uracil, adenosine, guanine. (11) Others:[37–40] ammonium formate, sucrose, 5-hydroxymethyl-furaldehyde, n-butyl-O-β-D-fructopyranose, mannitol, pyrophaeophorbideα, polygalitol. The main chemical constituents and chemical structures of I. indigotica are presented in Table 1 and Figures 1–6, respectively.[9–40]
Table 1.
Chemical constituents isolated from Isatis indigotica
| Classification | No. | Chemical constituents | Part of plant | Ref. |
|---|---|---|---|---|
| Alkaloids | 1 | Indigotin | whole herb | [11] |
| 2 | Indirubin | whole herb | [11] | |
| 3 | Isaindigotone | Whole herb | [11] | |
| 4 | Tryptanthrin | Whole herb | [11] | |
| 5 | 2,5-dihydroxy-indole | Root | [10] | |
| 6 | 2,3-dihydro-4-hydroxy-2-oxo-indole-3-acetonitrile | Root | [10] | |
| 7 | Indole-3-acetonitrile-6-O-Β-D-glucopyranoside | Root | [10] | |
| 8 | Hydroxyindirubin | Root | [10] | |
| 9 | Isatin | Root | [10] | |
| 10 | 2,4(1H,3H)-quinazolinedion | Aerial part | [11] | |
| 11 | 5-hydroxy-2-indolinone | Aerial part | [11] | |
| 12 | 10H-indole[3,2-b]quinoline | Aerial part | [11] | |
| 13 | Isatan A | Root | [10] | |
| 14 | 3-formyl-indole | Root | [10] | |
| 15 | Deoxyvascinone | Root | [10] | |
| 16 | 4(3H)-quinazolinone | Aerial part | [11] | |
| 17 | 3-(2'-hydroxyphenyl)-4(3H)-quinazolinone | Root | [10] | |
| 18 | 3-[2'-(5'-hydroxymethyl)furyl]-1(2H)-isoquinolinone-7-O-β-D-glucoside | Root | [10] | |
| 19 | 3-dihydro-1H-pyrrolo[2,1-c][1,4]benzodiazepine-5,11(10H,11aH)-dione | Root | [10] | |
| 20 | (E)-3-(3',5'-dimethoxy-4'-hydroxybenzylidene)-2-indolinone | Root | [10] | |
| Organic acids | 21 | Nicotic acid | Aerial part | [15] |
| 22 | Anthranilic acid | Aerial part | [15] | |
| 23 | 3-pyridinecarboxylic acid | Root | [30] | |
| 24 | Maleic acid | Root | [30] | |
| 25 | 2-hydroxy-1,4-benzenedicarboxylic acid | Root | [30] | |
| 26 | Benzoic acid | Root | [30] | |
| 27 | Palmitic acid | Root | [30] | |
| 28 | Salicylic acid | Whole herb | [15] | |
| 29 | Syringic acid | Whole herb | [15] | |
| 30 | Succinic acid | Whole herb | [15] | |
| 31 | 2-amino benzoic acid | Root | [30] | |
| 32 | 5-hydroxymethyl furoic acid | Root | [30] | |
| Flavonoids | 33 | Isovitexin | Whole herb | [17] |
| 34 | Neohesperidin | Root | [28] | |
| 34 | Liquiritigenin | Root | [28] | |
| 36 | Isoliquiritigenin | Root | [29] | |
| 37 | Linarin | Root | [29] | |
| 38 | Eupatorin | Root | [29] | |
| Lignans | 39 | (-)-lariciresinol | Aerial part | [18] |
| 40 | (+)-isolariciresinol | Whole herb | [18] | |
| 41 | lariciresinol-4-O-β-D-glucopyranoside | Root | [18] | |
| 42 | 4-(1,2,3-trihydroxypropyl)-2,6-dimethoxyphenyl-1-O-β-D-glucopyranoside | Root | [18] | |
| Nucleosides | 43 | Uridine | Whole herb | [36] |
| 44 | Adenosine | Whole herb | [36] | |
| 45 | Hypoxanthine | Whole herb | [36] | |
| 46 | Xanthine | Aerial part | [36] | |
| 47 | Uracil | Root | [36] | |
| 48 | Guanine | Root | [36] | |
| Steroids | 49 | Rosasterol | Aerial part | [20] |
| 50 | β-sitosterol[ | Whole herb | [20] | |
| 51 | Daucosterol | Root | [32] | |
| Amino acids | 52 | L-pyroglutamic acid | Aerial part | [21] |
| 53 | Arginine | Root | [21] | |
| 54 | Tyrosine | Root | [21] | |
| 55 | Valine | Root | [21] | |
| 56 | Glutamic acid | Root | [21] | |
| 57 | γ-aminobutyric acid | Root | [21] | |
| 58 | Tryptophan | Root | [35] | |
| 59 | Aspartic acid | Root | [35] | |
| 60 | L-threonine | Root | [35] | |
| 61 | Isoleucine | Root | [35] | |
| 62 | Histidine | Root | [35] | |
| 63 | Lysine | Root | [35] | |
| Others | 64 | Emodin | Root | [31] |
| 65 | Emodin-8-O-β-D-glucoside | Root | [31] | |
| 66 | Epigoitrin | Root | [34] | |
| 67 | Sucrose | Root | [37] | |
| 68 | 5-hydroxymethyl-furaldehyde | Root | [37] | |
| 69 | n-butyl-O-β-D- fructopyranose | Root | [37] | |
| 70 | Mannitol | Root | [37] | |
| 71 | 1-thiocyano-2-hydroxy-3-butenen | Root | [38] | |
| 72 | Sinigrin | Root | [38] | |
| 73 | Syringin | Root | [38] | |
| 74 | 4-(4'-hydroxy-3',5'-dimethoxyphenyl)-3-buten-2-one | Root | [38] | |
| 75 | Indoxyl-O-glucoside | Root | [38] | |
| 76 | (E)-2-[(3'-indole)cyanomethylene]-3-indolinone | Root | [38] | |
| 77 | 1-methoxy-3-acetonitrile indole | Root | [39] | |
| 78 | 3-acetate indole | Root | [39] | |
| 79 | 3- indole aldehyde | Root | [39] | |
| 80 | 1-methoxy-3-indolealdehyde | Root | [39] | |
| 81 | Qingdainone | Aerial part | [40] | |
| 82 | Linolenic | Root | [40] | |
| 83 | Erueic acid | Root | [40] |
Figure 1.
The chemical structures of alkaloids isolated from Isatis indigotica.
Figure 2.
The chemical structures of organic acids isolated from Isatis indigotica.
Figure 3.
The chemical structures of flavonoids and lignans isolated from Isatis indigotica.
Figure 4.
The chemical structures of nucleosides and steroids isolated from Isatis indigotica.
Figure 5.
The chemical structures of amino acids isolated from Isatis indigotica.
Figure 6.
The chemical structures of other compounds isolated from Isatis indigotica.
