Abstract
How epigenetic memory states are established and maintained is a central question in gene regulation. A major epigenetic process important for developmental biology involves Polycomb-mediated chromatin silencing. Significant progress has recently been made on elucidating Polycomb silencing in plant systems through analysis of Arabidopsis FLOWERING LOCUS C (FLC). Quantitative silencing of FLC by prolonged cold exposure was shown to represent an ON to OFF switch in an increasing proportion of cells. Here, we review the underlying all-or-nothing, digital paradigm for Polycomb epigenetic silencing. We then examine other Arabidopsis Polycomb-regulated targets where digital regulation may also be relevant.
Current Opinion in Plant Biology 2021, 61:102012
This review comes from a themed issue on Epigenetics
Edited by Mary Gehring and François Roudier
For a complete overview see the Issue and the Editorial
Available online 1st March 2021
https://doi.org/10.1016/j.pbi.2021.102012
1369-5266/© 2021 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Epigenetic switching and memory systems are fundamental for cell differentiation in development and to allow long term responses to the environment. Despite their importance in maintaining distinct gene expression programs in genetically identical cells, the mechanisms employed to switch and then maintain epigenetic memory states are still not fully elucidated. Epigenetic memory states must be robust to perturbations to maintain the integrity of the memory, yet controllable switching must also be possible to effect transitions between different gene expression programs.
The Polycomb silencing system is one of the most important epigenetic regulatory systems and is conserved from plants to flies to humans. Gene silencing is achieved through high levels of the histone modification H3K27me3, a mark that is added by Polycomb Repressive Complex 2 (PRC2) in a self-reinforcing, read-write feedback loop. In this review, we examine our current understanding of Polycomb switching and memory, inspired by cold-induced Polycomb silencing of FLC. Unpicking the underlying digital, all-or-nothing mechanism of this complex regulatory system required a tight integration of mechanistic mathematical modelling and experiments, including single cell approaches. We then review the mechanistic basis of silencing in other recently studied contexts, including for WUSCHEL in floral meristems and SPEECHLESS in stomatal lineage reprogramming, examining the potential relevance of a digital switching/silencing paradigm.
Digital switching and maintenance of Polycomb epigenetic states at a single locus
Most studies on Polycomb silencing analyse targets at the tissue level at different developmental stages. Inevitably, as with most molecular biology analyses, this averages together the effects from many cells. Quantitative changes in expression are frequently observed and often implicitly interpreted as reflecting graded changes in gene expression at each locus. However, a new paradigm has recently emerged where Polycomb targets can exist in two opposing expression states, active and silent [1•,2,3], with controllable switching between the two. Thus, Polycomb silencing involves cell-autonomous, digital switching to a transcriptionally silenced state and epigenetic maintenance of that state through subsequent cell cycles. In general terms, the switching is induced in response to an upstream signal, and overall leads to an analogue quantitative response to that signal at the cell population level, but this is based on the fraction of loci that have switched and not a graded response at each locus (Figure 1) [4,5].
Figure 1.
Digital switching and maintenance of Polycomb epigenetic states at a single locus. (a) Upstream signal that induces switching: a fluctuating, analogue signal undergoing a transient increase and eventually returning to basal levels. (b) Schematic of a single diploid cell depicting stochastic switching of an individual locus from a digital ON state (Green) to a digital OFF state (Red). (c) Stochastic switching off at individual loci, induced by the transient increase in the upstream signal. The probability of switching off at a locus depends on the strength of the signal. The digital expression states – both the ON and OFF states – are self-perpetuating (mitotically inherited). The OFF state is maintained even after the upstream signal inducing the switching has returned to basal levels. (d) At the population level, the digital switching at individual loci leads to an analogue quantitative response and memory of the transient increase in the upstream signal, based on the fraction of loci that have switched.
