Abstract
Objective
We aimed to evaluate the risk of hypercalcemia in patients with very high levels of 25‐hydroxy vitamin D (25(OH)D).
Methods
The distribution of patients who were screened for 25(OH)D in our hospital between January 2014 and December 2018 was evaluated and patients with serum concentrations of 25(OH)D >88 ng/mL were selected. Then, biochemical parameters of the cases with 25(OH)D >88 ng/mL were compared according to calcium status, vitamin D level (group 1, 88‐100 ng/mL; group 2, 100‐150 ng/mL, and group 3, >150 ng/mL), and gender.
Results
A total of 282 932 patients who underwent 25(OH)D tests in our hospital were evaluated. A total of 1311 (0.5%) patients had very high 25(OH)D levels (>88 ng/mL). Four hundred and ninety‐five patients who met our inclusion criteria and had complete data participated in the study. The median age was 58 years (interquartile range [IQR] = 41‐71 years) and the median level of 25(OH)D was 104.6 mg/mL (IQR = 94.9‐124.9 ng/mL). Most of the subjects (83.7%) with very high 25(OH)D levels were normocalcemic. A weak inverse correlation was observed between 25(OH)D level and intact parathyroid hormone (iPTH) level (r = −0.118, P = .01), but no correlation between 25(OH)D and calcium levels was observed. Alkaline phosphatase (ALP) levels were significantly higher in males (P = .032), and age and iPTH levels were higher in females (P < .001 and P = .004). ALP, phosphorus levels, and iPTH suppression rates were higher in hypercalcemic patients (P < .001, P < .001, and P < .001, respectively), while the iPTH level was significantly lower in hypercalcemic patients (P < .001) than in normocalcemic patients. Amongst the three groups with different 25(OH)D levels, no difference was found in levels of iPTH, calcium, phosphorus, ALP, or age.
Conclusion
Most patients with very high vitamin D levels were normocalcemic, but severe hypercalcemia was also observed. Vitamin D replacement therapy and follow‐up should be performed according to clinical guideline recommendations.
What's known
Vitamin D supplementation has increased awareness due to its many beneficial effects on health besides bone health. As a result of the increase in vitamin D awareness, hypervitaminosis D and intoxication cases due to excessive vitamin D supplementation have also increased. Cases of hypercalcemia due to Vitamin D over‐intake have been reported in the case series.
What's new
Our study shows the increase in the number of patients with vitamin D tests and very high vitamin D levels in a substantial population. The risk of hypercalcemia in patients with very high vitamin D levels was shown in the present study with the literature's most extensive case series. Although most of the patients are normocalcemic, severe hypercalcemia cases have also been observed. No significant correlation was observed between vitamin D level and calcium level.
1. INTRODUCTION
Vitamin D is an important steroid prohormone that maintains bone health. Its most important effect is on calcium, phosphorus metabolism, and bone mineralisation. 1 Vitamin D deficiency is recognised as a global epidemic. In addition to poor bone health, vitamin D deficiency is associated with autoimmune diseases, mental health problems, cardiovascular disease, insulin resistance, metabolic syndrome, immunodeficiency, neurocognitive dysfunction, and increased risk of extracellular complications such as cancer. 2 , 3 , 4 , 5 It is also a lifestyle biomarker as vitamin D deficiency affects the quality of life. 6 During the coronavirus pandemic, the importance of vitamin D has reappeared. 7 As a result of the increasing awareness of health problems associated with vitamin D deficiency, there has been a growing trend in vitamin D screening and treatment around the world. 8 As a result, vitamin D has become a popular supplementary agent all over the world. In recent years, after careless and uncontrolled use of vitamin D therapy, there has been a significant increase in the number of cases of vitamin D hypervitaminosis and intoxication. While vitamin D intoxication can often be asymptomatic, causing hypercalcemia, it may also produce symptoms ranging from mild, such as thirst or polyuria, to severe, such as coma and death. 9 In this study, we aimed to investigate the distribution pattern of patients’ serum concentrations of vitamin D during the last 5 years in our hospital in order to determine the burden of vitamin D hypervitaminosis and toxicity and compare these results with global trends.