Pharmacological Activities
Antiviral activity
Epigoitrin, an alkaloid from I. indigotica, can reduce the susceptibility to H1N1 virus and the production of pro-inflammatory cytokines to alleviate pneumonia in restraint-stressed mice.[41] Plant-derived compounds such as indigotin, sinigrin, aloe-emodin and hesperetin display anti-SARS coronavirus effects, effectively blocking the cleavage processing of the 3C-like protease.[40, 41] The injection of IIL extracts can inhibit the infection and proliferation of influenza A, encephalitis B, mumps viruses, etc.[42] The result from the hemagglutination titer test showed a direct inhibitory effect of IIL against influenza A virus.[43] However, there are few studies on its antiviral mechanism of action. 4(3H)-quinazolinone, a compound isolated from the leaves, has the capacity to inhibit influenza and coxsackie virus.[44] In the early stage of viral myocarditis (VMC), the leaves may improve and protect the myocardial cells by inhibiting the synthesis of the virus, enhancing the phagocytosis of leukocytes and reducing the permeability of capillaries.[45] The root aqueous extract can inhibit human H7N9 avian influenza virus in vitro possibly by blocking the absorption of H7N9 avian influenza virus to host cells by inhibiting the hemaglutinin of H7N9 avian influenza virus, so as to prevent the virus invading the host cells.[46] It has a good curative effect on virus-caused pharyngitis, acute upper respiratory tract infection and pneumonia, especially catarrhal inflammation such as cough, nasal obstruction, runny nose and sneeze.[47] Polysaccharides from I. indigotica can inhibit hepatitis B virus (HBV) in vitro, reduce extracellular and intracellular DNA level of HBsAg, HBeAg and HBV in HepG2.2.15 cells in a time and dose-dependent manner.[48, 49] Peptides reduces the mortality of mice infected with influenza virus and inhibits the proliferation of the virus.[50] Aqueous extract of leaves can antivirus such as HSV-II, Dengue virus II and Cytomegalovirus.[51, 53] Aqueous extract of roots can anti HSV-I, inhibits virus replication and proliferation in cells.[52]
Antibacterial activity
The aqueous, ethanol and n-butanol extracts of the leaves have antibacterial effects on Staphylococcus aureus and Escherichia coli.[53, 54] The leaf decoction showed an antibacterial effect in vitro on Staphylococcus aureus, Staphylococcus albus, Streptococcus A and Streptococcus B by use of disk diffusion test.[55] Tryptanthrin, a component isolated from the leaves, has strong inhibitory effects on Trichophyton mentagrophytes, Trichophyton rubrum, Trichophyton tonsurans, and Microsporum canis, which can cause tinea pedis.[56, 57] The roots have a broad-spectrum antibacterial effect, in which tryptanthrin is the main antibacterial active ingredient. The root aqueous extract can inhibit Escherichia coli, Staphylococcus epidermidis, Pneumococcus, Himophilus influenzae, and Streptococcus.[58] The total organic acids from roots also show strong antibacterial activity on Escherichiacoli by cylinder-plate test.[59, 60] Salicylic acid can inhibit excessive release of TNF-α and NO in serum of mice,[61] and the roots decoction can decrease the levels of TNF-α and IL-6 in peritoneal macrophages of mice.[62]
Anti-endotoxin
Bacterial endotoxin is the lipopolysaccharide component existing in the extracellular of gram-negative bacteria, which can stimulate the body’s defence system to release inflammatory factors, such as tumour necrosis factor and nitric oxide, causing fever, disseminated intravascular coagulation, multiple organ failure, and even death.[63, 64] The leaf extract can directly neutralize and degrade endotoxin to reduce the thermophilic and lethality of endotoxin in actinomycin D sensitized mice with endotoxin lethal attack.[65] The chloroform extract of the leaves has the anti-endotoxin effect on Escherichia coli O111B4 with dilution in vitro to 64 times still destroying the endotoxin, and the endotoxin dripped into the vein of rabbits is also destroyed, suggesting that the leaves contain anti-endotoxin active substances.[66, 67] IIR can significantly reduce the level of serum lipid peroxide and improve the activity of superoxide dismutase, suggesting its functions of anti-lipid peroxidation, scavenging free radicals and antagonizing endotoxin.[68] The result of bacterial endotoxin destruction test showed that the different pH value significantly affected the action intensity of the root aqueous extract against bacterial endotoxin, the reason being that the active ingredients contained in the roots against bacterial endotoxin are extracted more easily in an acid environment.[69]
Immunopotentiation
The leaf decoction can promote IL-2 secretion of spleen lymphocytes induced by concanavalin A in normal mice to enhance immunity but has no effect on TNF-α secretion of peritoneal macrophages and the activity of leukocytes, pathological damage and dysfunction.[70, 71] Polysaccharide of the roots has immunopotentiation effects, which can promote specific immune, non-specific immune, humoral immune or cellular immune effects.[72] Intraperitoneal injection of polysaccharide 50mg/kg significantly enhanced the immune function of normal mice with increasing the spleen weight and a total number of leukocytes and lymphocytes.[73, 74] However, it also markedly reduced spleen index and the total number of leukocytes and lymphocytes in the immunosuppressed mice induced by hydrocortisone, and inhibited the delayed anaphylaxis in immunosuppressed mice induced by dinitrochlorobenzene and cyclophosphamide.[75] Further study showed that lectin from the roots could bind to glycoprotein on the cell surface to promote the development of thymus and the proliferation of thymocytes, indirectly maintaining the microenvironment of the thymus, promoting the secretion of thymosin and cytokines by T-lymphocytes and thymic epithelial cells, and improving the immunity of the body.[76]
Anti-inflammation
The leaf decoction has a significant inhibitory effect on methanal induced arthritis in mice and suppresses the local inflammatory reaction and capillary permeability of rabbit skin caused by xylene.[77, 78] Total alkaloids and amino acids from the leaves also alleviate mouse ear oedema, suggesting the anti-inflammatory effects.[79] 70% ethanol extract of the roots can inhibit ear swelling of mice caused by xylene and foot swelling of rats caused by egg white to a certain extent.[80]
Anti-tumour
Indirubin, an alkaloid from I. indigotica, possesses an anti-tumour activity, which strongly inhibits transplanted tumour growth of animals and alleviates chronic myeloid leukaemia.[81, 82] Owing to poor water-soluble and liposoluble properties, the indirubin’s derivatives named derivative III were designed and synthesized to increase solubility with an inhibitory rate of 58% against leukaemia cells.[83] Indirubin is likely to participate in regulating the metabolism of lung cancer cells by inducing the activity of cytochrome P4501A1 and 1B1mRNA enzyme in MCF-7 lung cancer cells.[84, 85] Curdione isolated from the roots can inhibit the proliferation of hepatocarcinoma BEL-7402 cells and ovarian cancer A2780 cells, induce differentiation, reduce the telomerase activity and boost the conversion of tumour cells into normal cells.[86] Indirubin displays significant cytotoxicity in HL-60 cells, eliciting cell pyknosis, condensation and even lyses.[87]
Others
IIL also has a cholagogic effect, which can promote bile excretion and relieve pain.[88, 89] It can depress adenosine diphosphate-elicited platelet aggregation in rabbits due to the efficacy of promoting blood circulation and removing stasis.[90] Indigotin has a significant protective effect against liver injury caused by carbon tetrachloride[91, 92] and the leaves can detoxify the effects of lead poisoning mice.[93] All the pharmacological effects of this plant are summarized in Table 2.