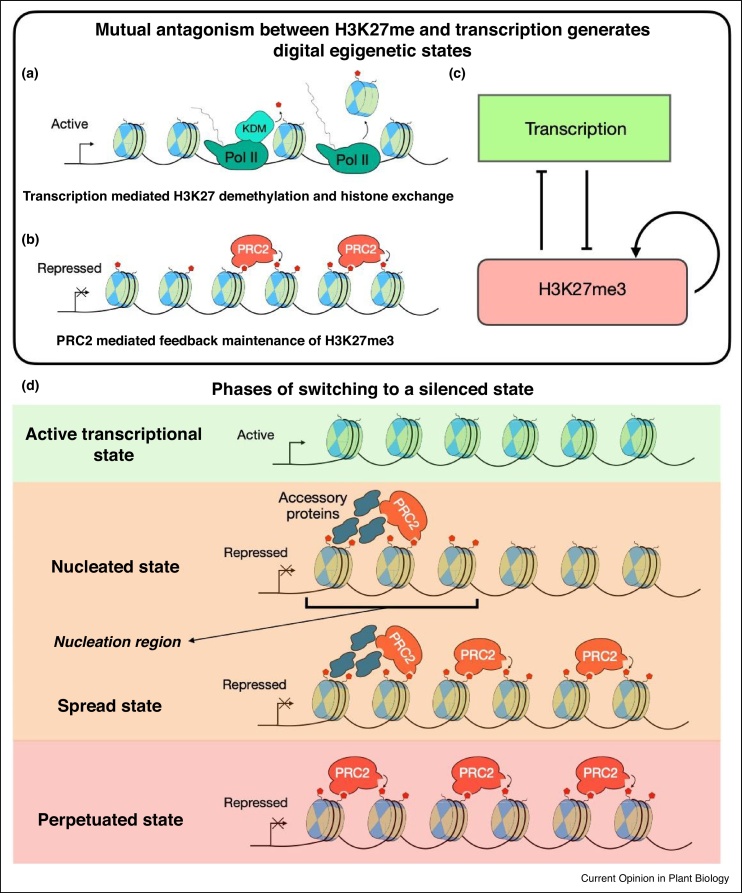
These two states, actively transcribed (low H3K27me3) and poorly transcribed (high H3K27me3) (Figure 2a–c) can emerge from the interplay between three regulatory features [1•]: PRC2-mediated positive read-write feedback in H3K27 methylation, transcription-mediated removal of H3K27 methylation, and H3K27me3-based repression of transcription. The self-reinforcing nature of the PRC2-mediated feedback can stabilise the silenced (high H3K27me3) state by restoring H3K27me3 levels following dilution at replication, where on average half of the parental histones are lost and replaced by histones without H3K27 methylation [6]. Similarly, transcription-mediated removal of H3K27 methylation can stabilise the active (low H3K27me3) state by preventing build-up of H3K27 methylation.
Figure 2.
Chromatin level schematic of digital epigenetic states and switching at an individual locus. (a) Active transcriptional state with transcription mediated antagonism of H3K27 methylation (KDM: H3K27 demethylase). (b) Silenced transcriptional state with H3K27me3 maintained by PRC2-mediated read-write feedback. (c) Such digital (all-or-nothing) epigenetic states can arise from the interplay between three core interactions: transcriptional antagonism of H3K27 methylation, H3K27me3-based repression of transcription, and PRC2-mediated self-reinforcing feedback acting to maintain H3K27 methylation. (d) Schematic depicting the different phases of digital switching from the transcriptionally active state to the silenced state. The locus has low H3K27me3 coverage in the active transcriptional state. In the first phase of silencing, PRC2 is targeted to the ‘nucleation region’ within the locus by accessory proteins. The nucleation is stochastic and digital at an individual locus, and results in transcriptional silencing. In the second phase of silencing, H3K27me3 (red pentagons) is spread across the locus, forming a fully stable ‘spread state’. In the last phase, the accessory proteins are lost from the nucleation region, leaving a ‘perpetuated state’ where H3K27me3 is maintained by PRC2-mediated read-write feedback alone.
In this paradigm, the establishment of H3K27me3 silencing at an active gene locus, in response to an upstream signal, proceeds through three distinct phases (Figure 2d) [7••,8••]. The first phase involves targeting of PRC2 activity to a specific region (the nucleation region) within the locus by accessory proteins, which nucleates H3K27me3 in this region. During this phase, nucleation occurs stochastically and digitally at an individual locus, that is, an all-or-nothing phenomenon rather than a gradual build-up of H3K27me3 [5]. The probability of nucleation depends on the strength of the upstream signal. The H3K27me3 ‘nucleation peak’ can itself enforce a silenced transcriptional state of the locus. Owing to the relatively small number of memory elements in the nucleation region, this silenced state is not fully mitotically stable, and has therefore been termed metastable. In the second phase of silencing, the H3K27me3 nucleation peak can spread H3K27 methylation across the whole locus, ‘pushing’ the locus into a fully stable mitotically inheritable ‘spread state’ [7••]. The third phase of silencing sees the accessory proteins lost from the nucleation region, leaving a ‘perpetuated’ epigenetic state of the locus where H3K27me3 is maintained across the locus solely by PRC2 mediated read/write feedback [8••].
