Abstract
Severe acute respiratory syndrome coronavirus 2 can lead to life‐threatening coronavirus disease 2019 (COVID‐19) infections in patients with hematologic malignancies, particularly among hematopoietic cell transplant (HCT) recipients. We describe two patients with COVID‐19 during the pre‐engraftment period after HCT and review previous reports of COVID‐19 in HCT recipients. Because of significant mortality from COVID‐19, primarily after allogeneic HCT, early, preemptive, and optimal directed therapy may improve outcomes and reduce the mortality rate but still needs to be established in clinical trials.
Keywords: COVID‐19, hematopoietic cell transplantation, outcomes, pre‐engraftment, stem cell therapy
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), secondary to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has become a worldwide pandemic, presenting unprecedented challenges to health‐care providers and systems. 1 The immunopathogenesis of COVID‐19 is intriguing as it involves virus‐driven tissue damage intertwined with an uncontrolled inflammatory response, contributing to severity of disease, acute respiratory distress syndrome (ARDS), and multiple organ failure. 2 Hematopoietic cell transplant (HCT) recipients are severely immunocompromised, with increased risk of severe COVID‐19 secondary to the myeloablative conditioning regimens, organ damage, and possible immune recovery leading to an exuberant inflammatory reaction. A comprehensive approach to manage HCT recipients with COVID‐19 at different stages after HCT (pre‐engraftment, early and late post‐engraftment) is lacking in terms of the potential role of early or pre‐emptive antiviral use and/or immunomodulators that may improve clinical outcomes. In this brief report, we present two patients with COVID‐19 pneumonia that occurred during the pre‐engraftment period after HCT at our comprehensive cancer center. Additionally, we review the previously reported cases of COVID‐19 in adults HCT recipients until December 31st, 2020, for whom sufficient data were available. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
1.1. Case 1
A 61‐year‐old man with multiple myeloma had an autologous HCT after a conditioning regimen consisting of busulfan and melphalan. On admission, the patient was asymptomatic, and a nasopharyngeal (NP) swab yielded negative real‐time reverse‐transcription polymerase chain reaction (rRT‐PCR) results for SARS‐CoV‐2. On day +3 post‐HCT and as part of an investigation of an employees’ cluster of cases, the patient, still asymptomatic, had a NP swab that was positive for COVID‐19. Computed tomography (CT) of the chest on day +4 showed no evidence of pneumonia (Figure 1A). On day +7, the patient had fevers up to 102°F (38.8°C) and diarrhea that prompted initiation of empiric antibiotics for febrile neutropenia. Further diagnostic workup, including blood cultures, was negative for infectious causes. Repeat chest CT on day +8 revealed new ground‐glass opacities (GGOs) suggestive of COVID‐19 pneumonia (Figure 1B). Although the patient had persistent fever and positive lung abnormalities, he was not short of breath or hypoxic and did not qualify for remdesivir therapy. He received one dose of tocilizumab (400 mg) on day +10 and a 4‐day course of dexamethasone (8 mg daily) starting on day +11 during engraftment for concerns of exuberant inflammatory reaction and engraftment syndrome that could lead to respiratory failure. The patient had a satisfactory clinical recovery with no further complications and was discharged home in stable condition on day +14 after HCT. The salient laboratory studies and clinical course are summarized in Figure 2 (top panel).
FIGURE 1.
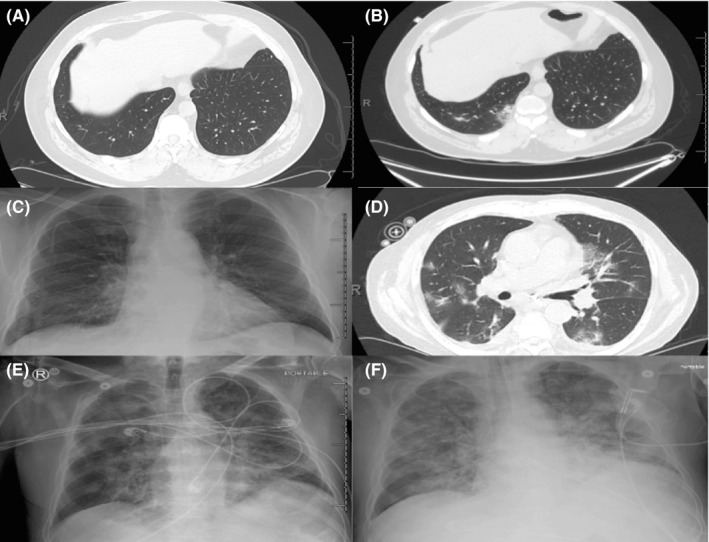
Chest imaging for the two cases. (A, B) Case 1. Panel A: CT scan obtained within 24h of COVID‐19 diagnosis showed no evidence of pneumonia. Panel B: CT scan at day+8 after HCT revealed new nodular ground‐glass opacities in the mid and lower lungs. (C‐F) Case 2. Panel C: Plain X‐ray at day+11 post HCT showed a clear lung field. Panel D: CT scan at day + 13 after HCT showed bilateral ground‐glass opacities with a peripheral predominance with focal scattered regions of consolidation. Panel E: X‐ray at the time of engraftment showed bilateral patchy multifocal interstitial and airspace opacities. Panel F: X‐ray at day + 19 after HCT with endotracheal tube in place showed progressive multilobar lung opacity
FIGURE 2.

Oxygen requirement, temperature curve, inflammatory markers, cell counts, and clinical course from time of transplant for patient 1 (top panel) and patient 2 (bottom panel). ALC: absolute lymphocyte count; CRP: C‐reactive protein; FiO2: fraction of inspired oxygen; IL‐6: interleukin‐6; Toci: tocilizumab; Tmax: temperature maximum in Celsius; WBC: white blood cell count
1.2. Case 2
A 69‐year‐old man with chronic lymphocytic leukemia underwent an allogeneic HCT with a reduced‐intensity conditioning regimen (fludarabine and melphalan) and received post‐transplant cyclophosphamide for graft‐versus‐host disease (GvHD) prophylaxis. On admission, the patient was asymptomatic and a NP swab yielded negative rRT‐PCR results for SARS‐CoV‐2. On day +11 after HCT, he developed fever associated with non‐productive cough that prompted further diagnostic workup, which included blood cultures that showed Enterococcus faecalis bacteremia, which was likely secondary to mucosal‐related bloodstream infection and was treated with vancomycin. A plain X‐ray of the chest showed no evidence of pneumonia (Figure 1C). However, in light of persistent fever after 48h of appropriate antibacterial therapy, new onset of cough, and given the concern for a nosocomial acquisition of SARS‐CoV 2 infection in the setting of a cluster of employees diagnosed with COVID‐19 in the same location, a repeated NP swab on day +13, yielded a positive rRT‐PCR results for SARS‐CoV‐2. A CT scan of the chest showed new bilateral GGOs (Figure 1D). The patient required oxygen supplementation and received convalescent plasma, two doses of tocilizumab (400 mg) on days +16 and +17, methylprednisolone (1 mg/kg) started on day +16, anakinra (100 mg daily) started on day +18, and a 5‐day course of remdesivir started on day +19 (day +6 after COVID‐19 diagnosis, when it became available at our institution). At day +15 when the patient started engrafting, he required a rapid increase in oxygen supplementation and showed increased bilateral pulmonary opacities (Figure 1E) that led to endotracheal intubation on day +19 and mechanical ventilation. The patient continued worsening and had a severe clinical course and ARDS (Figure 1F), with no improvement. Three weeks after onset of illness and despite full supportive treatment, the patient died. The patient's laboratory studies and clinical course are outlined in Figure 2 (bottom panel).
2. DISCUSSION
Severe COVID‐19 infection has been reported among patients with underlying medical comorbidities such as cardiovascular diseases, hypertension, diabetes mellitus, or cancer and in the elderly people. 14 , 15 The spectrum of infection among those with hematologic malignancies and particularly patients who have undergone HCT is not well described, and such patients are likely at greater risk of morbidity and mortality from COVID‐19, underscoring the need for effective preventive and therapeutic strategies. To date, there is a paucity of data on the impact of COVID‐19 on HCT recipients, and the reported cases have mainly described patients who developed COVID‐19 after engraftment. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
Overall, 276 HCT recipients with COVID‐19 have been reported in the medical literature (Table 1). Of these, 171 (62%) were men and 105 (38%) women. The median age was 57 y [interquartile (IQR), 54‐61]. The median elapsed time from HCT to COVID‐19 diagnosis was 309 d (IQR, 140‐506). Of the 276 patients, 157 (57%) and 119 (43%) received an allogeneic HCT and an autologous HCT, respectively. Of the 157 allogeneic HCT recipients, 135 (86%) were on immunosuppressant drugs for chronic or acute GvHD at the time of COVID‐19 diagnosis. Seventy‐four of 276 patients (27%) had severe COVID‐19 pneumonia and progressed to respiratory failure and ARDS. Overall mortality rate from COVID‐19‐related complications occurred in 48 (17%) patients. Of these, 30 (62%) and 18 (37%) patients had received an allogeneic and an autologous HCT, respectively.
TABLE 1.