Table 2.
Pharmacological activities of Isatis indigotica
| Pharmacological effect | Tested substance | Model | Tested living system/organ/cell | Result | Dose | Ref. |
|---|---|---|---|---|---|---|
| Anti-virus | Epigoitrin | H1N1 | KM mice | Reduces the production of pro-inflammatory cytokines to alleviate pneumonia. | 88 mg/kg (ig) | [41] |
| Indigotin | SARS-coronavirus | SARS-CoV 3C-like protease | Blocks the cleavage processing of the 3C-like protease | 1, 10, 100 μg/mL | [41] | |
| Alkaloid | Influenza A virus | ICR mice | Prolongs the survival time of infected mice. | 0.65 g/kg (ig) | [42] | |
| Indirubin | Influenza virus | NCI-H292 cells | Inhibits transcription and production of RANTES. | 0.01, 0.1, 1, 10 μM/mL | [43] | |
| 4(3H)-quinazolinone | Escherichia coli | Rabbit | Reduces high body temperature in rabbits caused by endotoxin. | 5 mL/kg (ip) | [44] | |
| Alkaloid | Newcastle disease virus | Chicken embryo fibroblasts | Blocks the absorption of virus, protects cells and reduces virus infection. | 7.8–31.3 μg/mL | [45] | |
| Root aqueous extract | H7N9 avian influenza virus | Chicken embryos | Inhibit human H7N9 avian influenza virus in vitro by blocking the absorption of H7N9 avian influenza virus to host cells. | IC-50 = 5000 μg/mL | [46] | |
| Unnamed Compounds from leaves | Respiratory syncytial virus | Hep-2 cells | Inhibits the proliferation of respiratory syncytial virus after invading Hep-2 cells. | 10–120 μg/mL | [47] | |
| Polysaccharide | HSV-II | BALB/C mice | Reduces the incidence rate, mortality and prolongs the average survival time in mice. | 0.5 and 1.0 mg/kg (ip) | [48] | |
| HBV | HepG2/2–15 cells | Reduces extracellular and intracellular levels of HBsAg, HBeAg and HBV DNA in cells. | 50, 100 and 200 μg/mL | [49] | ||
| Peptides | H1N1 | KM mice | Reduces the mortality of mice infected with influenza virus and inhibits the proliferation of virus. | 50, 100 and 200 mg/kg (ig) | [50] | |
| Leaf aqueous extract | HSV-II | Vero cells | Inhibits the replication and Inhibits proliferation of HSV-II in cells. | 0.25–16 mg/mL | [51] | |
| Root aqueous extract | HSV-I | Hep-2 cells | Inhibits biosynthesis of HSV-I in vitro. | 2–128 mg/mL | [52] | |
| Leaf aqueous extract | Dengue virus II | C6/36 cells | Inhibits virus replication and proliferation in cells | 0.5–4.0 mg/mL | [53] | |
| Leaf ethanol extract | Cytomegalovirus | Guinea pig embryo lung cells | Antiguinea pig cytomegalovirus activity. | 3 g·mL−7–3 g·mL−1 | [54] | |
| Antibacterial | Leaf aqueous extract | Shigella Castellani | Tube method | Obvious inhibitory effect | 25–400 mg/kg | [55, 56] |
| Streptococcus pneumoniae | ||||||
| Staphylococcus aureus | ||||||
| Organic acid | ||||||
| Alkaloid | Escherichia coli | Oxford Cup | Components have strong antibacterial activity. | 2.0 g/mL | [57, 58] | |
| Nucleoside | ||||||
| Anthraquinone | ||||||
| Salicylic acid | Lipopolysaccharide | Balb/c mice | Inhibits excessive release of TNF-α and NO in serum of mice. | 20 mL/kg (ip) | [59] | |
| Root decoction | Lipopolysaccharide | Peritoneal macrophage | Decreases the levels of TNF-α and IL-6 in peritoneal macrophages of mice. | 1 g/mg | [60] | |
| Immunomodulatory | Polysaccharide | Lymphocyte | KM mice | Enhances peripheral blood lymphocytes in mice. | 2 mg/mL | [61] |
| Balb/c mice | Promotes the humoral immune response of the body and produces immune effect. | 4 mg/mL | [62] | |||
| Fructopyrano-(1→4)-glucopyranose | Macrophage phagocytosis | KM mice | Enhance the phagocytic function of peritoneal macrophages in mice. | 100, 200 mg/kg (ig) | [63] | |
| Root ethanol extract | Lipopolysaccharide | RAW264.7 cells | Inhibits the release of PGE 2 and TNF-α. | 0.1,0.5,1.0,2.5 mg/mL | [64] | |
| Antitumor | Polysaccharide | S-180 cells | ICR mice | Enhances the immune function of tumor bearing mice and prolongs the survival time of tumor bearing mice | 50,100 mg/kg (ig) | [65] |
| Indirubin-3'-oxime | MV4-11 cells | BALB/c nude mice | Increases the anti-proliferative efficacy of MV4-11 cells | 20 mg/kg (ig) | [66] | |
| Indirubin | leukemia | HL-60 cells | Elicits pyknosis, condensation and lyses in cells. | 25, 50, 100, 200, 400 μg/mL | [67] | |
| Leaf ethanol extract | Medicated serum | K562 cells | The drug containing serum inhibits the proliferation of cells. | 1 g/mL | [68] |
Toxicity
I. indigotica is generally considered nontoxic, however, the adverse reactions of its leaves occur from time to time as reported in the literature.[94, 95] The extracts of roots of I. indigotica, also called Banlangen, can induce the micronucleus rate of polychromatic erythrocytes in mouse bone marrow and increase the sperm deformity rate of mice, suggesting certain genotoxicity in mammalian somatic cells and germ cells.[96, 97]
Clinical Application
Hepatitis
The leaves of I. indigotica show significantly improvement effects on acute common infectious hepatitis. 32 cases of icterohepatitis were treated with the leaves of I. indigotica in combination with roots of Salviae miltiorrhizae, roots of Curcumae longae, roots of Dryopteridis crassirhizomatis and fruits of Ziziphus jujuba, and the effective rate was 94%.[98, 99] Yigan-Jiedu decoction composed of the leaves and roots of I. indigotica, roots of Salviae miltiorrhiza, roots of Astragalus membranaceus, and the whole herb of Lysimachia christinae apparently improved the symptoms and signs of 86 cases with chronic hepatitis B when compared with the control group.[100] Another injection named Shu-gan-ning, composed of roots of I. indigotica, Ganoderma lucidum, fruits of Kochia scoparia, fruits of Gardenia jasminoides, and roots of Scutellaria baicalensis, quickly alleviated jaundice symptoms of 45 cases with acute icteric hepatitis, and the clinical effective rate was 91%.[101, 102] Qinggan-Lidan decoction, consisted by the roots of I. indigotica, whole herb of Artemisia carvifolia, fruits of Gardenia jasminoides, barks of Phellodendri chinensis, the whole herb of Bupleurum chinense, Poria cocos, roots of atractylodis macrocephalae, and semens of Coix lacryma-jobi, treated 100 cases with acute icteric hepatitis and the effective rate was 100%. The compound decoction is simple, easy to use, economical and cheap, and has few reported side effects.[103]