The digital nature of the silencing – from an active transcriptional state with little H3K27me3 to the spread/perpetuated state with full H3K27me3 coverage, via a nucleation peak which is also digital – gives multiple advantages relative to an analogue/gradual H3K27me3 build-up. Firstly, as the efficiency of switching to the spread state likely depends on the level of the nucleation peak, a digital nucleation peak – where the high level of the peak is independent of the duration of the signal – provides a memory mechanism at a population level that can register even signals of short duration, allowing reliable perception of fluctuating signals [5]. Secondly, mitotic inheritance of all-or-nothing states is simpler to achieve. While self-reinforcing feedbacks are essential to restore the epigenetic state following replication, they cannot reliably restore intermediate states without additional memory elements. Such feedback mechanisms characteristically tend to establish and maintain all-or-nothing states [1•]. Thirdly, digital epigenetic states maintained by self-reinforcing feedbacks are robust to fluctuations, for instance in the level of trans regulators, preventing such fluctuations from triggering loss of epigenetic memory [1•].
Within this paradigm, resetting of H3K27me3 silencing at a locus (for instance before the next generation) is conceptually simpler than establishing quantitative silencing – significant downregulation of H3K27 methyltransferase activity, upregulation of H3K27me3 demethylase activity, insertion of specific histone variants [9•], or a combination of these mechanisms [10•], are all capable of removing enough silencing marks to allow a switch back to an active transcriptional state.
FLC as a test case for digital epigenetic silencing
A combination of mathematical modelling with genetic and molecular analysis has enabled a detailed characterization of the epigenetic switch and memory occurring at Arabidopsis thaliana FLC. This work inspired the general theoretical paradigm outlined above. FLC encodes a floral repressor that quantitatively influences flowering time through regulation of genes required to switch the meristem to a floral fate [11]. FLC is epigenetically silenced during the prolonged exposure to winter cold in a process called vernalization, which enables floral activation in the spring [11]. Key experiments that informed the FLC modelling included population-level single gene quantification of mRNA levels and of histone modifications by ChIP-qPCR (Figure 3a) [7••]. However, single cell experiments to resolve the cell autonomous, digital switching and memory were also crucial, both for RNA using single molecule RNA FISH [8••] and at the protein level (Figure 3b). In the latter case, distinct mitotically inheritable transcriptional states of different gene copies were demonstrated by combining different fluorescent protein tags at different gene copies [12] (Figure 3c), a technique which was also subsequently used in mammalian systems to resolve cis-based Polycomb dynamics [13].
Figure 3.
Cis-mediated epigenetic switching at FLC (reproduced from Ref. [7••]). (a) H3K27me3 ChIP across the FLC locus before cold and after a 6-week cold treatment in wild-type (Col-FRI) and lhp1 mutant shows that spreading of H3K27me3 is disrupted in lhp1. FLC silencing was found not to be stably maintained in lhp1. (b) FLC-Venus imaged 14 days after a 10-week cold treatment in wild-type (Col-FRI) and lhp1. H3K27me3 loss observed at the population level in lhp1 mutants in (a) reflects stochastic reactivation of FLC expression at the single-cell level in (b). (c) FLC-Venus and FLC-mCherry intensities in root meristems, imaged 10 days after a 6-week cold treatment in the lhp1 mutant, showing that the two copies are switched independently; thus nucleation is cis-regulated. v: FLC-Venus ON cells, c: FLC-mCherry ON cells, b: both FLC-Venus and FLC-mCherry ON. Scale bars in (b) and (c) are 50 μm.
