To the Editor:
Efficacy of vaccines against SARS‐CoV‐2 virus is of great importance to mitigate the Covid‐19 pandemic. 1 The BNT162b2 mRNA vaccine (Comirnaty) is the first approved by both FDA and EMA, due to its efficacy in apparently healthy adults. 2 Recently, an assessment of the first vaccination dose effects among nursing facility residents and staff showed that there is some protection after the first injection. 3 Here we present the kinetics of anti‐SARS‐CoV‐2 Spike‐Receptor Binding Domain (RBD) IgGs and SARS‐CoV‐2 neutralizing antibodies (NAbs) development in health workers and octogenarians, who participated in a prospective study (NCT04743388) studying the efficacy of vaccination for the prevention of Covid‐19.
Major inclusion criteria for participation in this study included: (a) age above 18 years; (b) ability to sign the informed consent form, and (c) eligibility for vaccination, according to the national program for Covid‐19 vaccination. Major exclusion criteria included the presence of: (a) autoimmune disorder under immunosuppressive therapy; (b) active malignant disease and (c) end‐stage renal disease.
Anti‐Spike‐RBD IgG antibodies (representing response to either prior infection or vaccine) and NAbs against SARS‐CoV‐2 were measured using FDA approved methods, that is, the Elecsys Anti‐SARS‐CoV‐2 S assay (Roche Diagnostics GmbH, Mannheim, Germany) and the cPas SARS‐CoV‐2 NAbs Detection Kit (GenScript, Piscataway, NJ, USA), 4 respectively; the latter allows the indirect detection of potential SARS‐CoV‐2 NAbs in the blood, by assaying the antibody (independent of class)‐mediated inhibition of SARS‐CoV‐2 RBD binding to human host receptor angiotensin converting enzyme 2 (ACE2). Time‐points for blood collection and serum isolation were day 1 (D1; first BNT162b2 dose), D8, D22 (second dose), D36 and D50 for health workers and D1, D22 and D50 for octogenarians. After vein puncture, serum was separated within 4 h from blood collection and stored at −80°C until the day of measurement. Stored samples from different time points of the same donor were measured in parallel assays. The on‐going study also includes a follow‐up of the antibodies' titers every 3 months till month 18, post D22.
The study was approved by the respective Ethical Committee of Alexandra Hospital, in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. All patients and controls provided informed consent before entering the study.
Study population included 255 health workers (92 M/163F; median age: 49 years, range: 25–70 years) of Alexandra General Hospital in Athens, Greece (group one) and 112 volunteered octogenarians (51 M/61F; median age: 85 years, range: 80–95 years) who were vaccinated in the same hospital vaccine center (group two).
From group one, seven (2.74%) and 21 (8.23%) individuals had anti‐Spike‐RBD IgGs titer >0.8 U/mL (positivity threshold) and NAbs inhibition titer >30% (positivity threshold), respectively, on D1. Whereas, from group two, one (0.89%) was positive for anti‐Spike‐RBD IgGs and 10 (8.92%) for Nabs. All participants who were positive for anti‐Spike‐RBD IgGs on D1 were positive for NAbs; interestingly, positivity for NAbs did not always correlate with increased anti‐Spike‐RBD IgGs titers (data not shown) indicating the existence of NAbs to distinct non‐RBD epitopes on the Spike protein. 5 Also, in agreement to recent findings, 6 anti‐Spike‐RBD IgGs and NAbs titers were significantly expanded on D8 suggesting the presence of robust immune memory responses in Covid‐19 healers.
The anti‐Spike‐RBD IgGs titer in health workers who were negative on D1, increased on D22, reached high values after the second dose of the vaccine (D36; values above the measuring range following 10‐fold dilution of the sample are reported as 2500 U/mL) and started to gradually decline on D50 (Figure 1A1). NAbs titers, in negative volunteers from group one on D1, were found to increase significantly on D22, plateaued 2 weeks (D36) after the second dose of the vaccine [median NAbs inhibition (all donors) ≥95%] and remained (despite a slight decline) at high levels on D50 [median NAbs inhibition (all donors) ≥95%] (Figure 1B). The increase of anti‐Spike‐RBD IgGs was less prominent for ages 51–70 years vs 25–50 years on D22 and D36 and significantly less pronounced (vs other age groups) for octogenarians on D22 and D50 (Figure 1A1). This age‐dependent pattern of immune responses was likewise evident for NAbs on D22 and D50 by comparing individuals aged 25–50 vs 51–70 years, and for both age groups vs octogenarians (Figure 1B).