Parotitis
Total 92 cases of children mumps were treated with the formula containing the leaves combined with ganciclovir. The time of fever abatement, parotid swelling abatement and parotid pain abatement was significantly shortened in the treatment group when compared with the control group, and their effective rates were 97.83% and 80.43%, respectively.[104, 105] The formula comprised of the roots of I. indigotica, borneolum syntheticum and cactus cured all 45 cases of epidemic parotitis, with 15 cases cured in two days, accounting for 33%, 21 cases in three days accounting for 47%, 9 cases in four days accounting for 20%.[106] The external application of jinhuang ointment combined with the oral administration of the root granules has an effective rate of 100% when treating 60 cases of children mumps and no adverse reactions and complications were reported in any of the patients.[107]
Upper respiratory tract infection
Total 56 cases of upper respiratory tract infection were treated with the root granules, and the effective rate was 98.21%, which is higher than that of 80.36% observed in the control group treated with ribavirin only.[108, 109] A similar result for the root granules was observed in another 60 cases of upper respiratory tract infection, with the effective rate of 100% versus 87% in the control group treated with ribavirin only.[110] Oseltamivir phosphate combined with the root granules showed significant clinical efficacy in the treatment of influenza A (H1N1) when compared the control group of patients received oseltamivir phosphate alone, and the total effective rate was 97.14%.[111]
Others
The decoction comprised of the leaves and roots of I. indigotica, herba lysimachiae and radix et rhizoma rhei displayed significant improvement effects in the treatment of pointed condyloma 28 cases, among whom, 14 cases were cured, 12 improved and 2 ineffective, having an effective rate of 92.8% when oral decoction was combined with fumigation and washing.[112] 35 cases of palmoplantar pustulosis were treated topically with the formula consisting of the leaves, herba violae, flos lonicerae, radix sophorae flavescentis, fructus kochiae, fructus cnidii, semen plantaginis, rhizoma atractylodis, and alum, and the total effective rate was 68.57%.[113] 136 cases of epidemic kerato-conjunctivitis were treated with the root granules in combination with herba houttuyniae injection, 110 cases recovered, and the cure time was 2–15 days, averaging 5.6 days.[114] The compound granule could treat viralmyocarditis, which consists of the leaves and roots of I. indigotica, fructus forsythiae, and rhizoma bistortae, and the effective rate was 85.5%, among whom, 23 cases were excellent, 77 fine, 17 ineffective for ventricular premature beats symptom.[115]
Conclusions and Perspectives
Natural agents which are commonly derived from plants or herbs could not only give us essential foods for living, including sugars, lipids, proteins and vitamins, but also supply us some precious medicinal secondary metabolites for preventing various diseases, such as berberine, artemisinin, emodin, and taxol.[116–118] As a natural plant, I. indigotica contains alkaloids, organic acids, flavonoids, lignans, nucleosides, amino acids, and steroids. Previous pharmacological researches indicated that I. indigotica possesses promising antivirus, antibacterial, immunoregulatory, anti-inflammation, and cholagogic effects. Importantly, it can inhibit various viruses, such as influenza, hepatitis B, mumps, herpes simplex, cytomegalovirus, and coxsachievirus. Clinically, it is frequently used to treat various viral diseases like viral influenza, parotitis and viral hepatitis. Consequently, I. indigotica may be beneficial for the prevention and treatment of coronavirus disease 2019 (COVID-19). I. indigotica has the function of immune regulation, which reinforces its anti-virus effects in turn. Therefore, I. indigotica may be effective for the prevention and treatment of COVID-19, however, this need to be investigated further. Although numerous chemical constituents have been isolated and identified from I. indigotica, the active components, mechanisms of action and their target remain unknown. As the clinic application of Chinese medicines is characterized by compatibility, the therapeutic mechanism of I. indigotica combined with other medicines should be investigated further. However, it is rather difficult to clarify the mechanism at the molecular level based on the compatibility of the crude extracts or components. The compound-based Chinese medicine formula (CCMF) may be promising for clarification of the mechanism and target due to its clear composition of compounds derived from Chinese medicines. The action targets of compounds can be investigated through such techniques as CETSA, DARTS, and MST. When the mechanism of compatibility for CCMF is defined, the scientific connotation for the TCM compatibility theory will probably be clarified.
Funding
This work was supported by funds from the National Natural Science Foundation of China (No. 81773941), National Key Subject of Drug Innovation (2019ZX09201005-007), National key R & D program for key research project of modernization of traditional Chinese medicine (2019YFC1711602) and Xinglin Scholar Discipline Promotion Talent Program of Chengdu University of Traditional Chinese Medicine (no. BSH2018006).
Author Contribution
QC and HYL reviewed the literature and wrote the manuscript, WP and KR revised the manuscript, QCL, XL and HZ conceived and designed the study and revised the manuscript.
Conflicts of Interest
The authors confirm that this article content has no conflicts of interest.
References
- 1. Liang ZB, Li HY, Li XL, et al. Study on the effective components of Isatis indigotica root, stem and leaf. Guangzhou Chem 2016; 44: 156–7. 10.3969/j.issn.1001-9677.2016.11.054 [DOI] [Google Scholar]