Silencing at FLC starts with transcriptional repression during early cold exposure, which involves increased expression of COOLAIR, a set of long non-coding antisense RNAs initiated in proximity of the major poly(A) site of FLC transcripts [14]. COOLAIR transcription is mutually exclusive with FLC transcription and occurs independently at each FLC gene copy [15]. Subsequently, H3K27me3 is deposited at each FLC copy independently, in a cis-mediated all-or-nothing digital fashion, by PRC2. This occurs in the FLC nucleation region, covering approximately 3 nucleosomes over exon 1 and the beginning of intron 1. The H3K27me3 deposition requires the core PRC2 plus the PRC2 accessory proteins VIN3 and VRN5 [4,5,7••,16,17]. VIN3 expression is induced by cold and increases in a graded (analogue) manner and thus corresponds to the upstream regulator of the previous section (Figure 1a). This upregulation is itself driven by many temperature-dependent processes [7••,18,19], including a long-term thermosensory input through accumulation of the direct regulator NTL8: as cell division slows down in the cold the level of NTL8 increases due to reduced growth-dependent dilution [20]. Through NTL8, longer cold thus leads to increased VIN3 expression, which in turn generates a higher probability of stochastic nucleation and switching at FLC. The stochastic nature of the nucleation and switching process means that different FLC gene copies switch at different times, and the fraction of gene copies that are digitally PRC2-nucleated increases with increased duration of cold exposure.
Spreading of H3K27me3 across the FLC gene body to allow long-term stable silencing is observed within four days after return to warm conditions, in a process which requires an active cell cycle and which is genetically separable from nucleation [7••]. In the spreading mutants clf (defective in one of the histone K27 methyltransferases) and lhp1 (defective in LIKE HETEROCHROMATIN PROTEIN 1), the metastable nucleated state can be maintained for tens of cell cycles after vernalization before silencing is lost. Post-cold, the dynamics of VRN5 binding at the FLC nucleation site match the loss of H3K27me3 nucleation in spreading mutants, consistent with the idea that VRN5 is a defining factor in the metastability of the nucleated state [7••]. On the other hand, VIN3 is rapidly depleted as plants are returned to warm conditions: its central role is instead in triggering cold-induced nucleation, as described above.
Analysis of FLC epigenetic silencing in the A. thaliana accession Lov-1 collected from Northern Sweden, which requires a much longer period of cold for vernalization, has recently revealed an additional phase in the Polycomb silencing mechanism [8••], following the spread state. In this ‘perpetuated’ phase, the accessory proteins are no longer present at the nucleation region, but the H3K27me3 modifications over the gene body remain. Although first identified in Lov-1 [8••], this perpetuated state appears to be generic, including in the laboratory wild type Col-FRI. However, specific to Lov-1 is that even after spreading, and in the perpetutated state, silencing is not stably maintained through subsequent cell cycles. A combination of non-coding single nucleotide polymorphisms (SNPs) in Lov-1 FLC were identified as causal for a large proportion of this instability, which was again shown to occur digitally in individual cells. Most of the SNPs are located in the nucleation region, placing nucleation of H3K27me3 not only in setting up the OFF state at FLC, but also in maintaining it through multiple cell cycles. This is strongly reminiscent of the requirement in Drosophila for the Polycomb Responsive Element (PRE) to maintain H3K27me3 following DNA replication [21,22].
The perpetuated phase of the silencing mechanism may contribute to the seasonal FLC dynamics in perennial Brassicaceae [23,24], where FLC silencing occurs during the cold but then re-activates enabling many of the meristems to remain vegetative. The contribution of non-coding SNPs to the seasonal regulation of FLC expression patterns in perennials remains to be investigated. However, in the perennial Arabis alpina, the introduction of FLC from the closely related annual species Arabis montbretiana, which is stably silenced following vernalization, recapitulates the stable silencing of AmFLC but not of the endogenous FLC orthologue AaPEP1 [25,26]. This also implicates cis-regulatory mechanisms in the gene regulation of FLC in perennial plants.
The seasonal dynamics of histone modifications at FLC were recently studied in a natural population of the perennial Arabidopsis halleri [27••,28]. Mathematical modelling was also used to interpret the H3K27me3 dynamics in terms of cis-mediated digital nucleation and spreading: like in the closely related A. thaliana, AhFLC shows H3K27me3 nucleation close to the transcriptional start site during the winter months and delayed spreading of the repressive mark across the locus with increasing temperatures in late winter and spring. However, unlike in annual A. thaliana, this is followed by a synchronised decrease in H3K27me3 and FLC reactivation after bolting/flowering, where the rate of FLC reactivation anti-correlated with the cell population level of H3K27me3 attained during the cold [28].
