Abstract
Background
Whilst research efforts have focussed on treatment during the acute phase, little work has focussed on the long‐term sequelae of COVID‐19 infection. This case described a patient who remained symptomatic several weeks after discharge from hospital with what was diagnosed as a COVID‐19 infection. There were significant deficits shown in his functional exercise testing, his pulmonary functions testing and there was evidence of fibrotic changes on his radiology.
Methods
As part of a multidisciplinary clinic, he was started on steroids and a tailored pulmonary rehabilitation course over a course of 6–8 weeks. Thereafter, his exercise testing, pulmonary function tests and radiology were all repeated to see progress.
Results
On completing the course of corticosteroids and concurrent personalised pulmonary rehabilitation, there was a dramatic improvement in the patient's symptom severity, radiology and pulmonary function. The most significant improvement noted was in his exercise testing, namely a 6‐min walk test and 1 min of sit‐to‐stands. Before treatment, he had a Medical Reserch Council (MRC) score of 2, and after it returned to his baseline of 0.
Discussion
Using corticosteroids and exercise training that allowed quantitative evaluation throughout the stages of recovery was a valuable tool to gauge progress and response to treatment. These therapies present opportunity to prevent the development of irreversible pulmonary fibrosis that could prove to be a major breakthrough in reducing long‐term morbidity and improving the quality of life of those affected.
Keywords: breathlessness, physiotherapy, pulmonary rehabilitation, rehabilitation services
1. INTRODUCTION
Since the start of the 2019 coronavirus pandemic, more than 88 million infected people have been admitted to hospitals and over 63 million discharged. While there have been breakthroughs for various vaccines and treatments in the acute phase, little work has focussed on the long‐term sequelae of COVID‐19 infection.
Early reports from China showed developing lung fibrosis in studies analysing the radiology (Shi et al., 2020) and cadavers (Tian et al., 2020) of those infected with COVID‐19. Pulmonary fibrosis has been noted as a long‐term sequelae of coronavirus infection in the past two major outbreaks of viruses in this family: severe acute respiratory syndrome (SARS) and middle eastern respiratroy syndrome (MERS) (Das et al., 2017; Xie et al., 2005). This condition affects patients’ quality of life and also infers increased risk of early mortality (Brown et al., 2020; Ojo et al., 2020)
When previously given during active infection for SARS and MERS, corticosteroids led to enhanced viral replication, reduced viral clearance and had no positive effect on survival (Arabi et al., 2018; Stockman et al., 2006). Yet conversely, the use of methylprednisolone reduced lung remodelling and eventual mortality in those patients that progress to acute respiratory distress syndrome from COVID‐19 infection (Gentile et al., 2020; Wu et al., 2020). Furthermore, it is thought that extended courses of low‐dose corticosteroids may reduce lung remodelling in post‐COVID‐19 patients (Gentile et al., 2020). Randomised control trials are currently being conducted to establish the efficacy of prednisolone in treating post‐COVID‐19 fibrosis (Rashad, 2020).
Pulmonary rehabilitation programmes have been shown to improve exercise capacity, dyspnoea and quality of life in those interstitial lung disease and idiopathic pulmonary fibrosis (Dowman et al., 2014). Early guidance from China advocated for pulmonary rehabilitation in COVID‐19 survivors (Yang & Yang, 2020).
2. CASE STUDY
A 51‐year‐old gentleman presented to a London District General Hospital during the COVID‐19 pandemic with 1‐week history of fatigue, dry cough and worsening dyspnoea.
His medical history included hypertension and chronic kidney disease stage 2. At his functional baseline, he was independent. He did not smoke or drink alcohol and had an MRC dyspnoea score of 0. At his baseline exercise tolerance, he would regularly run a distance of 5 km up to three times a week and would also carry out a home‐based exercise workout once a week.
On examination at admission, he was haemodynamically stable and was maintaining saturations on 2 L of oxygen via nasal cannula. A chest x‐ray (CXR) demonstrated slight coarsening of bronchovascular markings but no acute consolidation, effusion or collapse. The arterial blood gas (ABG) on 2 L showed pH 7.48, pCO2 5.93 and pO2 11.2. He was commenced on IV antibiotics for suspected superadded bacterial infection. His laboratory results revealed a raised C‐reactive protein (CRP), slight increase in creatinine from baseline and a lymphopenia. His notable haematology and biochemistry results throughout the case are displayed in Table 1.