- 2. Pei Y. Pharmaceutical research on the medicinal parts of Isatis indigotica and Indigo indigotica[D]. Heilongjiang Univ Trad Chin Med 2007. [Google Scholar]
- 3. Qu RJ, et al. Selection of reference genes for the quantitative real-time PCR normalization of gene expression in Isatis indigotica fortune. BMC Mol Biol 2019; 20: 9. 10.1186/s12867-019-0126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan ZH. Clinical practical Chinese medicine[M]. Beijing: People’s Health Publishing House; 1984. ISBN: 14048–519. [Google Scholar]
- 5. Li HY, Li YG, Liang QY, et al. Extraction and content comparison of indigo and indirubin in Isatis indigotica root, stem and leaf. Guangdong Chem 2016; 43: 27–8. https://doi.org/CNKI:SUN:GDHG.0.2016-02-012 [Google Scholar]
- 6. Yu YP, Gong D, Ye Z, et al. Quality consistency evaluation of Isatidis Folium combined with equal weight quantified ratio fingerprint method and determination of antioxidant activity. J Chromatogr B 2018; 1095: 149–56. 10.1016/j.jchromb.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 7. Liao BL, Pan LW, Pan YJ, et al. Four natural compounds separated from Folium isatidis: crystal structures and antibacterial activity. Chem Biodiver 2018; 15–15. 10.1002/cbdv.201800152 [DOI] [PubMed] [Google Scholar]
- 8. Li X. Study on Chemical composition and quality control of isatis root[D]. Shanxi Medical University, 2010. 10.7666/d.Y1686322 [DOI]
- 9. Zheng H Z, Dong Z H, She J.. Modern research and application of traditional Chinese medicine[M]. Beijing: Xueyuan Press, 1993. [Google Scholar]
- 10. Li L, Yang GJ. Chemical constituents of Isatis indigotica. Chin Herb Med 1996; 027(007): 389–91. 10.7501/j.issn.0253-2670.1996.7.218 [DOI] [Google Scholar]
- 11. Deng X Y, Gao GH, Li FM, et al. Chemical constituents of Folium isatidis. J Shenyang Pharm Univ 2009; 26: 274–8. https://doi.org/CNKI:SUN:SYYD.0.2009-04-007 [Google Scholar]
- 12. Liu J F, Zhang XM, Xue DQ, et al. Chemical constituents of Folium isatidis. Chin J Trad Chin Med 2006; 31(23): 1961–5. 10.3321/j.issn:1001-5302.2006.23.012 [DOI] [PubMed] [Google Scholar]
- 13. Liu J F, Jiang ZY, Wang RR, et al. Isatisine A, a novel alkaloid with an unprecedented skeleton from leaves of Isatis indigotica. Org Lett 2007; 9: 4127–9. 10.1021/ol701540y [DOI] [PubMed] [Google Scholar]
- 14. Li W, Chen FK, Yin XW, et al. Chemical constituents of Folium isatidis. J Shenyang Pharm Univ 2005; ( 01): 15–6. 10.3969/j.issn.1006-2858.2005.01.005 [DOI] [Google Scholar]
- 15. Liu R, Yuan B, Liu ZQ, et al. Identification of five chemical components in the aqueous ex-tract of Folium isatidis by HPLC-MS2. Trad Chin Med 2005; 018(009): 33–5. 10.13863/j.issn1001-4454.2005.09.013 [DOI] [Google Scholar]
- 16. Ruan JL, Zou JH, Cai YL. Chemical constituents of Folium isatidis. Chin J Trad Chin Med 2005; 30(19): 49–50. 10.3321/j.issn:1001-5302.2005.19.012 [DOI] [PubMed] [Google Scholar]
- 17. Gao GH. Study on the separation, identification and determination of flavonoids in folium isatidis[D]. Shenyang Pharmaceutical University, 2008. [Google Scholar]
- 18. Chen XH. Study on the extraction and refining technology of effective components from Isatis indigotica[D]. Chengdu University of Technology, 2005. [Google Scholar]
- 19. Wu X, Liu Y, Sheng W, et al. Chemical constituents of Isatis indigotica. Planta Med 1997; 63: 55–7. https://doi.org/CNKI:SUN:KFYZ.0.1999-03-039 [DOI] [PubMed] [Google Scholar]
- 20. Li WJ. Study on the extraction and refining process of the effective compo-nents of Isatis indigotica leaves. Biotechnol World 2016; 222. https://doi.org/CNKI:SUN:SWJJ.0.2016-03-183 [Google Scholar]
- 21. Gong M G. Studies on the dynamics of the synthesis and accumulation of the active components of isatis indigotica and the differences of their contents[D]. Northwest Agricultural and Forestry University of Science and Technology, 2005. [Google Scholar]
- 22. Lv WY, Lv P. Determination of eight inorganic elements in the Folium isatidis of Flos Lonicerae and Forsythia suspense. Microelement Health Res 2003; ( 06): 26–7. 10.3969/j.issn.1005-5320.2003.06.012 [DOI] [Google Scholar]
- 23. Liu YH, Qin GW, Ding SP, et al. Chemical constituents of isatis root (I). Chin Herb Med 2001; 32(12): 4–7. https://doi.org/CNKI:SUN:ZCYO.0.2001-12-000 [Google Scholar]
- 24. Ding SP, Liu YH, Li J, et al. Study on chemical constituents of isatis root (II). Med J 2001; 020(008): 475–6. 10.3870/j.issn.1004-0781.2001.08.001 [DOI] [Google Scholar]
- 25. Fang JG, Wang SB, Xu H, et al. Chemical constituents of isatis root (I). Chin Herb Med 2004; 35(8): 9–10. 10.7501/j.issn.0253-2670.2004.8.387 [DOI] [Google Scholar]
- 26. Liu HL, Wu LJ, Li H, et al. Chemical constituents of Radix isatidis. J Shenyang Pharm Univ 2002; ( 02):93–5. 10.3969/j.issn.1006-2858.2002.02.005 [DOI] [Google Scholar]
- 27. Liang Y N, Zhu J, Tang ZS, Sun XC, et al. Optimization of ultrasonic extraction process and antioxi-dant activity of total flavonoids from Radix isatidis by box Behnken re-sponse surface methodology. J Changchun Univ Trad Chin Med 2019; 35: 119–24. 10.13463/j.cnki.cczyy.2019.01.036 [DOI] [Google Scholar]
- 28. Zhang H J, He LW, Tang M. Molecular docking study on anti influenza virus of flavonoids in isatis root. Chem Time 2018; 32: 19–21. 10.16597/j.cnki.issn.1002-154x.2018.03.007 [DOI] [Google Scholar]
- 29. Zhao WT. Optimization of ultrasonic assisted extraction technology and antioxidant activity of Flavonoids from Isatis indigotica root by response surface methodology. Chin Modern Appl Pharm 2016; 33: 313–7. 10.13748/j.cnki.issn1007-7693.2016.03.013 [DOI] [Google Scholar]
- 30. Liu YH, Ding SP, Wu XY, et al. Study on chemical constituents of isatis root (III). Chin Herb Med 2002; 33(002): 3–5. 10.7501/j.issn.0253-2670.2002.2.041 [DOI] [Google Scholar]
- 31. Liu YH, Qin GW, Wu XY, et al. Study on chemical constituents of isatis root (IV). Med J 2003; 22(9): 591–4. https://doi.org/CNKI:SUN:YYDB.0.2003-09-000 [Google Scholar]