Do other Polycomb targets follow the same rules?
We now have a relatively detailed theoretical framework for Polycomb switching and silencing, inspired by and applied to Arabidopsis cold-induced FLC dynamics. However, the FLC system is somewhat special as it involves a quantitative epigenetic response to an environmental signal, and does not involve cell fate changes. The question remains whether the digital nucleation and spreading paradigm can be applied to other Polycomb targets, particularly in developmental cell fate contexts. We next discuss two plant systems where this important question could be further explored, building on important prior work, although we emphasise that there are many other potential applications [29].
The first example is silencing of the homeobox gene WUSCHEL (WUS). WUS is involved in maintaining the stem cell pool in the floral meristem and is silenced during floral meristem termination after the initiation of the floral organs [30,31]. A key player in the silencing process is KNUCKLES (KNU), which integrates both transcriptional repression and Polycomb-mediated silencing of WUS [32••]. The use of transgenic lines with inducible KNU activity to assay the temporal progression of silencing over several hours revealed that KNU is initially required for the transcriptional repression of WUS involving eviction of the chromatin remodelling factor SPLAYED, which is followed by slower PRC2-mediated deposition of H3K27me3 at the WUS locus (Figure 4a). Direct protein interactions of KNU with the PRC2 component FIE suggest a role for KNU in Polycomb recruitment which goes beyond merely setting the stage for Polycomb activity by mediating transcriptional repression. An intriguing question is therefore whether KNU mediates a digital silencing mechanism at WUS, with KNU being the upstream regulator analagous to VIN3 at FLC. ChIP experiments have already shown that KNU nucleates in the WUS promoter proximal region and subsequently mediates H3K27me3 deposition downsteam in the gene body [32••]. This is very reminiscent of VIN3/FLC except that the H3K27me3 and its upstream inducer are entirely spatially separated at WUS. It will be interesting to see whether other factors are needed for H3K27me3 spreading at WUS such as LHP1 and/or an active cell cycle, as at FLC. Using the inducible KNU system, it might also be possible to address digital WUS silencing at the single cell level using fluorescent protein fusions following an intermediate level and/or duration of induced KNU activity, potentially leading to only a fraction of fully WUS silenced cells.
Figure 4.
Silencing of the Polycomb targets WUSCHEL (WUS) and SPEECHLESS (SPCH). (a)WUS is expressed in the stem cell pool in the floral meristem and is silenced during floral meristem termination. KNUCKLES (KNU) mediates transcriptional repression, involving eviction of the chromatin remodelling protein SPLAYED, as well as Polycomb (PRC2) silencing of WUS (red pentagons = H3K27me3). (b) Cell stage transitions during stomatal development are sequentially mediated by the master regulator transcription factors SPEECHLESS (SPCH), MUTE and FAMA. FAMA interacts with RBR to direct PRC2 activity towards stomatal genes during stomatal lineage progression. Mutations in FAMA (FAMALGK) that abrogate interaction with RBR result in the reinitiation of cell identity patterns of the early stomatal lineage. SPCH, required for entry into the stomatal lineage, is silenced during later stages and shows increased expression in reprogrammed guard cells (FACS-isolated) in FAMALGK lines. Not all cell stages within the stomatal lineage progression are shown (double arrows).
A second example is the silencing of the transcription factor SPEECHLESS (SPCH) during stomatal lineage progression (Figure 4b) [33••,34]. SPCH expression is required for cells to enter the stomata lineage [35], but is downregulated during the progression towards guard mother cells, where the transcription factor FAMA is upregulated [36, 37, 38]. FAMA operates in a protein complex with RETINOBLASTOMA-RELATED (RBR), and this interaction is required for the maintenance of guard cell identity as shown by the introduction of point mutations in FAMA (FAMALGK) that abolish the interaction with RBR [34]. Guard cells in FAMALGK lines fail to stably sustain their terminal identity and revert to stomatal precursor identity, as evidenced by increased expression of several stomatal precursor genes including SPCH [33••,34]. Because RBR directly interacts with PRC2 components [39,40], it was hypothesised that the FAMA–RBR module is involved in PRC2 recruitment to enable maintenance of guard cell identity via H3K27me3 deposition at SPCH and other early stomatal lineage genes [34]. Guard cell-specific expression and H3K27me3 profiles were assayed using the FAMA promoter to drive expression of YFP to perform fluorescence-activated cell sorting (FACS). This indeed revealed lower levels of SPCH expression in wild-type guard cells versus reprogrammed guard cells in FAMALGK lines [33••], with H3K27me3 at SPCH only detectable in the wild type, although at lower levels than in aerial tissue. These lower levels were attributed to the slow kinetics of H3K27me3 re-establishment [33••]. Together with ChIP data showing that FAMA and RBR bind to the SPCH promoter [34], this suggests that the FAMA–RBR interaction is required for the stable silencing of SPCH during guard cell differentiation. It is therefore possible that FAMA–RBR could play the role of the upstream regulator, analagous to VIN3 and possibly KNU, in digitally nucleating H3K27me3 at SPCH. However, unlike in FLC silencing, where a quantitative accumulation of silencing within a cell population underlies the regulation of the floral transition in response to environmental signals, SPCH silencing occurs as a single cell event in the stomatal progenitor cell to allow its differentiation.
Conclusions
In this review, we have emphasised a digital paradigm for Polycomb-based epigenetic switching and memory. This framework was constructed through interdisciplinary investigation of the FLC locus, but it may apply much more widely to switching at other developmental Polycomb targets. Indeed, beyond epigenetics, all-or nothing switching at a fraction of loci has been proposed as a general mode of transcriptional regulation [41].
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We acknowledge financial support from the UK Biotechnology and Biological Sciences Research Council Institute Strategic Programme grant GEN (BB/P013511/1), ERC Advanced Grant EPISWITCH and UKRI Physics of Life grant EP/T00214X/1.
References
- 1•.Berry S., Dean C., Howard M. Slow chromatin dynamics allow polycomb target genes to filter fluctuations in transcription factor activity. Cell Syst. 2017;4:445–457. doi: 10.1016/j.cels.2017.02.013. e448. [DOI] [PMC free article] [PubMed] [Google Scholar]; Theoretical model for H3K27me3 based epigenetic memory where all-or-nothing transcriptional states emerge from mutual antagonism between transcription and H3K27 methylation, reinforced by PRC2 read-write feedback.
- 2.Dodd I.B., Micheelsen M.A., Sneppen K., Thon G. Theoretical analysis of epigenetic cell memory by nucleosome modification. Cell. 2007;129:813–822. doi: 10.1016/j.cell.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 3.Ringrose L., Howard M. Dissecting chromatin-mediated gene regulation and epigenetic memory through mathematical modelling. Curr Opin Syst Biol. 2017;3:7–14. [Google Scholar]
- 4.Angel A., Song J., Dean C., Howard M. A Polycomb-based switch underlying quantitative epigenetic memory. Nature. 2011;476:105–108. doi: 10.1038/nature10241. [DOI] [PubMed] [Google Scholar]
- 5.Angel A., Song J., Yang H., Questa J.I., Dean C., Howard M. Vernalizing cold is registered digitally at FLC. Proc Natl Acad Sci U S A. 2015;112:4146–4151. doi: 10.1073/pnas.1503100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alabert C., Barth T.K., Reverón-Gómez N., Sidoli S., Schmidt A., Jensen O.N., Imhof A., Groth A. Two distinct modes for propagation of histone PTMs across the cell cycle. Genes Dev. 2015;29:585–590. doi: 10.1101/gad.256354.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Yang H., Berry S., Olsson T.S.G., Hartley M., Howard M., Dean C. Distinct phases of Polycomb silencing to hold epigenetic memory of cold in Arabidopsis. Science. 2017;357:1142–1145. doi: 10.1126/science.aan1121. [DOI] [PubMed] [Google Scholar]; This study uses extensive time-course ChIP analysis and imaging of Polycomb mutants to demonstrate that the nucleation and spreading phases of Polycomb silencing at FLC are genetically and mechanistically separate.
- 8••.Questa J.I., Antoniou-Kourounioti R.L., Rosa S., Li P., Duncan S., Whittaker C., Howard M., Dean C. Noncoding SNPs influence a distinct phase of Polycomb silencing to destabilize long-term epigenetic memory at Arabidopsis FLC. Genes Dev. 2020;34:446–461. doi: 10.1101/gad.333245.119. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combined experimental and modelling study that that reveals the post-cold perpetuated state of Polycomb silencing at FLC. This study identifies noncoding SNPs as a causal factor in the instability of the perpetuated state, leading to reactivation of Lov-1 FLC.