FIGURE 1.
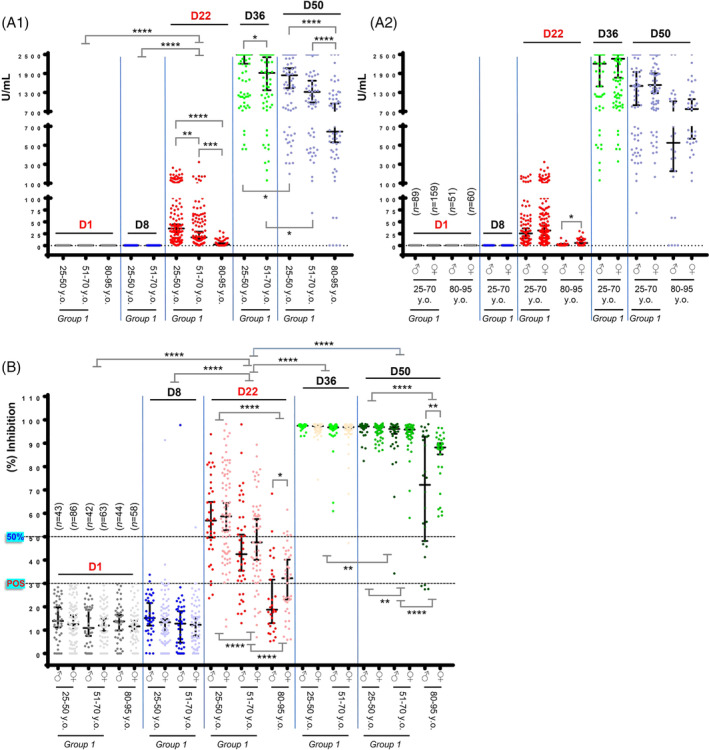
Anti‐Spike‐RBD and neutralizing antibodies (NAbs) against SARS‐CoV‐2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. (A1) Anti‐Spike‐RBD IgG antibodies in donors of group one increased on D22 (day of second vaccination), reached a plateau 2 weeks after the second dose of the vaccine (D36; median U/mL for ages 25–50, 2500, and for ages 51–70, 1918.5) and started to decline at D50 (D50; median U/mL for ages 25–50, 1844, and for ages 51–70, 1319). The efficacy of the vaccine‐mediated anti‐Spike‐RBD IgG immune responses is age‐dependent as evident on both D22 (median U/mL for ages 25–50, 35.94; for ages 51–70, 17.36; and for octogenarians, 2.28) and on D50 (median U/mL for ages 25–50, 1844; for ages 51–70, 1319; and for octogenarians, 644.5). (A2) Development of anti‐Spike‐RBD IgG antibodies on D22 tended to be more intense in females vs males in the younger ages (males, median U/mL, 25.55; females, median U/mL, 31.46) and was significant in octogenarians (males, median U/mL, 0.793; females, median U/mL, 5.36). On D50, the noted difference for male vs female octogenarians (males, median U/mL, 526; females, median U/mL, 778) did not reach statistical significance. All positive for anti‐Spike‐RBD IgG antibodies participants on D1 have been removed (see text) to show only [n values in (A2)] the vaccine‐mediated effects; values are not paired with those shown in B. Median ages per group are reported in B,; for D1, D8 vs D36 or D50 in (A1) p < .001 (not indicated). (B) NAbs in donors of group 1 increased on D22, plateaued 2 weeks after the second dose of the vaccine (D36) [median NAbs (%) inhibition (all donors), 97.22] and remained (with a slight decline) at high levels on D50 [median NAbs (%) inhibition (all donors), 96.62] indicating that NAbs are likely more durable vs anti‐RBD IgGs (see A1) following BNT162b2 vaccination. On D22 the kinetics of accumulating NAbs (%) inhibition titers were age dependent as evident by comparing donors aged 25–50 [median NAbs (%) inhibition, 57.55] vs donors aged 51–70 (median NAbs (%) inhibition, 44.39) and for both age groups vs octogenarians (median NAbs (%) inhibition, 23.20); similar age‐dependent readouts were observed on D50. Immune responses tended to be more robust in females vs males reaching statistical significance on D22 and D50 in octogenarians, suggesting gender dependent humoral immune responses, at least in the elderly individuals. To demonstrate only the vaccine effect, all positive for NAbs donors (see text) on D1 have been removed. Shown n values denote the number of initially enrolled (negative for NAbs) participants in this on‐going study. For D1 or D8 vs D36, D50, p < .001 (not indicated). 25–50 years old.: ♂ Median Age: 39, ♀ Median Age: 39; 51–70 years old: ♂ Median Age: 60, ♀ Median Age: 59; 80–95 years old: ♂ Median Age: 84.5, ♀: Median Age: 85. In both A,B, * p ANOVA <.05, ** p‐ANOVA <.01, *** p‐ANOVA <.001, **** p‐ANOVA <.001). Plotted are Median values with 95% Confidence Interval (GraphPad Prism 7)