- 32. Chen Y, Fan CL, Wang Y, et al. Chemical constituents of Radix isatidis. Chin J Trad Chin Med 2018; 43: 2091–6. 10.19540/j.cnki.cjcmm.20180116.001 [DOI] [PubMed] [Google Scholar]
- 33. Liu LF, Li FZ. Chemical composition, pharmacology and quality control management of Radix Isatidis. Chin Health Indu 2018; 15: 36–7. 10.16659/j.cnki.1672-5654.2018.13.036 [DOI] [Google Scholar]
- 34. Sun Q, Zhao J, Zhang SJ, et al. Chemical constituents of isatis root Radix isatidis. Chin J Exp Pharmacol 2012; 18: 74–5. 10.1007/978-1-4020-9623-5-5 [DOI] [Google Scholar]
- 35. Wang XL, Chen MH, Wang F, et al. Studies on the chemical constituents of Radix isatidis. In: 9th Natural organic chemistry academic conference of China Chemical Society, Haikou, Hainan, China, 2012. [Google Scholar]
- 36. Liu YH, Wu XY, Fang JG, et al. Study on chemical constituents of Radix isatidis (V). Zhongnan Pharm 2003; 1(5): 302–5. https://doi.org/CNKI:SUN:ZNYX.0.2003-05-017 [Google Scholar]
- 37. Yan J. Study on chemical constituents and activity evaluation of radix isatidis and Flaxseed[D]. Jilin University, 2011. [Google Scholar]
- 38. Wang T, Wang X, Zhuo Y, et al. Antiviral activity of a polysaccharide from Radix isatidis (Isatis indigotica Fortune) against hepatitis B virus (HBV) in vitro via acti-vation of JAK/STAT signal pathway. J Ethnopharmacol 2020;257: 112782. 10.1016/j.jep.2020.112782 [DOI] [PubMed] [Google Scholar]
- 39. Kurihara H, Luo Z, Liu LF, et al. Isatis indigotica Epigoitrin, an alkaloid from reduces H1N1 infection in stress-induced susceptible model in vivo and in vitro. Front Pharmacol 2019; 10: 78. 10.3389/fphar.2019.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsai FJ, Hsieh CC, Tsai CH, et al. Anti-SARS coronavirus 3C-like protease effects of Isatis ndigotica root and plant-derived phenolic compounds. Antiv Res 2005; 68: 36–42. 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peng W, Qin R, Li X, et al. Polygonum cuspidatum Sieb. et Zucc.: a review of its botany, phytochemistry, pharmacology, and potential applications. J Ethnopharmacol 2013, 148: 729–45. 10.1016/j.jep.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 42. Zhou LN, Yuan B, Su X, et al. Study on the in vitro antibacterial effect and anti endotoxin effect of compound Folium isatidis injection. J Shenyang Pharm Univ 2006; ( 04): 247–50. https://doi.org/CNKI:SUN:SYYD.0.2006-04-013 [Google Scholar]
- 43. Liu S, Chen WS, Qiao CZ, et al. Anti influenza A virus effects of different germplasm of isatis root and Folium isatidis. J Second Milit Med Univ 2000; ( 03) 204–6. 10.16781/j.0258-879x.2000.03.003 [DOI] [Google Scholar]
- 44. Xu T. Anti-viral effects of 4(3H)quinazolinone from Folium isatidis against influenza virus A and PRRS virus in vitro[D]. Gansu Agricultural University, 2008. [Google Scholar]
- 45. Li XQ. Mechanism of Astragalus Membranaceus and Folium isatidis in the treatment of viral Myocarditis in mice[D]. The Fourth Military Medical University, 2002. [Google Scholar]
- 46. Li ZT, Li L, Wang YT, et al. Study on the efficacy of aqueous extract of Radix isatidis in inhibiting human H7N9 avian influenza virus in vitro. J Moder Chin Wester Med 2016; 25: 3877–9. 10.3969/j.Issn.1008-8849.2016.35.001 [DOI] [Google Scholar]
- 47. Yan XS, Zhou LR, Cuo J, et al. Clinical observation on 179 cases of influenza A H1N1 treated with non Tamiflu drugs. Med Innov Chin 2010;7(8): 130–1. 10.3969/j.issn.16744985.2010.08.070 [DOI] [Google Scholar]
- 48. Zheng JL, Wang MH, Yang XZ, et al. Study on bacteriostasis of extracts from Folium isatidis and Radix isatidis. Chin J Microbiol 2003; 015(001): 21–2. 10.3969/j.issn.1005-376X.2003.01.008 [DOI] [Google Scholar]
- 49. Zhang LT, Qu SC, Lv JH, et al. Antibacterial effect of Folium isatidis in vitro. Lishizhen Med Mater Med Res 2002; ( 05): 283–4. 10.3969/j.issn.1008-0805.2002.05.013 [DOI] [Google Scholar]
- 50. Hou JY, Fang TH.. Pharmacology of traditional Chinese Medicine. Beijing: China Press of Traditional Chinese Medicine, 2007, 54–5. [Google Scholar]
- 51. Ma JM. Analysis of modern pharmacology and clinical application of Radix isatidis. Chin Health Standard Manag 2014; 5: 65–6. 10.3969/J.ISSN.1674-9316.2014.06.038 [DOI] [Google Scholar]
- 52. Hu XY, Liu MH, Sun Q, et al. Study on the spectrum effect relationship of the antibacterial active parts of Radix isatidis. Chin Herba Med 2013; 44: 1615–20. 10.7501/j.issn.0253-2670.2013.12.018 [DOI] [Google Scholar]
- 53. Yao XY. Study on the regulatory mechanism of CD11b activation on the pathogenesis of endotoxic shock and related immune cell activation[D]. Jinan University, 2019. [Google Scholar]
- 54. Lejars M, Hajnsdorf E. The world of as RNAs in gram-negative and gram-positive bacteria. BBA-Gene Regul Mech 2020; 1863–3. 10.1016/j.bbagrm.2020.194489 [DOI] [PubMed] [Google Scholar]
- 55. Fang JG, Shi CY, Tang J, et al. Screening of anti-endotoxin active parts of Folium isatidis. Chin Herb Med 2004; 35(1): 64–6. https://doi.org/CNKI:SUN:ZCYO.0.2004-01-025 [Google Scholar]
- 56. Li Y. Study on the basis of antiviral active substances of folium isatidis[D]. Chengdu University of Traditional Chinese Medicine, 2006. [Google Scholar]
- 57. Tang J, Shi CY, Fang JG, et al. Tang effect of Radix isatidis on serum LPO and SOD levels in rabbits with endotoxic DIC. Herald Med 2004; ( 01): 4–5. 10.3870/j.issn.1004-0781.2004.01.003 [DOI] [Google Scholar]
- 58. Hu WB, Wu JL, Li WS. The destructive effect of Radix isatidis extracted by different methods on bacterial endotoxin. Res Prac Chin Med 2003; 17(4): 60–1. 10.3969/j.issn.1673-6427.2003.04.026 [DOI] [Google Scholar]
- 59. Zhao H, Zhang SJ, Ma LR. In vitro study on the regulation of IL-2 and TNF-α secretion by mouse immune cells by Folium isatidis decoction. Shaanxi J Trad Chin Med 2003; 24(008): 757–9. 10.3969/j.issn.1000-7369.2003.08.064 [DOI] [Google Scholar]
- 60. Jing XP, Pei M, He L. Immunomodulatory mechanism of Astragalus polysaccharide and Isatis polysaccharide based on antibody chip technology. Chin J Trad Chin Med Pharm 2013; 28: 3420–3. https://doi.org/CNKI:SUN:BXYY.0.2013-11-079 [Google Scholar]