TABLE 1.
Serial biochemistry and haematology results throughout admission
| Admission | Day 2 | Day 9 | Discharge | 6/52 follow‐up clinic | |
|---|---|---|---|---|---|
| WCC | 8.2 | 10.3 | 8.9 | 7.9 | 4.7 |
| Haemoglobin | 155 | 141 | 128 | 136 | 149 |
| Lymphocytes | 0.5 | 0.9 | 1.0 | 1.5 | 2.0 |
| Neutrophils | 7.3 | 9.1 | 6.9 | 5.4 | 2.0 |
| Creatinine | 144 | 123 | 116 | 103 | 105 |
| CRP | 121 | 224 | 60 | 31 | 2 |
Abbreviations: CRP, C‐reactive protein; WCC, White cell count.
TABLE 2.
Comparison of physiotherapy assessment parameters at 6‐ and 12‐week post‐discharge
| 6MWT | Desaturation | MRC score | FACIT score | Recovery (s) | Max BORG | STS | Desaturation | |
|---|---|---|---|---|---|---|---|---|
| 6‐week post‐discharge | 490 | 8 | 2 | 19 | 300 s | 3 | 23 | 6 |
| 12‐week post‐discharge | 645 | 5 | 0 | 0 | 140 | ‐ | 37 | 5 |
On day 2, he developed a rapidly increasing oxygen requirement and a worsening type 2 respiratory failure (T2RF), pH 7.445, pCO2 6.53 and pO2 10.4. His first COVID‐19 viral PCR swab, sputum and blood cultures, as well as an atypical pneumonia screen all returned negative.
On day 8, his oxygen requirement further increased to 15L via a non‐rebreathe mask (NRB) and an arterial gas showed a further deteriorating T2RF pH 7.451, pCO2 6.27, pO2 9.38 and HCO3 31.2. His antibiotics were escalated, and he was referred to critical care who reviewed him on day 9 and believed he did not require admission to intensive care. A repeat COVID‐19 PCR swab again returned negative.
He continued to require 15 L NRB for further 2 days after which his oxygenation began to improve. Inpatient physiotherapy teams taught breathlessness management, educated him on oxygen delivery and stressed the need for early mobilisation.
His oxygen was slowly able to be titrated, and on repeat physiotherapy assessment, he was able to demonstrate maintenance of his independent function and mobility despite his increased oxygen demand and prolonged admission. He was, therefore, discharged from physiotherapy with no acute inpatient therapy needs identified. He was successfully weaned entirely off oxygen over the next 3 days, maintaining saturations on room air after 16 days of admission. He was then discharged with follow‐up imaging, pulmonary function testing and to be followed up in a multidisciplinary post‐COVID clinic.
Despite the absence of a positive COVID‐19 PCR, this was still deemed a COVID‐19 infection owing to the presentation during the peak surge in our hospital, high clinical suspicion, consistent radiology and biochemistry, and absence of evidence for other precipitating causes.
3. 6‐WEEK FOLLOW‐UP POST‐DISCHARGE
This gentleman was reviewed in the post‐COVID‐19 clinic 6 weeks post‐discharge. He reported slowly resolving fatigue and breathlessness. His MRC dyspnoea scale was now 2 from a baseline of 0. A CXR showed significant resolution of the COVID‐19 pneumonitis with persistent volume loss and early fibrotic changes. It was also noted that he had returned to work at a reduced capacity and hours due to his ongoing symptoms.
He had a thorough physiotherapy assessment that included a sit‐to‐stand test and the 6‐min walk test (6MWT). He also completed the FACIT‐Dyspnoea Scale 10‐item Short Form of which he scored 19/60 reporting severe shortness of breath on ‘walking for ½ mile without stopping’ and moderate shortness of breath on ‘walking up 20 stairs without stopping’ and completing household activities such as ‘sweeping or mopping’ as per the outcome measure. Throughout the testing monitoring of objective markers including saturations (SpO2), heart rate (HR) and respiratory rate (RR) were taken. In addition to this, a score on the Modified Borg Dyspnoea RPE scale and a 0–10 ‘fatigue of legs’ scale were taken at rest and post‐testing. Time to recover to baseline resting observations and RPE were also measured. Time was given between the two tests as per the trust Standard Operating Procedure for exercise testing in post‐COVID‐19 patients.
For the 6MWT, he achieved a total distance of 490 m. During the testing, we recorded a significant acute desaturation of 8% from 98% to 90%. His saturations then took 50 s to recover to be within normal resting range (>96%). His HR rose from 89 beats per minute (bpm) to 138 bpm during testing and reached this peak at his greatest desaturation. He had a raised resting respiratory rate of 24, which rose to 26 on testing; however, he recovered quickly. His cardiovascular response to the exercise testing took a total of 5 min to recover post‐testing. He also reported a perceived exertion of ‘moderate’ (3) on the Modified Borg Dyspnoea RPE scale throughout the testing which was ‘nothing at all’ (0) at rest.
He achieved a total of 23 sit‐to*stands in 1 min. For comparison, the median number for his age group would be 50 (Strassmann et al., 2013). His saturations were from 97% at rest to 91% during the assessment, and it then took 45 s for his saturations to recover to baseline. His RPE went from ‘nothing at all’ (0) at rest to ‘very severe’ (7) and took approximately 2 min and 45 s to return to his baseline resting RPE. His heart rate rose from 96 to 130 bpm, however, recovered within 1 min and 15 s. His level of fatigue within his legs rose to ‘very severe’ (7) on completion of the tests.
Pulmonary function tests (PFTs) were performed 8 weeks post‐discharge, which demonstrated a reduced FEV1 of 2.07 (60% of predicted) and reduced FVC of 2.45 (58% of predicted) with a normal FEV1/FVC ratio and moderately reduced TLCO of 5.61 (58% of predicted), suggestive of a restrictive defect (Figure 1). An echocardiogram demonstrated no abnormalities. High‐resolution CT (HRCT) scan (Figure 2a) showed bilateral predominantly peripheral atelectatic band seen in the mid and lower zones. There was some evidence that airways change in the lower zones suggestive of fibrosis. The cardinal fact, however, was that the patient was very symptomatic at this time, from a baseline of good health.
FIGURE 1.
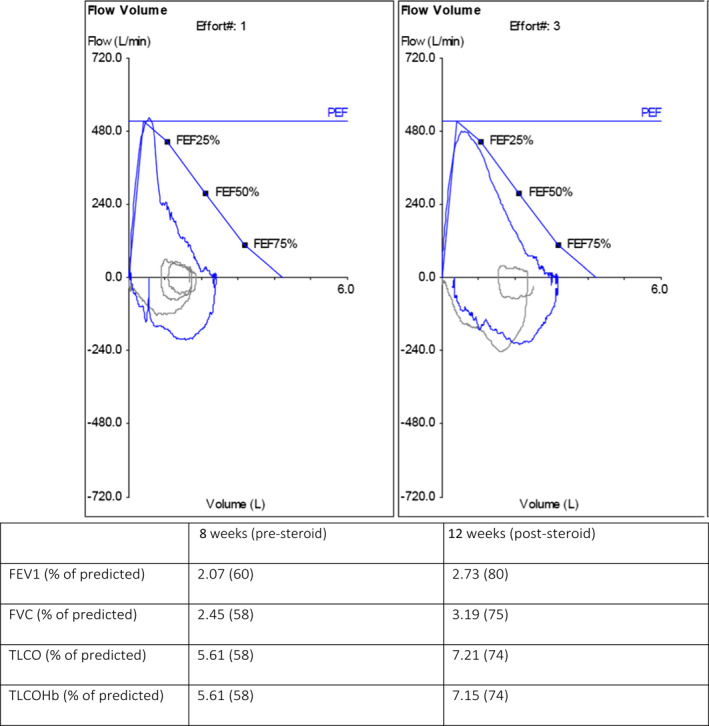
Pulmonary function testing at 8‐week post‐discharge and 12‐weeks post‐discharge
FIGURE 2.