- 9•.Yan A., Borg M., Berger F., Chen Z. The atypical histone variant H3.15 promotes callus formation in Arabidopsis thaliana. Development. 2020;147 doi: 10.1242/dev.184895. [DOI] [PubMed] [Google Scholar]; The authors demonstrate that the deposition of the histone variant H3.15, characterized by the absence of lysine residue K27, which is methylated by PRC2, contributes to the release from PRC2-mediated gene repression.
- 10•.Borg M., Jacob Y., Susaki D., LeBlanc C., Buendia D., Axelsson E., Kawashima T., Voigt P., Boavida L., Becker J. Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat Cell Biol. 2020;22:621–629. doi: 10.1038/s41556-020-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that global resetting of paternal H3K27me3 before plant embryogenesis can be ascribed to the interplay between cell-type specific modulation of histone writer/reader activity and the deposition of a sperm-specific histone variant which is immune to K27 methylation.
- 11.Michaels S.D., Amasino R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry S., Hartley M., Olsson T.S., Dean C., Howard M. Local chromatin environment of a Polycomb target gene instructs its own epigenetic inheritance. eLife. 2015;4 doi: 10.7554/eLife.07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng K.K., Yui M.A., Mehta A., Siu S., Irwin B., Pease S., Hirose S., Elowitz M.B., Rothenberg E.V., Kueh H.Y. A stochastic epigenetic switch controls the dynamics of T-cell lineage commitment. eLife. 2018;7 doi: 10.7554/eLife.37851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swiezewski S., Liu F., Magusin A., Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009;462:799–802. doi: 10.1038/nature08618. [DOI] [PubMed] [Google Scholar]
- 15.Rosa S., Duncan S., Dean C. Mutually exclusive sense–antisense transcription at FLC facilitates environmentally induced gene repression. Nat Commun. 2016;7 doi: 10.1038/ncomms13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung S., Amasino R.M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- 17.Greb T., Mylne J.S., Crevillen P., Geraldo N., An H., Gendall A.R., Dean C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol. 2007;17:73–78. doi: 10.1016/j.cub.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Hepworth J., Antoniou-Kourounioti R.L., Bloomer R.H., Selga C., Berggren K., Cox D., Collier Harris B.R., Irwin J.A., Holm S., Säll T. Absence of warmth permits epigenetic memory of winter in Arabidopsis. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoniou-Kourounioti R.L., Hepworth J., Heckmann A., Duncan S., Qüesta J., Rosa S., Säll T., Holm S., Dean C., Howard M. Temperature sensing is distributed throughout the regulatory network that controls FLC epigenetic silencing in vernalization. Cell Syst. 2018;7:643–655.e649. doi: 10.1016/j.cels.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y., Antoniou-Kourounioti R.L., Calder G., Dean C., Howard M. Temperature-dependent growth contributes to long-term cold sensing. Nature. 2020;583:825–829. doi: 10.1038/s41586-020-2485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman R.T., Struhl G. Causal role for inheritance of H3K27me3 in maintaining the OFF state of a Drosophila HOX gene. Science. 2017;356 doi: 10.1126/science.aai8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laprell F., Finkl K., Müller J. Propagation of Polycomb-repressed chromatin requires sequence-specific recruitment to DNA. Science. 2017;356:85. doi: 10.1126/science.aai8266. [DOI] [PubMed] [Google Scholar]
- 23.Wang R., Farrona S., Vincent C., Joecker A., Schoof H., Turck F., Alonso-Blanco C., Coupland G., Albani M.C. PEP1 regulates perennial flowering in Arabis alpina. Nature. 2009;459:423–427. doi: 10.1038/nature07988. [DOI] [PubMed] [Google Scholar]
- 24.Aikawa S., Kobayashi M.J., Satake A., Shimizu K.K., Kudoh H. Robust control of the seasonal expression of the Arabidopsis FLC gene in a fluctuating environment. Proc Natl Acad Sci U S A. 2010;107:11632. doi: 10.1073/pnas.0914293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiefer C., Severing E., Karl R., Bergonzi S., Koch M., Tresch A., Coupland G. Divergence of annual and perennial species in the Brassicaceae and the contribution of cis-acting variation at FLC orthologues. Mol Ecol. 2017;26:3437–3457. doi: 10.1111/mec.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyun Y., Vincent C., Tilmes V., Bergonzi S., Kiefer C., Richter R., Martinez-Gallegos R., Severing E., Coupland G. A regulatory circuit conferring varied flowering response to cold in annual and perennial plants. Science. 2019;363:409. doi: 10.1126/science.aau8197. [DOI] [PubMed] [Google Scholar]
- 27••.Nishio H., Buzas D.M., Nagano A.J., Iwayama K., Ushio M., Kudoh H. Repressive chromatin modification underpins the long-term expression trend of a perennial flowering gene in nature. Nat Commun. 2020;11 doi: 10.1038/s41467-020-15896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combined experimental and modelling study that examines the dynamics of H3K27me3 silencing in the perennial Arabidopsis halleri FLC, including reactivation in the spring. Their results suggest a possible role for an interaction between H3K4me3 and H3K27me3 in registering long-term temperature trends at FLC.
- 28.Nishio H., Iwayama K., Kudoh H. Duration of cold exposure defines the rate of reactivation of a perennial FLC orthologue via H3K27me3 accumulation. Sci Rep. 2020;10:16056. doi: 10.1038/s41598-020-72566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo Y., Yan Z., Zhang B., Wang X. Recruitment of Polycomb repressive complex 2 is essential to suppress the target chromatin in Arabidopsis. Crit Rev Plant Sci. 2016;35:131–145. [Google Scholar]
- 30.Mayer K.F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 31.Sun B., Xu Y., Ng K.-H., Ito T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 2009;23:1791–1804. doi: 10.1101/gad.1800409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Sun B., Zhou Y., Cai J., Shang E., Yamaguchi N., Xiao J., Looi L.S., Wee W.Y., Gao X., Wagner D. Integration of transcriptional repression and Polycomb-mediated silencing of WUSCHEL in floral meristems. Plant Cell. 2019;31:1488–1505. doi: 10.1105/tpc.18.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study the authors uncover the multi-step mechanisms underlying the silencing of WUSCHEL in a spatiotemporal manner during the termination of floral stem cells.
- 33••.Lee L.R., Wengier D.L., Bergmann D.C. Cell-type-specific transcriptome and histone modification dynamics during cellular reprogramming in the Arabidopsis stomatal lineage. Proc Natl Acad Sci U S A. 2019;116:21914–21924. doi: 10.1073/pnas.1911400116. [DOI] [PMC free article] [PubMed] [Google Scholar]; On the basis of fate transitions within the stomatal lineage, this study highlights the relevance of generating cell-type specific H3K27me3 profiles to uncover the role of chromatin modifications in cell identity maintenance.
- 34.Matos J.L., Lau O.S., Hachez C., Cruz-Ramírez A., Scheres B., Bergmann D.C. Irreversible fate commitment in the Arabidopsis stomatal lineage requires a FAMA and RETINOBLASTOMA-RELATED module. eLife. 2014;3 doi: 10.7554/eLife.03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacAlister C.A., Ohashi-Ito K., Bergmann D.C. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- 36.Ohashi-Ito K., Bergmann D.C. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell. 2006;18:2493. doi: 10.1105/tpc.106.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee L.R., Bergmann D.C. The plant stomatal lineage at a glance. J Cell Sci. 2019;132 doi: 10.1242/jcs.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillitteri L.J., Sloan D.B., Bogenschutz N.L., Torii K.U. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- 39.Jullien P.E., Mosquna A., Ingouff M., Sakata T., Ohad N., Berger F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosquna A., Katz A., Shochat S., Grafi G., Ohad N. Interaction of FIE, a Polycomb protein, with pRb: a possible mechanism regulating endosperm development. Mol Genet Genomics. 2004;271:651–657. doi: 10.1007/s00438-004-1024-6. [DOI] [PubMed] [Google Scholar]
- 41.Alamos S., Reimer A., Niyogi K.K., Garcia H.G. Quantitative imaging of RNA polymerase II activity in plants reveals the single-cell basis of tissue-wide transcriptional dynamics. bioRxiv. 2020 doi: 10.1038/s41477-021-00976-0. 2020.2008.2030.274621. [DOI] [PMC free article] [PubMed] [Google Scholar]