Interestingly, the anti‐Spike‐RBD IgGs titer on D22 were higher in females vs males in octogenarians (Figure 1A2); a trend for more robust female anti‐Spike‐RBD immune responses was also observed in the other age groups. Similarly, NAbs' titers were found higher in females vs males in octogenarians on both D22 and D50 (Figure 1B).
We conclude that the BNT162b2 mRNA vaccine is particularly effective in producing high anti‐SARS‐CoV‐2 anti‐RBD IgGs and NAbs titers in healthy individuals with no presence of active malignancy, autoimmune disease under immunosuppressive therapy or end‐stage renal dysfunction. Interestingly, this humoral immune response is age‐dependent and gender‐dependent (especially at octogenarians); it peaks at the highest level (independently of age or gender) 2 weeks post D22 (second dose) and starts to decline (also independently of age or gender) 4 weeks post D22. Our data also support an increased production of anti‐RBD IgGs and NAbs titers after the first dose, which is similarly more robust in younger ages and in female octogenarians. These findings indicate that the second timely vaccination is critical, especially in the elderly population. Given its 18 months duration, age stratification, demographics, and combined Abs assays, our on‐going study has the potential to provide critical information on the duration of the BNT162b2 mRNA vaccine‐mediated protection.
ACKNOWLEDGMENTS
We thank Tina Bagratuni, PhD, Dimitrios Patseas, PhD, Mrs Nikoletta‐Aikaterini Kokkali and Mrs Stamatia Skourti for administrative, technical, and/or material support. We also thank Roche Diagnostics GmbH (Germany), SYN‐ENOSIS (Greece), AEGEAS (Greece) and IEMBITHEK (Greece) for partially funding this study, as well as all of the study participants for donating their time and samples.
Evangelos Terpos and Ioannis P. Trougakos are equal contribution as first authors
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions
REFERENCES
- 1. Trougakos IP, Stamatelopoulos K, Terpos E, et al. Insights to SARS‐CoV‐2 life cycle, pathophysiology, and rationalized treatments that target COVID‐19 clinical complications. J Biomed Sci. 2021;28(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gharpure R, Patel A, Link‐Gelles R. First‐dose COVID‐19 vaccination coverage among skilled nursing facility residents and staff. JAMA. 2021;325(16):1670–1671. 10.1001/jama.2021.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan CW, Chia WN, Qin X, et al. A SARS‐CoV‐2 surrogate virus neutralization test based on antibody‐mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38(9):1073‐1078. [DOI] [PubMed] [Google Scholar]
- 5. Rogers TF, Zhao F, Huang D, et al. Isolation of potent SARS‐CoV‐2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saadat S, Tehrani ZR, Logue J, Newman M, Frieman MB, Harris AD. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS‐CoV‐2. JAMA. 2021;325(14):1467‐1469. 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions