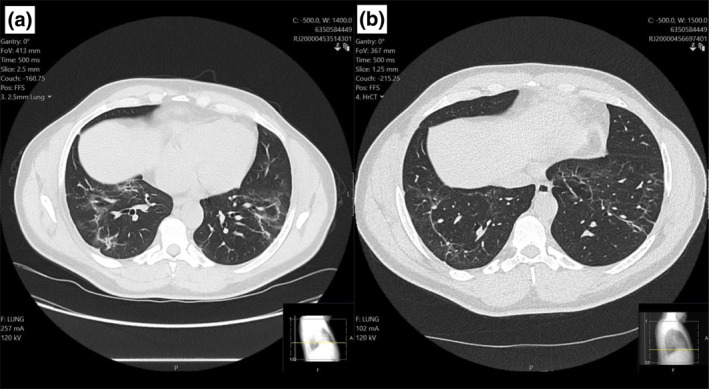
(a) HRCT scans at 6‐week post‐discharge. (b) 12‐week post‐discharge
FIGURE 3.
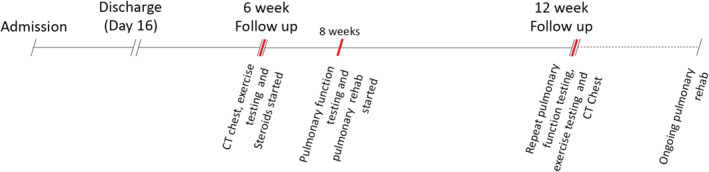
Timeline of significant events
Following the assessment, he was provided with a self‐directed exercise booklet and referred to community pulmonary rehabilitation services for further input. He received particular advice on breathlessness management, pacing, exercise progression and nutrition. The approach to exercise prescription was based on adapted principles of rehabilitation for survivors of critical illness (Berney et al., 2012) and the hospital's own developed approach to rehabilitation of COVID‐19 patients in acute and community settings.
4. 12‐WEEK FOLLOW‐UP POST‐DISCHARGE
This gentleman was then reviewed at 12‐week post‐discharge and due to his poor physiotherapy outcomes, established fibrosis on HRCT and reduced lung volumes and transfer factor, he was commenced on a 3‐week course of oral prednisolone (30 mg OD for 7 days, 20 mg OD for 7 days, 10 mg OD for 7 days).
On completion of this initial course of steroids, he was then re‐assessed by our physiotherapy team and repeated the previous 6MWT and 1‐min sit‐to‐stand test. He scored 0/60 on the FACIT‐Dyspnoea Scale 10 item short form. He reported having increased his work capacity and hours and was close to returning to full time.
For his 6MWT, he had a significant increase of 155 m compared to his previous test. Desaturation did occur, although only reduced by 5% from 98% to 93%. Max HR was 130 bpm with a much faster recovery of 2 min and 20 s to return to baseline. This was also the time it took for his RPE and fatigue scores to recover to baseline of ‘nothing at all’ (0) from ‘moderate to severe’ (4).
For his 1‐min sit‐to‐stand test, he was able to achieve 37 sit‐to‐stands, which is a significant increase of 14 sit‐to‐stands. He did again, desaturate from 98% to 93%. Overall, his recovery was within 2 min and 50 s for all subjective and objective markers. His RPE was reduced from a previous ‘very severe’ (7) to at a ‘severe’ (5) on retesting which was in keeping with the self‐reported improved fitness and conditioning levels.
He was therefore able to achieve a significant increase in distance walked, sit‐to‐stands achieved and recovery time for subjective and objective markers. The distance walked and sit‐to‐stands achieved demonstrate a significant difference for both tests, although the minimal clinically importance difference (MCID) has not been standardised in the post‐COVID‐19 population as of yet.
This retesting showed considerable improvement although he continued to acutely desaturate. It was decided that he would be continued on a further 4‐week course of prednisolone 10 mg OD.
Repeat PFTs at 12‐week post‐discharge (Figure 1) demonstrated a marked improvement from his initial testing; FEV1 was 2.73 (80% of predicted), FVC was 3.19 (75% of predicted) and TLCO was 7.21 (74% of predicted). A repeat HRCT (Figure 2b) at 12‐week post‐discharge showed partial resolution of the previously noted fibrosis and ground glass opacifications. Residual fibrosis and mild traction bronchiectasis were seen scattered peripherally in both lung fields. However, both modalities confirmed notable improvement documented after repeat exercise testing.