2. METHODS
The information of patients who underwent 25‐hydroxy vitamin D (25(OH)D) tests in our hospital between January 2014 and December 2018 was accessed from the database of our institute. The study protocol was approved by the ethics committee of our institute and the privacy of patients was preserved throughout the study. We determined the distribution of 25(OH)D concentrations and included those patients with 25(OH)D levels >88 ng/mL (220 nmol/L), the upper limit of the normal range of vitamin D concentration in our hospital, in our study. For patients with multiple vitamin D assessments, only the initial test was taken into consideration for the study. Patients who met inclusion criteria and whose serum calcium, phosphorus, iPTH, and ALP levels were analysed at the same time as their 25(OH)D levels were included in our study (Figure 1). Patients with hyperparathyroidism, hypoparathyroidism [iatrogenic (post‐surgical) or idiopathic)], lymphoma, lymphoproliferative disease granulomatosis disease (sarcoidosis, tuberculosis, fungal diseases, leprosy, giant cell polymyositis, or berylliosis) or creatinine clearance ≤40 mL/min, as well as women with creatinine concentration >1.3 mg/dL or men with creatinine concentration >1.1 mg/dL, were excluded from the study. Changes in 25(OH)D reports, patients who underwent 25(OH)D tests, and patients with 25(OH)D levels >88 ng/mL during the 5‐year period were evaluated. Patients with 25(OH)D levels >88 ng/mL were grouped according to 25(OH)D concentration: 88‐100 ng/mL (group 1), 100‐150 ng/mL (group 2), and >150 ng/mL (group 3). Subgroup analysis of patients with very high 25(OH)D levels was performed according to calcium status, gender, and vitamin D levels.
FIGURE 1.
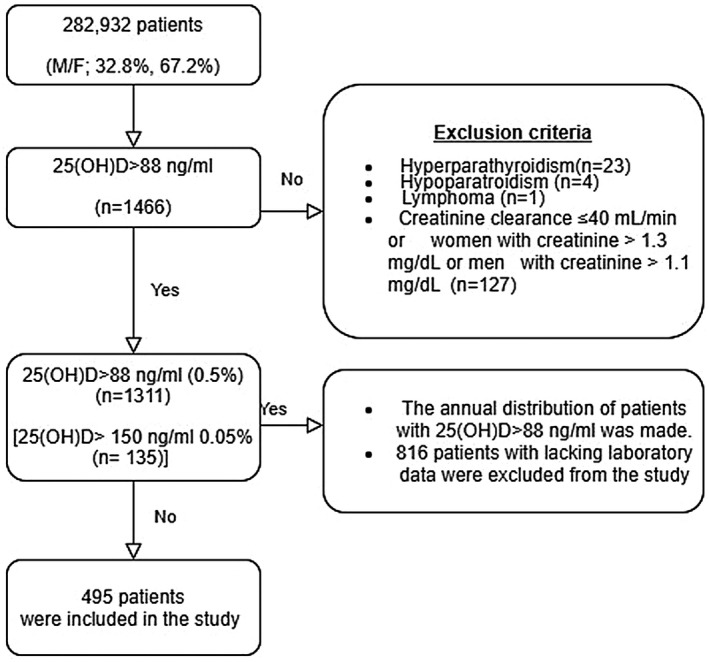
A flow diagram of the study design
2.1. Biological assessment
Serum calcium and phosphate (inorganic) levels were measured with Cobas CA2 and Cobas PHOS2 on COBAS C702 analyser (Roche Diagnostics, Mannheim, Germany). ALP levels were measured with Architect I1000SR and Architect CI8200 (Abbott Diagnostics, US). Vitamin D serum concentrations were measured by electrochemiluminescence‐protein‐binding assay (ECLIA) (Cobas e602, Roche Diagnostics, Germany) and serum levels of iPTH were measured by solid‐phase, two‐site chemiluminescent enzyme‐labelled immunometric assay (IMMULITE 2000 Siemens, Los Angeles, CA, USA).
2.2. Statistical analysis
The variables in the study were evaluated in terms of the normal distribution with the one‐sample Kolmogorov–Smirnov test and the data were presented as median (interquartile range (IQR)). Categorical variables were shown as counts and percentages. Categorical data were appropriately analysed by Chi‐square (χ 2) test or Fisher's exact test. Discrepancies between the groups were analysed by Mann–Whitney U test and Kruskal–Wallis test because of the non‐normal distribution. Correlation analyses were performed by calculating Spearman's and Pearson's correlation coefficients. The results were evaluated at a 95% confidence interval and P < .05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 22.0 (SPSS Inc, Chicago, IL).
3. RESULTS
A total of 282 932 patients (32.8% male, 67.2% female) who underwent 25(OH)D testing in our hospital were evaluated in terms of vitamin D distribution. Most patients were 40‐60 y old (34.1%); the percentages belonging to other age groups were 9.2% (<20 y), 27.2% (20‐40 y), and 29.5% (>60 y). It was observed that 357 526 25(OH)D tests were performed in these patients within 5 years and the number of tests increased over the years. There was also a significant increase in the number of patients who underwent the vitamin D test over the years; it was 7.02% in 2014, 17.23% in 2015, 21% in 2016, 25.2% in 2017, and 29.55% in 2018 (P < .001) (Figure 2). Patients with very high 25(OH)D levels (>88 ng/mL) that met our inclusion criteria were observed at a rate of 0.5% (n = 1311) (21.7% male, 78.3% female), 0.05% of whom (n = 135) had 25(OH)D concentration >150 ng/L. Patients >60 y old had a higher rate of hypervitaminosis D (39.5%, n = 518) than those <20 y old (11.7%, n = 153), 20‐40 y old (18.4%, n = 241), and 40‐60 y old (30.4%, n = 399). The number of patients with very high 25(OH)D levels also increased over the years. The distribution of patients with very high 25(OH)D levels by year was 5% (n = 65) in 2014, 20.5% in 2015 (n = 269), 14.9% (n = 195) in 2016, 23.9% (n = 313) in 2017, and 35.8% (n = 469) in 2018 (Figure 3). Four hundred and ninety‐five patients whose complete biochemical data were collected at the same time the 25(OH)D test was performed were included in our study (Figure 1).
FIGURE 2.
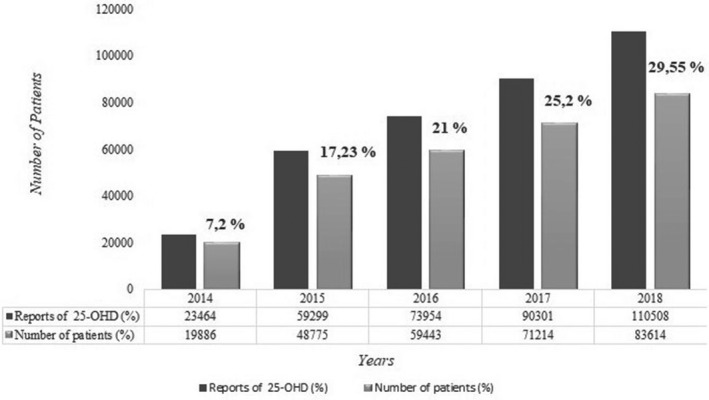
Distribution of 25(OH)D reports and patients who underwent vitamin 25(OH)D tests by years (2014‐2018)
FIGURE 3.
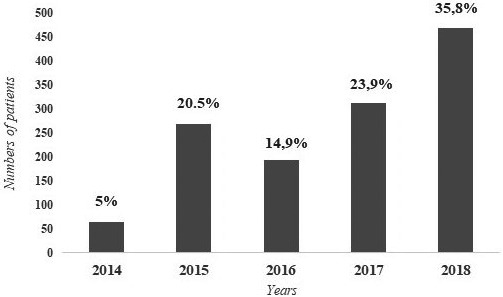
Distribution of the number of patients with 25(OH)D level >88 ng/mL by years (2014‐2018) (n:1311)
Patients with very high 25(OH)D levels were mostly female (80%, n = 396); male comprised 20% of these patients (n = 99). The median age was 58 y (IQR = 41‐71), median vitamin D level was 104.6 ng/mL (IQR = 94.9‐124.9, range = 88.3‐686.6), median calcium level was 9.7 mg/dL (IQR = 9.4‐10, range = 8‐16.95), median phosphorus level was 3.6 mg/dL (IQR = 3.3‐4.04, range = 1.69‐7.63), median iPTH level was 33.1 pg/mL (IQR = 23.7‐45.11, range = 2‐86), and median ALP level was 67 U/L (IQR = 53‐91.75, range = 27‐3631) (Table 1).
TABLE 1.
Demographic and laboratory characteristics of the hypercalcemic and normocalcemic patients with 25(OH)D levels >88 ng/mL
| Parameters median (IQR) | All (n = 495) | Hypercalcemic (n = 80) | Normocalcemic (n = 415) | P value |
|---|---|---|---|---|
| Gender (M/F) | 99/396 (20%/80%) | 19/61 (23.8%/76.2%) | 80/335 (19.3%/80.7%) | .360 |
| Age | 58 (41‐71) | 61 (31‐70.75) | 57 (43‐72) | .531 |
| 25(OH)D | 104.6 (94.9‐124.9) | 104.79 (94.9‐126.75) | 104.61 (94.93‐124.73) | .174 |
| iPTH (pg/mL) | 33.1 (23.7‐45.11) | 27.6 (18.07‐45.96) | 34.17 (24.33‐44.97) | <.001 |
| Calcium (mg/dL) | 9.7 (9.4‐10) | 10.4 (10.3‐10.6) | 9.6 (9.36‐9.8) | .017 |
| Phosphorus (mg/dL) | 3.6 (3.3‐4.04) | 4.08 (3.5‐4.93) | 3.6 (3.26‐3.91) | <.001 |
| ALP (U/L) | 67 (53‐91.75) | 86.5 (53‐85) | 65 (64‐185.25) | <.001 |
| PTH suppression rate | 4.2% (n = 21) | 16.2% (n = 13) | 1.9% (n = 8) | <.001 |
| Hypercalcemia rate | 16.2% (n = 80) |
Bold values indicate statistically significant (P < .05).
Although there was no difference in age, gender, or 25(OH)D level between hypercalcemic and normocalcemic patients, ALP level, phosphorus level, and iPTH suppression rates were significantly higher in hypercalcemic patients (P < .001, P < .001, and P < .001, respectively), while the iPTH level was significantly lower in hypercalcemic patients (P < .001) than in normocalcemic patients (Table 1). There was no significant correlation between 25(OH)D level and calcium, phosphorus, and ALP levels (r = −0.001, r = 0.011, and r = 0.024, respectively); however, a weak negative correlation (r = −0.118, P = .01) was observed between vitamin D level and iPTH level. The majority of cases (83.8%, n = 415) were normocalcemic (Calcium level <10.2 mg/dL). The proportion of mild hypercalcemia (10.2‐12 mg/dL) was 15.2% (n = 75), moderate hypercalcemia (12‐14 mg/dL) was 0.6% (n = 3), and severe hypercalcemia (>14 mg/dL) was 0.4% (n = 2). Amongst cases with 25(OH)D >150 ng/mL (n = 56), 75% (n = 42) were normocalcemic, 23.2% (n = 13) had mild hypercalcemia, 1.8% (n = 1) had moderate hypercalcemia, and severe hypercalcemia was not observed (Figure 4).
FIGURE 4.
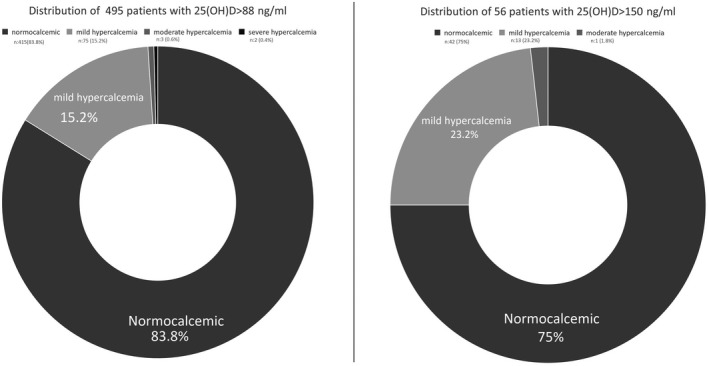
Distribution of hypercalcemia in patients with 25(OH)D >88 ng/mL
Amongst the three groups defined by 25(OH)D levels, there was no significant difference in age, gender, calcium level, iPTH level, phosphorus level, ALP level, hypercalcemia rate or iPTH suppression rate, although the PTH suppression rate and hypercalcemia rate were both higher in group 3 (Table 2).
TABLE 2.
Demographic and laboratory characteristics of patients with 25(OH)D levels >88 ng/mL according to serum 25(OH)D levels
| Parameters median (IQR) | Group 1 (n:191) (>88 ng/mL) | Group 2 (n:248) (88‐15 088 ng/mL) | Group 3 (n:56) (>150 ng/mL) | P value |
|---|---|---|---|---|
| Gender (%) | 40/151 (20.9%/79.1%) | 43/205 (17.3%/82.7%) | 15/40 (28.6%/71.4%) | .151 |
| Age | 61 (38‐73) | 62 (44‐74) | 57.5 (37.25‐72.25) | .907 |
| 25(OH)D | 93.2 (90.7‐97.8) | 115.32 (105.24‐127.59) | 188.3 (163.1‐210.38) | <.001 |
| iPTH (pg/mL) | 32.2 (23.7‐45.6) | 33.6 (23.8‐44.81) | 32.77 (22.6‐40.4) | .160 |
| Calcium (mg/dL) | 9.7 (9.4‐9.97) | 9.7 (9.33‐10) | 9.74 (9.5‐10.05) | .107 |
| Phosphorus (mg/dL) | 3.6 (3.3‐4) | 3.6 (3.22‐4.03) | 3.65 (3.4‐4.43) | .388 |
| ALP (U/L) | 72 (55‐94) | 67 (53‐87) | 72.5 (54.5‐108.5) | .725 |
| Hypercalcemia rate | 15.2% (n = 29) | 14.9% (n = 37) | 25% (n = 14) | .162 |
| PTH suppression rate | 4.2% (n = 8) | 3.2% (n = 8) | 8.9% (n = 5) | .160 |
Bold values indicate statistically significant (P < .05).
Table 3 presents demographic and laboratory data of the patients by gender. ALP levels were significantly higher in males (P = .032), and age and iPTH levels were higher in females (P < .001 and P = .004, respectively). There was no difference in terms of 25(OH)D level, calcium, phosphorus level, iPTH suppression, or hypercalcemia rate.
TABLE 3.
Comparison of demographic and laboratory characteristics of patients with 25(OH)D levels >88 ng/mL by gender
| Parameters median (IQR) | Male (n = 99) | Female (n = 396) | P value |
|---|---|---|---|
| Age | 51 (26‐67) | 60 (44‐72) | <.001 |
| 25(OH)D | 105.7 (95.1‐128.9) | 104.56 (95.32‐124) | .676 |
| iPTH (pg/mL) | 28.5 (22.2‐38.1) | 34.23 (24.35‐45.57) | .004 |
| Calcium (mg/dL) | 9.75 (9.6‐10.1) | 9.65 (9.4‐9.98) | .084 |
| Phosphorus (mg/dL) | 3.5 (3.1‐4.5) | 3.61 (3.35‐4) | .443 |
| ALP (U/L) | 75 (53.5‐165) | 66 (53‐85) | .032 |
| iPTH suppression rate | 5.1% (n = 5) | 4% (n = 16) | .656 |
| Hypercalcemia rate | 19.2% (n = 19) | 15.4% (n = 61) | .360 |
Bold values indicate statistically significant (P < .05).
4. DISCUSSION
As our hospital has one of the largest patient capacities in the country, with an average of one million patient cases per year, our study offers an overview both in our country and in the Middle East. The most important result of the study was that most cases with very high 25(OH)D levels were normocalcemic. In our study, there was an increase in the number of patients with very high 25(OH)D levels in parallel with the increase in the number of 25(OH)D tests and patients during the 5‐year study period. Patients with very high 25(OH)D levels were mostly female and elderly. In addition, the rate of hypercalcemia and iPTH suppression was higher in patients with 25(OH)D levels >150 ng/mL, although the difference was not statistically significant.
Vitamin D is an important steroid prehormone for overall body health, supporting bone health and offering additional benefits as well. In the 21st century, there was an increase in vitamin D deficiency all over the world, including developed countries. Amongst the most important reasons for this increase is the increased awareness of and testing for vitamin D deficiency. In addition, there has been a significant increase in studies investigating the role of vitamin D metabolism in the pathophysiology of various diseases. As a result, there has been a growing demand for measuring vitamin D metabolite levels and taking vitamin supplements around the world, especially in developed countries. 10 In a study conducted in Canada, it was observed that the number of 25(OH)D tests increased 25 times from 2004 to 2010. 11 In addition, a study conducted in the United Kingdom showed that the number of 25(OH)D tests increased from 7537 in 2007 to 46 000 in 2010. 8 Similarly, another study in Australia showed that the number of tests increased seven times between 2001 and 2010. 10 Similar to these studies, our review showed that the number of 25(OH)D tests in our hospital increased approximately five times between 2014 and 2018.
As a result of increased rates and awareness of D vitamin deficiency across the world, there has been also a large increase in prescriptions for vitamin D deficiency symptoms. Ergocalciferol (D2) and cholecalciferol (D3) are the most common form of dietary supplements of vitamin D. The recommended daily physiological vitamin D supplementation amount is 400 IU for infants, 600 IU for adults under 70 y old, and 800 IU for adults over 70 y old. The safe daily vitamin D limit is 4000 IU. Acute vitamin D toxicity is usually observed in cases of 10 000 IU/d intake. 12 , 13
While bolus‐ and single‐dose treatments were previously recommended to treat vitamin D deficiency in a rapid way, recent studies showed that bolus‐dose use of vitamin D is associated with increased risk of fractures and falls, and this approach has been abandoned. 14 However, bolus‐dose use is still widespread in developing countries and it was observed that prolonged supra‐physiological use leads to vitamin D hypervitaminosis. 15 In our country, vitamin D hypovitaminosis and the use of vitamin D at a dose of 300 000 IU in osteoporosis, especially in the elderly, are common. 16 One of the reasons for common vitamin D excess in the elderly in our study may be the widespread and uncontrolled use of these preparations in this age group. In the literature, most patients with vitamin D hypervitaminosis are elderly and women. 17 Similarly, most patients who were tested for vitamin D in our study were female and elderly. The reason for this may be that elderly people are more often examined because of the symptoms of vitamin D deficiency, osteoarthritis, osteoporosis, and post‐menopausal screening. In a meta‐analysis of studies from our region, vitamin D deficiency was most frequently observed in elderly women. 18 , 19 We thought that as a result of uncontrolled use of supraphysiological doses of vitamin D because of deficiency, vitamin D hypervitaminosis was observed at a high rate in elderly and female patients. In the present study, the proportion of female patients with very high vitamin D levels was high because of the high rate of female patients who underwent vitamin D testing. However, no significant difference was found between males and females in vitamin D and calcium levels. It has been observed that women with very high vitamin D levels are older and generally post‐menopausal. Oestrogen deficiency in post‐menopausal women causes abnormalities in calcium homeostasis, decreased ALP, increased bone resorption, and increased parathyroid hormone secretion. 20 In our study, we thought that ALP levels were lower but iPTH levels were higher in women than in men because of their advanced age and post‐menopausal status. In addition, because of higher bone mass in men, the normal range of ALP is higher than in women. Because most of them were female (mostly post‐menopausal), increases in calcium levels and iPTH suppression may not be evident in patients with very high vitamin D levels.
The increased use of vitamin D supplementation and continuation of therapeutic doses without monitoring caused increases in vitamin D hypervitaminosis and the number of cases of toxicity. For vitamin D hypervitaminosis, there are different criteria. Some publications consider 25(OH)D level >88 ng/mL (>220 nmol/L) to indicate vitamin D hypervitaminosis, as in our hospital 21 , 22 ; others consider 25(OH)D level >100 ng/mL (>250 nmol/L) as a criterion. 23 In addition, many authorities determined 25(OH)D level >150 ng/mL (>375 nmol/L) as vitamin D toxicity. Dudenkov et al showed in their study that the incidence of 25(OH)D >50 ng/mL (>125 nmol/L) increased from 9 to 233 between 2002 and 2011, and similar to our study, the majority of cases consisted of women and older adults. 17 In our study, the large proportion of female patients with very high vitamin D levels reflected the large proportion of female patients amongst those who underwent vitamin D testing. According to data from the National Poison Data System, toxic exposure to vitamin D increased from 196 cases between 2000 and 2005 to 4335 cases between 2005 and 2011. 24 Assay rates of 25(OH)D level >150 ng/mL (>375 nmol/L) were 27 (~0.05%) in a study of 60 237 tests in the United States, 15 (~0.03%) in another study that included 57 433 tests, and 8 (~0.02%) in a study of 41 177 tests in Australia. 12 , 25 In our study, vitamin D intoxication (>150 ng/mL) ratio was 0.05%, consistent with the literature, and increased over the years.
As a result of excessive intake of vitamin D, serum levels of vitamin D metabolites (25(OH)D; 24,25(OH)2D; 25,26 (OH)2D; and 25(OH)D‐26,23‐lactone) increase and exceed vitamin D binding protein binding capacity, causing the increase of free active metabolite 1,25(OH)2D. This vitamin D metabolite reaches the vitamin D receptor (VDR) in the nucleus of cells and increases calcium absorption and bone mobilisation by causing exaggerated gene expression. As a result, it causes hypercalcemia and hyperphosphatemia, resulting in iPTH suppression. 26 While the most common cause of vitamin D‐associated hypercalcemia is iatrogenic supplementation, production of ectopic 1,25‐dihydroxyvitamin D (sarcoidosis, tuberculosis, fungal diseases, granulomatous diseases such as leprosy, and lymphoma) can cause inactivating mutations of the CYP24A1 gene. 27 Therefore, long‐term follow‐up is required in vitamin D intoxication in terms of symptoms and findings associated with hypercalcemia. In addition to hypercalcemia, vitamin D intoxication can cause several symptoms such as depression, stupor, and coma psychiatrically; short QT, bradyarrhythmia, and hypertension in the cardiovascular system; nausea, vomiting, constipation, peptic ulcer, and pancreatitis in the gastrointestinal tract; and hypercalciuria, nephrocalcinosis, and renal failure in the kidneys. 28 In addition to 25(OH)D level >150 ng/mL biochemically, vitamin D intoxication can be symptomatic; hypercalcemia, hypercalciuria, and parathyroid hormone suppression can be seen. 26 Some studies have observed that in cases with vitamin D intoxication and hypervitaminosis, vitamin D level and serum calcium levels were not associated, and most of the cases were normocalcemic. 29 , 30 Some control mechanisms are thought to prevent very high vitamin D levels from causing hypercalcemia. These include the enzyme system in the liver that metabolises vitamin D to inactive metabolites and the storage of excess vitamin D in adipose tissue. In addition, we think that feedback mechanisms may play a role in personalised VDR polymorphisms, receptor, and post‐receptor interactions in tissues. Excessive vitamin D that exceeds these control mechanisms may cause symptoms depending on the dose and duration of supplementation. Apart from its hypercalcemic effects, there is no publication in the literature on other potential effects on the body because of its storage in adipose tissue. In the studies conducted on vitamin D levels, vitamin D was directly proportional to the calcium level and inversely proportional to the parathyroid hormone level, but the same situation was not observed in vitamin D hypervitaminosis and intoxication cases. In a series of 5557 patients studied in India, 151 cases had vitamin D intoxication, and similar to our study, 69.5% of the cases were normocalcemic. 30 Although the rate of normocalcemia was high, cases with severe hypercalcemia that could cause serious symptoms were observed in our study, albeit at a low rate. İn addition, factors such as calcium‐containing supplements, diet, and the duration of vitamin D supplementation may also have contributed to hypercalcemia.
This study has potential limitations since it is a retrospective study. Information on the patients’ supplementation levels, hypercalciuria rates, specific treatments received for hypercalcemia or hypocalcemia, diet, lifestyle, level of exposure to the sun, presence and duration of symptoms, and treatment for intoxication is missing. Vitamin D intoxication studies usually include a small number of cases, and there is a need for prospective studies examining extensive case series.
5. CONCLUSION
This study aims to emphasise that in recent years, there has been a tendency towards a secular increase in the number of 25(OH)D tests and an increase in iatrogenic vitamin D hypervitaminosis cases as a result of increased widespread supplementation use. This study shows that despite the high prevalence of vitamin D deficiency in developing countries, such as our country, vitamin D hypervitaminosis is not a rare clinical reality. Vitamin D hypervitaminosis and intoxication are usually iatrogenic and can be prevented. Although most of these patients become normocalcemic as a result of some possible control mechanisms in the liver and adipose tissue, they can experience severe hypercalcemia, as in our study, which can cause serious symptoms. Especially regimens including intramuscular and supra‐physiological doses, empirical, or long‐term vitamin D supplementations without follow‐up should not be recommended, and treatment and follow‐up planning should be performed according to the clinical guideline recommendations.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Institutional review board/Ethics Committee has approved the study.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study. The article has not been presented at any conference or meeting.
DISCLOSURES
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENT
We thank the biochemistry department team, data scientist Nazım Topaç for his data arrangements and Dilek Gogas Yavuz for his consultancy support.
Batman A, Saygili ES, Yildiz D, et al. Risk of hypercalcemia in patients with very high serum 25‐OH vitamin D levels. Int J Clin Pract. 2021;75:e14181. 10.1111/ijcp.14181
Contributor Information
Adnan Batman, Email: dradnan54@hotmail.com.
Yuksel Altuntas, Email: yukselaltuntas@yahoo.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491‐499. 10.1093/ajcn/88.2.491S [DOI] [PubMed] [Google Scholar]
- 2. Hiemstra TF, Lim K, Thadhani R, Manson JE. Vitamin D and atherosclerotic cardiovascular disease. J Clin Endocrinol Metab. 2019;104(9):4033‐4050. 10.1210/jc.2019-00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dutta D, Mondal SA, Choudhuri S, et al. Vitamin‐D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103(3):e18‐e23. 10.1016/j.diabres.2013.12.044 [DOI] [PubMed] [Google Scholar]
- 4. Tamer G, Mesci B. Role of vitamin D in the immune system. Turk J Endocrinol Metab. 2013;17(1):5‐7. 10.4274/Tjem.1938 [DOI] [Google Scholar]
- 5. Iyidir OT, Altinova AE. Vitamin D and diabetes mellitus. Turk J Endocrinol Metab. 2012;16(4):89‐95. 10.4274/Tjem.1968 [DOI] [Google Scholar]
- 6. Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9(6):337‐347. 10.1038/nrneph.2013.74 [DOI] [PubMed] [Google Scholar]
- 7. Ali N. Role of vitamin D in preventing of COVID‐19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373‐1380. 10.1016/j.jiph.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sattar N, Welsh P, Panarelli M, Forouhi NG. Increasing requests for vitamin D measurement: costly, confusing, and without credibility. Lancet. 2012;379(9811):95‐96. 10.1016/S0140-6736(11)61816-3 [DOI] [PubMed] [Google Scholar]
- 9. Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients. 2013;5(9):3605‐3616. 10.3390/nu5093605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galior K, Ketha H, Grebe S, Singh RJ. 10 years of 25‐hydroxyvitamin‐D testing by LC‐MS/MS‐trends in vitamin‐D deficiency and sufficiency. Bone Rep. 2018;8:268‐273. 10.1016/j.bonr.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilinski K, Boyages S. The rise and rise of vitamin D testing. BMJ. 2012;345:e4743. 10.1136/bmj.e4743 [DOI] [PubMed] [Google Scholar]
- 12. Quaggiotto P, Tran H, Bhanugopan M. Vitamin D deficiency remains prevalent despite increased laboratory testing in New South Wales, Australia. Singapore Med J. 2014;55(5):271‐280. 10.11622/smedj.2014071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13(8):466‐479. 10.1038/nrendo.2017.31 [DOI] [PubMed] [Google Scholar]
- 14. Bischoff‐Ferrari HA, Dawson‐Hughes B, Orav EJ, et al. Monthly high‐dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175‐183. 10.1001/jamainternmed.2015.7148 [DOI] [PubMed] [Google Scholar]
- 15. Masood MQ, Khan A, Awan S, et al. Comparison of vitamin D replacement strategies with high‐dose intramuscular or oral cholecalciferol: a prospective intervention study. Endocr Pract. 2015;21(10):1125‐1133. 10.4158/EP15680.OR [DOI] [PubMed] [Google Scholar]
- 16. Cigerli O, Parildar H, Unal AD, Tarcin O, Erdal R, Demirag NG. Vitamin D deficiency is a problem for adult out‐patients? A university hospital sample in Istanbul, Turkey. Public Health Nutr. 2013;16(7):1306‐1313. 10.1017/S1368980012003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dudenkov DV, Yawn BP, Oberhelman SS, et al. Changing incidence of serum 25‐hydroxyvitamin d values above 50 ng/mL: a 10‐year population‐based study. Mayo Clin Proc. 2015;90:577‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassil D, Rahme M, Hoteit M, Gel‐H F. Hypovitaminosis D in the Middle East and North Africa: prevalence, risk factors and impact on outcomes. Dermatoendocrinology. 2013;5(2):274‐298. 10.4161/derm.25111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lips P, Cashman KD, Lamberg‐Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23‐P54. 10.1530/EJE-18-0736 [DOI] [PubMed] [Google Scholar]
- 20. Khosla S, Melton LJ III, Riggs BL. Osteoporosis: gender differences and similarities. Lupus. 1999;8(5):393‐396. 10.1177/096120339900800513 [DOI] [PubMed] [Google Scholar]
- 21. Vieth R. Vitamin D supplementation, 25‐hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842‐856. 10.1093/ajcn/69.5.842 [DOI] [PubMed] [Google Scholar]
- 22. Shea RL, Berg JD. Self‐administration of vitamin D supplements in the general public may be associated with high 25‐hydroxyvitamin D concentrations. Ann Clin Biochem. 2017;54(3):355‐361. 10.1177/0004563216662073 [DOI] [PubMed] [Google Scholar]
- 23. Glade MJ. A 21st century evaluation of the safety of oral vitamin D. Nutrition. 2012;28(4):344‐356. 10.1016/j.nut.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 24. Klingberg E, Oleröd G, Konar J, Petzold M, Hammarsten O. Seasonal variations in serum 25‐hydroxy vitamin D levels in a Swedish cohort. Endocrine. 2015;49(3):800‐808. 10.1007/s12020-015-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ladizesky M, Lu Z, Oliveri B, et al. Solar ultraviolet B radiation and photoproduction of vitamin D3 in central and southern areas of Argentina. J Bone Miner Res. 1995;10(4):545‐549. 10.1002/jbmr.5650100406 [DOI] [PubMed] [Google Scholar]
- 26. Marcinowska‐Suchowierska E, Kupisz‐Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity—a clinical perspective. Front Endocrinol. 2018;9:550. 10.3389/fendo.2018.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tebben PJ, Singh RJ, Kumar R. Vitamin D‐mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37(5):521‐547. 10.1210/er.2016-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Potts JT Jr, Juppner H. Disorders of the parathyroid gland and calcium homeostasis. In: Longo DL, Fauci AS, Dl Kasper, Sl Hauser, Jl Jameson, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. Vol. 2. 18th ed. New York, NY: McGraw Hill; 2012:3096‐3129. [Google Scholar]
- 29. Vogiatzi MG, Jacobson‐Dickman E, DeBoer MD, Drugs, and Therapeutics Committee of the Pediatric Endocrine Society . Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99(4):1132‐1141. 10.1210/jc.2013-3655 [DOI] [PubMed] [Google Scholar]
- 30. Sharma LK, Dutta D, Sharma N, Gadpayle AK. The increasing problem of subclinical and overt hypervitaminosis D in India: an institutional experience and review. Nutrition. 2017;34:76‐81. 10.1016/j.nut.2016.09.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