- 61. Geng CJ, Liu ZM, Chi XX, et al. Immunomodulatory effect of Radix isatidis polysaccharide on immunosuppressive mice. Agri Prod Process 2012; 4: 36–9. https://doi.org/10.3969.jigsn.1671–9646(x).2012.04.009 [Google Scholar]
- 62. Wang HY, Ma YF, Zhou WX. Toxicity test of Isatis polysaccharide and its effect on immune system of mice. J Mianyang Norm Univ 2012; 31: 75–80. 10.3969/j.issn.1672-612X.2012.02.021 [DOI] [Google Scholar]
- 63. Zhang J, Hu AJ, Bi YN, et al. Two way immunoregulation of Isatis polysaccharide on cyclophosphamide model rats. Drug Eva Res 2016; 39: 531–8. 10.7501/j.issn.1674-6376.2016.04.005 [DOI] [Google Scholar]
- 64. Wang L, Zhou CL, Ding YQ. Experimental study on antipyretic, anti-inflammatory, analgesic and bacteriostatic effects of new compound folium isatidis tablets. Chin J Trad Med Sci Technol 2007; 14: 412–3. 10.3969/j.issn.1005-7072.2007.06.014 [DOI] [Google Scholar]
- 65. Shi GJ, Zhang J. Experimental study on the pharmacological effect of ethanol precipitate of Folium isatidis. J Henan Univ Chin Med 2006; 21(4): 15–6. 10.16368/j.Issn.1674-8999.2006.04.009 [DOI] [Google Scholar]
- 66. Wei CL, Yan XL. Anti inflammatory effect of isatis root. J Henan Univ Chin Med 2000; 019(004): 53–4. 10.15991/j.cnki.41-1361/r.2000.04.044. [DOI] [Google Scholar]
- 67. Yu MF. Synthesis and antitumor activity of indirubin analogues[D]. Shang Hai JiaoTong University, 2009. [Google Scholar]
- 68. Wu KM, Zhang MY, Fang Z, et al. Synthesis of indirubin, indigo and isoindigo derivatives. Acta Pharm Sin 1985;11: 821–6. 10.16438/j.0513-4870.1985.11.005 [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Sha F, Chen YH, et al. Research progress on antitumor and neuroprotective effects of indirubin and its analogues. Chin Med Herb 2014; 45: 2404–11. 10.7501/j.issn.0253-2670.2014.16.024 [DOI] [Google Scholar]
- 70. Liang YH, Hou HX, Li DR, et al. Anticancer activity of Radix isatidis diketone B in vitro. Chin Med Herb 2000; 31(7): 53–5. https://doi.org/CNKI:SUN:ZCYO.0.2000-07-028 [Google Scholar]
- 71. Hsuan SL, Chang SC, Wang SY, et al. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol 2009; 123: 61–7. 10.1016/j.jep.2009.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tian DH. Pratical dictionary of traditional Chinese medicine. Beijing: People’s Medical Publishing House, 2002. [Google Scholar]
- 73. Yu S, Yao XS, Chen YJ, et al. Study on the active components of promoting blood circulation in Radix isatidis. Bull Chin Mater Med 1988; 13(2): 31–2. [Google Scholar]
- 74. Hou JY, Fang TH. Pharmacology of traditional Chinese medicine[M]. Beijing: Chin Press Trad Chin Med 2007: 54–5. [Google Scholar]
- 75. Zhao L, Liu N, Sun ZC. Antagonistic effect of Mungbean and Folium isatidis on lead toxicity. Chin J Public Heal 2004; 20(008): 74. 10.11847/zgggws2004-20-08-44 [DOI] [Google Scholar]
- 76. Pang ZL, Tang J, Zhu WY, et al. Effect of Radix isatidis on genotoxicity of experimental mice. Acad J Guangzhou Med Coll 2000; 28(3): 41–4. 10.3969/j.issn.1008-1836.2000.03.013 [DOI] [Google Scholar]
- 77. Jin MZ, Ren DX, Meng FP, et al. Effect of Radix isatidis on immune function and influenza virus FM1. Lishizhen Med Mater Med Res 2007; 02: 394–6. 10.3969/j.issn.1008-0805.2007.02.073 [DOI] [Google Scholar]
- 78. Hou XB. Study on the Discovery and Mechanism of Potential Pharmacodynamic Components of Radix isatidis[D]. Nanjing University of Chinese Medicine, 2017. [Google Scholar]
- 79. Liu QW. Quality Standard Research of Isatis indigotica[D]. Gansu Agricultural University, 2018. [Google Scholar]
- 80. He LW, Wu XP, Yang JY, et al. Extraction and purification of total alkaloids from Radix isatidis and their antiviral pharmacological effects. Chin Patent Drug 2014; 36: 2611–4. 10.3969/j.issn.1001-1528.2014.12.039 [DOI] [Google Scholar]
- 81. Xu YF, Liu JG, Zhao B, et al. Effect of acid of Radix isatidis alkaloid on adsorption and release of Newcastle disease virus. J Nanjing Agri Univ 2010; 33: 90–4. 10.7685/j.issn.1000-2030.2010.06.017 [DOI] [Google Scholar]
- 82. Zuo Y, Zhu HJ, Liu J, et al. Experimental study on the anti herpes simplex virus type II effect of Isatis polysaccharide. West Chin J Pharm Sci 2013; 28: 267–9. https://doi.org/CNKI:SUN:HXYO.0.2013-03-018 [Google Scholar]
- 83. Mak NK, Leung CY, Wei XY, et al. Inhibition of RANTES expression by indirubin in influenza virus-infected human bronchial epithelial cells. Biochem Pharmacol 2004; 67: 167–74. 10.1016/j.bcp.2003.08.020 [DOI] [PubMed] [Google Scholar]
- 84. Liu XJ, Lin SJ. Study on anti-virus effect of peptides from Isatis indigotica on the mice infected by influenza virus. Chin Pharm 2014; 25: 590–2. 10.6039/j.issn.1001-0408.2014.07.05 [DOI] [Google Scholar]
- 85. Yu SQ, Chen XY, Yu L. In vitro experimental study on the anti herpes simplex virus type II effect of extracts from Folium isatidis. Herald Med 2008; 027(004): 394–6. 10.3870/j.issn.1004-0781.2008.04.011 [DOI] [Google Scholar]
- 86. Fang JG, Hu Y, Tang J, et al. Effect of Radix isatidis on herpes simplex virus type I in vitro. Chin Trad Herb Drugs 2005; 30(17): 242–4. 10.3321/j.issn:1001-5302.2005.17.011 [DOI] [PubMed] [Google Scholar]
- 87. Li XQ, Zhang GC, Xu DL, et al. A comparative study of Astragalus and Folium isatidis in the treatment of viral myocarditis in mice. Chin J Contemp Pediatr 2003; 05(05): 439–42. https://doi.org/CNKI:SUN:DDKZ.0.2003-05-011 [Google Scholar]
- 88. Liu Z, Yang ZQ, Xiao H. Experimental study on the effect of effective monomer of Folium isatidis on respiratory syncytial virus. Lishizhen Med Mater Med Res 2009; 20: 1977–9. 10.3969/j.issn.1008-0805.2009.08.070 [DOI] [Google Scholar]
- 89. Hong WY, Tang BH, Liu JH, et al. In vitro experimental study on the anti dengue virus type II effect of extracts from Folium isatidis. Chin J Moder Drug Appl 2010; 4: 161–2. 10.3969/j.issn.1673-9523.2010.20.146 [DOI] [Google Scholar]
- 90. Liu HZ, Chen SH, Wang XR, et al. Preliminary study on the effect of Folium isatidis on cyto-megalovirus induced cytopathy. Chin J Birth Heal Heredity 2006; 14(1): 58–60. https://doi.org/CNKI:SUN:ZYYA.0.2006-01-031 [Google Scholar]
- 91. Chang XB. Study on the chemical basis of endotoxin and pharmaco dynamics of Radix isatidis and resistance. Jilin Med J 2013; 34: 5539. 10.3969/j.issn.1004-0412.2013.27.001 [DOI] [Google Scholar]
- 92. Chu YF, Li XR, Hu YL. Effects of Chinese herbal medicinal ingredient on cells mediated immunity in mice. J Nanjing Agri Univ 2004; ( 01): 97–100. 10.3321/j.issn:10002030.2004.01.024 [DOI] [Google Scholar]
- 93. Xu YQ. Chemical separation of Isatis polysaccharide and its immunoenhancement activity. Biotechnol World 2015;007: 140. https://doi.org/CNKI:SUN:SWJJ.0.2015-07-126 [Google Scholar]
- 94. Liu MH, Li M, Xiao SH, et al. Effects of Fructopyrano-(1→4)-glucopyranose extracted from Radix isatidis on tumor growth and immune function in tumor-bearing mice. Chin Pharm J 2012; 47: 1542–6. 10.3969/j.issn.1008-0805.2012.06.013 [DOI] [Google Scholar]
- 95. Ma YM, Li N, Liu CW, et al. Comparative study on anti-inflammatory and analgesic activities of different extracts of Radix isatidis. Chin Trad Herb Drugs 2014; 45: 2517–21. 10.7501/j.issn.0253-2670.2014.17.017 [DOI] [Google Scholar]
- 96. Li JP, Zhu GH, Yuan Y, et al. Experimental study on antitumor effect and immune function regulation of Isatis polysaccharide in vivo. Nat Prod Res Develop 2017; 29: 2010–6. [Google Scholar]
- 97. Jeong P, Moon Y, Lee JH, et al. Discovery of orally active indirubin-3’-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia. Eur J Med Chem 2020; 195: 112205. 10.1016/j.ejmech.2020.112205 [DOI] [PubMed] [Google Scholar]
- 98. Jian XS, Yin YZ, Chen JQ, et al. In vitro antitumor activity of ethanol extract of Folium isatidis containing serum. J Chin Med Mater 2013; 36: 633–5. https://doi.org/CNKI:SUN:ZYCA.0.2013-04-041 [Google Scholar]
- 99. Huo XY. 32 cases of icteric hepatitis treated with Folium isatidis mixture. Shaanxi J Trad Chin Med 1985; 05: 222. [Google Scholar]
- 100. Yan Q. Clinical observation on 86 cases of chronic hepatitis B treated with Yiganjiedu Decoction and entecavir. Clin J Chin Med 2017; 9:42–3. 10.3969/j.issn.1674-7860.2017.02.020 [DOI] [Google Scholar]
- 101. Tai J, Liu F, Yang XR, et al. Clinical analysis of 45 cases of acute icteric hepatitis treated with Shuganning injection. J Clin Int Med 2007; 24(02): 103. 10.3969/j.issn.1001-9057.2007.02.024 [DOI] [Google Scholar]
- 102. Zhang JL, Yue AP, Huang DH, et al. 6th National Congress of difficult and severe liver diseases, Lanzhou, Gansu, China, 2011. [Google Scholar]
- 103. Liu Y, Lu P. Clinical study of compound Folium isatidis mixture combined with ganciclovir in the treatment of children mumps. Mod Pharm Clin 2019; 34: 1414–7. https://doi.org/CNKI:SUN:GWZW.0.2019-05-032 [Google Scholar]
- 104. Han H. Application of JinHuang ointment combine with Radix isatidis granules in children mumps. Chin Forei Med Treatment 2012; 31: 117. 10.3969/j.issn.1674-0742.2012.18.089 [DOI] [Google Scholar]
- 105. Hu XY, Liu MH, Zhang SJ, et al. Study on the spectral activity relationship of the antibacterial active parts of Isatidis Radix. Chin Trad Herbal Drugs 2013; 44:1615–20. https://doi.org/CNKI:SUN:ZCYO.0.2013-12-017 [Google Scholar]
- 106. Li J, Liu YH, Huang MS, et al. Anti-endotoxic effects of 4(3H)-quinazolinone from Radix isatidis. West Chin J Pharm Sci 2008; 23(01): 7–9. 10.3969/j.issn.1006-0103.2008.01.003 [DOI] [Google Scholar]
- 107. Li J, Tang J, Xie W, et al. Anti-endotoxic effects of salicylic acid from Radix isatidis. Chin J Hosp Pharm 2007; 027(010): 1349–52. 10.3321/j.issn:1001-5213.2007.10.006 [DOI] [Google Scholar]
- 108. Huang SX. Clinical observation on the treatment of upper respiratory tract infection with Radix isatidis granules. J North Pharm 2018; 15:149. https://doi.org/CNKI:SUN:BFYX.0.2018-05-128 [Google Scholar]
- 109. Zhan Y. Radix isatidis, Borneolum syntheticum and cactus in the treatment of epidemic Parotitis. Moder J Integr Trad Chin West Med 2002; 011(020): 2033. 10.3969/j.issn.1008-8849.2002.20.053 [DOI] [Google Scholar]
- 110. Liu CJ. Clinical observation on the treatment of upper respiratory tract infection with Radix isatidis granules. Asia-Pacific Trad Med 2012; 8:82–3. 10.3969/j.issn.1673-2197.2012.03.045 [DOI] [Google Scholar]
- 111. Huang YQ. Observation on the efficacy of oseltamivir phosphate combined with Radix isatidis Granules in the treatment of influenza A(H1N1) and discussion on nursing care. Strait Pharm J 2019; 31:233–4. https://doi.org/CNKI:SUN:HAIX.0.2019-01-131 [Google Scholar]
- 112. Luo CY, Hu NY. “Root and leaf Decoction” in the treatment of 28 cases of male condyloma acuminatum. J Integr Chin West Med 1990; ( 09):537. 10.7661/CJIM.1990.9.537 [DOI] [Google Scholar]
- 113. Zhang DL, He CX, Zhang HB, et al. Clinical efficacy and mechanism of Folium isatidis for pasfulosis palmaris et plantaris: a pilot study. J Dermatol Venereol 2019;41: 476–8. https://doi.org/CNKI:SUN:PFBX.0.2019-04-005 [Google Scholar]
- 114. Dai LB, Yang GY, Zhen CF. Herba houttuyniae injection and radix isatidis granule in the treatment of epidemic kerato-conjunctivitis. Chin Naturopath 2001; 09(005): 47–8. 10.3969/j.issn.1007-5798.2001.05.066 [DOI] [Google Scholar]
- 115. Ma WY, Lu RY, Xu F. An observation on the therapeutic efficacy of compound radix isatidis on viralmyocarditis. Prac J Cardiac Cere Pneumal Vascular Dis 2003; 011(003): 135–8. 10.3969/j.issn.1008-5971.2003.03.003 [DOI] [Google Scholar]
- 116. Zhang Q, et al. A network pharmacology approach to investigate the anticancer mechanism and potential active ingredients of Rheum palmatum L. against lung cancer via induction of apoptosis. Front Pharmacol 2020; 11:528308. 10.3969/j.issn.1002-2090.2020.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Long Y, Zhang JP. Nose to brain drug delivery-a promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol Res 2020; 159: 104795. https://doi.org/CNKI:SUN:ZLXZ.0.2020-14-145 [DOI] [PubMed] [Google Scholar]
- 118. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016; 79: 629–61. https://doi.org/CNKI:SUN:ZCYO.0.2016-10-000 [DOI] [PubMed] [Google Scholar]