5. PULMONARY REHABILITATION
He was referred to pulmonary rehabilitation following his initial assessment in the post‐COVID‐19 clinic. The course commenced 8 weeks after discharge for 8 weeks where he was also given guidance on how to continue beyond the course with a tailored exercise programme. Due to the current climate and change of practices, he completed his course via telephone, video and programmes being sent to him via email. His pulmonary rehabilitation was divided into two sections: Aerobic exercises and strength exercises. He began all aerobic exercises on level 3 (no weights) and progressed with all of them to level 4 (weights) and some to level 5. He started his strength exercises for upper limbs on level 5 and he managed to progress his weights further during the course. His lower limb strength exercises were started on a level 4 and progressed to level 5. He completed the exercises two times per week, next to his own weightlifting programme 1–2 per week and running twice weekly.
On completion of his pulmonary rehabilitation course, he has now returned to his pre COVID‐19 exercise baseline with an MRC dyspnoea score of zero. At his baseline exercise tolerance, he would regularly run 5 km and would also carry out a home‐based exercise workout once a week. He is now achieving a 45‐min run twice weekly and is able to achieve a higher weight at increased number repetitions than previously for his home exercise programme. He is now back to full time working with no reported dyspnoea, fatigue or other limiting factors.***
6. CONCLUSION
This case report of a patient with established COVID‐19 fibrosis represents a dramatic improvement in the patient's symptom severity, radiology and PFTs after completing a course of corticosteroids. The authors hypothesise that this dramatic improvement enabled the patient to participate fully in pulmonary rehabilitation and realise the long‐term benefits of improved exercise tolerance, dyspnoea and quality of life. However, given significant possible side effects including avascular necrosis, hyperglycaemia and psychosis, corticosteroids must be used with caution.
Quantitative evaluation throughout the stages of recovery was a valuable tool to gauge progress and response to treatment. The 6MWT, CT imaging and PFTs are all currently used in research to assess and monitor progression of COVID symptomatology. Though no tests are specifically validated, 6MWT and CT imaging in particular have been used to good effect in prognostications and safe ongoing care of COVID‐19 patients (Fuglebjerg et al., 2020; Rai et al., 2020).
Initial application of an adapted version of the principles demonstrated by Berney et al (2012) on the role of exercise prescriptions and rehabilitation for survivors of critical illness were applied in this case. Pulmonary rehabilitation continued in keeping with current guidelines, and he excelled beyond the level of exercise prescription available.
The authors acknowledge that it is difficult to prove causality from this single case. If further studies replicated the results seen in this report, the use of corticosteroids and pulmonary rehabilitation in those with ongoing COVID‐19 associated pneumonitis, to prevent the development of irreversible pulmonary fibrosis, could prove to be a major breakthrough in reducing long‐term morbidity and improving the quality of life of those affected.
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
AUTHOR CONTRIBUTIONS
Adhnan Omar, Jack Fanshawe and Jack Howell drafted and revised the manuscript. Thomas Simpson, Jack Howel and Megan Piper were all involved in the care given to the patient. Thomas Simpson critically revised the manuscript and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ETHICAL APPROVAL
Ethical approval not required for this report, and patient written consent obtained.
ACKNOWLEDGEMENT
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Fanshawe, J. , Howell, J. , Omar, A. , Piper, M. , & Simpson, T. (2021). Corticosteroids and pulmonary rehabilitation reducing long‐term morbidity in a patient with post‐COVID‐19 pneumonitis: A case study. Physiotherapy Research International, 26(3), e1903. 10.1002/pri.1903
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Arabi, Y. M. , Mandourah, Y. , Al‐Hameed, F. , Sindi, A. A. , Almekhlafi, G. A. , Hussein, M. A. , Jose, J. , Pinto, R. , Al‐Omari, A. , Kharaba, A. , Almotairi, A. , Al Khatib, K. , Alraddadi, B. , Shalhoub, S. , Abdulmomen, A. , Qushmaq, I. , Mady, A. , Solaiman, O. , Al‐Aithan, A. M. , … Saudi Critical Care Trial Group . (2018). Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. American Journal of Respiratory and Critical Care Medicine, 197(6), 757–767. 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- Berney, S. , Haines, K. , Skinner, E. H. , & Denehy, L. (2012). Safety and feasibility of an exercise prescription approach to rehabilitation across the continuum of care for survivors of critical illness. Physical Therapy, 92(12), 1524‐1535. 10.2522/ptj.20110406 [DOI] [PubMed] [Google Scholar]
- Brown, K. K. , Martinez, F. J. , Walsh, S. L. , Thannickal, V. J. , Prasse, A. , Schlenker‐Herceg, R. , Goeldner, R. G. , Clerisme‐Beaty, E. , Tetzlaff, K. , Cottin, V. , & Wells, A. U. (2020). The natural history of progressive fibrosing interstitial lung diseases. European Respiratory Journal, 55(6), 2000085. 10.1183/13993003.00085-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, K. M. , Lee, E. Y. , Singh, R. , Enani, M. A. , Al Dossari, K. , Van Gorkom, K. , Larsson, S. G. , & Langer, R. D. (2017). Follow‐up chest radiographic findings in patients with MERS‐CoV after recovery. Indian Journal of Radiology and Imaging, 27(3), 342. 10.4103/ijri.ijri_469_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowman, L. , Hill, C. J. , & Holland, A. E. (2014). Pulmonary rehabilitation for interstitial lung disease. Cochrane Database of Systematic Reviews, 10, CD006322. [DOI] [PubMed] [Google Scholar]
- Fuglebjerg, N. , Jensen, T. O. , Hoyer, N. , Ryrsø, C. K. , Lindegaard, B. , & Harboe, Z. B. (2020). Silent hypoxia in patients with SARS CoV‐2 infection before hospital discharge. International Journal of Infectious Diseases, 99, 100‐101. 10.1016/j.ijid.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile, F. , Aimo, A. , Forfori, F. , Catapano, G. , Clemente, A. , Cademartiri, F. , Emdin, M. , & Giannoni, A. (2020). COVID‐19 and risk of pulmonary fibrosis: The importance of planning ahead. European Journal of Preventive Cardiology, 27(13), 1442‐1446. 10.1177/2047487320932695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo, A. S. , Balogun, S. A. , Williams, O. T. , & Ojo, O. S. (2020). Pulmonary fibrosis in COVID‐19 survivors: Predictive factors and risk reduction strategies. Pulmonary Medicine, 2020, 1–10. 10.1155/2020/6175964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai, D. K. , Sharma, P. , & Kumar, R. (2020). Post COVID‐19 pulmonary fibrosis—Is it reversible? Indian Journal of Tuberculosis. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7654356/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashad, A. (2020). Short term low dose corticosteroids for management of post covid‐19 pulmonary fibrosis. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04551781
- Shi, H. , Han, X. , Jiang, N. , Cao, Y. , Alwalid, O. , Gu, J. , Fan, Y. , & Zheng, C. (2020). Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: A descriptive study. The Lancet Infectious Diseases, 20(4), 425‐434. 10.1016/s1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman, L. J. , Bellamy, R. , & Garner, P. (2006). Sars: Systematic review of treatment effects. PLoS Medicine, 3(9), e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann, A. , Steurer‐Stey, C. , Dalla Lana, K. , Zoller, M. , Turk, A. J. , Suter, P. , & Puhan, M. A. (2013). Population‐based reference values for the 1‐min sit‐to‐stand test. International Journal of Public Health, 58(6), 949‐953. 10.1007/s00038-013-0504-z [DOI] [PubMed] [Google Scholar]
- Tian, S. , Xiong, Y. , Liu, H. , Niu, L. , Guo, J. , Liao, M. , & Xiao, S. Y. (2020). Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Modern Pathology, 33(6), 1007‐1014. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Chen, X. , Cai, Y. , Xia, J. , Zhou, X. , Xu, S. , Huang, H. , Zhang, L. , Zhou, X. , Du, C. , Zhang, Y. , Song, J. , Wang, S. , Chao, Y. , Yang, Z. , Xu, J. , Zhou, X. , Chen, D. , Xiong, W. , … Song, Y. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 934‐943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, L. , Liu, Y. , Fan, B. , Xiao, Y. , Tian, Q. , Chen, L. , Zhao, H. , & Chen, W. (2005). Dynamic changes of serum SARS‐coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respiratory Research, 6(1), 1‐7. 10.1186/1465-9921-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L.‐L. , & Yang, T. (2020). Pulmonary rehabilitation for patients with coronavirus disease 2019 (COVID‐19). Chronic Diseases and Translational Medicine, 6(2), 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