Characteristics of COVID‐19 infections among hematopoietic cell transplantation recipients
| Ref. | Month/Year | No. of Pts. |
Median age, yr |
Gender | Type of Cancer | Medical Comorbidities | Type of HCT | Cond. Reg./GvHD Primary Prophylaxis | rRT‐PCR | Median time from HCT, days | GVHD Treatment |
Prog. to ARDS |
Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | June/2020 | 1 | 47 | M | AITL | None | Allo/MUD | NR | Yes | 510 | Rux | No | HCQ | Alive |
| 4 | July/2020 | 7 | 61 |
F (5), M (2) |
AML (3), MDS (1), MF (1), B‐ALL (1), HL (1) |
HTN (1), obesity (2), DM (1) |
Allo (6) [MUD (4), Haplo (2)]; Auto (1) |
Non‐MAC (5)/PTCy (4); Bu/Flu/Alem (1); BEAM (1) |
Yes (7) | 98 |
Tacro + MMF (2); Tacro (1); CG + infliximab (1); CG (1) |
Yes (2) | HCQ (1) | Alive (4); Dead (3) [Allo (2), Auto (1)] |
| 5 | July/2020 | 1 | 59 | M | MF | DM | Allo/MRD | NR | Yes | 379 | Rux | Yes |
Lop/r, HCQ |
Alive |
| 6 | June/2020 | 2 | 60 |
F (1), M (1) |
B‐ALL (1), AML (1) |
CAD + HTN + COPD + obesity (1) |
Allo (2) [Haplo (1), MUD (1)] |
MAC + PCTy (1); Non‐MAC (1) |
No [CRISPR (2)] |
238 |
CG/Ritu (2); ibritunib (1) |
Yes (1) | CCP (1) |
Alive (1), Dead (1) |
| 7 | May/2020 | 7 | 61 |
F (2), M (5) |
MM (5), ALL (1), DLBCL (1) |
HTN (2); DM + HTN + obesity (2); CM (1); HTN + stroke (1) |
Auto (5), Allo (2) | NR (7) | Yes (7) | NR (7) | NR (2) |
Yes (3) [Allo (2)] |
Ana (1); Lop/r (1); HCQ/AZT + Toci (1) |
Dead (1) [Auto] |
| 8 | Aug/2020 | 34 | 57 |
F (12), M (22) |
AL (16), MM (9), Lym (6), others (3) |
Obesity (11), smoking (20), HTN (15), DM (13) |
Auto (14); Allo (20) |
MAC (21) | Yes (34) | 529 | ISD (15) | Yes (14) |
HCQ (15), Toci (6), Remd (5), CCP (2), Lop/r (1), Ana (1), sarilumab (1), Rib (1) |
Alive (27); Dead (7) [Allo (5), Auto (2)] |
| 9 | Aug/2020 | 2 | 57 | F (2) | CML (1), AML (1) | HTN (2) | Allo (2) [MRD (1), MUD (1)] | MAC (2) | Yes (2) | 107 |
CG + Cyclo (1); MMF + Cyclo (1) |
No | HCQ (2), AZT (1) | Alive |
| 10 | Dec/2020 | 72 | 62 |
M (46) F (26) |
MM (28), NHL (15), A/C L (19), MDS (4), HL (4), AL (1), MPD (1) |
1 a 2 + a |
Allo (35) [ MRD (9), MUD (8), MisMUD (7), UCB (7), Haplo (4)]; Auto (37) |
MAC (13), Non‐MAC (5), RIC (17), PTCy (7) |
Yes (72) | N/A |
CNI/MMF (9); CNI/MTX (10) |
Yes (15) | HCQ (23), AZT (18), Methylp (13), CCP (11), Toci (7), Remd (3) |
Dead (13) [ Allo (9), Auto (4)] Alive (59) |
| 11 | Oct/2020 | 113 | 54 |
M (64) F (49) |
NR | NR |
Allo (71), Auto (42) |
NR | Yes | 495 |
CNI (23), MMF (4), mTOR (8), CG (44) |
Yes (26) |
HCQ (74), AZT (33), CG (22), Toci (19), Anakinra (3) |
Dead (18) [ Allo (11), Auto (7)] |
| 12 | Oct/2020 | 32 | 56 |
M (25) F (7) |
MM (11), NHL (7), HL (2), AML (5), ALL (3), CML (3), AA (1) |
DM (7), HTN (18), CAD (5), Respiratory diseases (10) |
Allo (12) [MRD (9), MUD (3)], Auto (20) |
NR | Yes | NR | Cyclo (4), Tacro (2) | Yes (11) | Lop/r (2), HCQ (24), Favipiravir (12), Oseltamivir (12) | Dead (5) [Allo (2), Auto (3)] |
| 13 | Nov/2020 | 5 | 30 |
M (4) F (1) |
AA (1), AML (2), ALL (2) |
NR | Allo (5) [ MRD (5)] | MAC (5), PTCy (3) | Yes | 240 |
Cyclo (4) + CG (4), MMF (1) |
Yes (1) | CG (1) | Alive (5) |
Abbreviation: AA: aplastic anemia; AITL: angioimmunoblastic T‐cell lymphoma; AL: acute leukemia; A/C L: acute/chronic leukemia; AL: AL amyloidosis; ALL: acute lymphoblastic leukemia; Allo: allogeneic; AML: acute myeloblastic leukemia; Ana: anakinra; ARDS: acute respiratory distress syndrome; Aug: August; Auto: autologous; AZT: azithromycin; BEAM: carmustine + etoposide + cytarabine + melphalan; Bu/Flu/Alem: busulfan/fludarabine/alemtuzumab; CCP: convalescent plasma; CG: corticosteroids; CLL: chronic lymphoblastic leukemia; CM: cardiomyopathy; CML: chronic myeloblastic leukemia; CNI: calcineurin inhibitors; Cond. Reg.: conditioning regimen; COPD, chronic obstructive pulmonary disease; Cyclo: cyclosporine; Dec: December; DLBCL: diffuse large B‐cell lymphoma; DM: diabetes mellitus; dys: dyslipidemia; F: female; Haplo: haploidentical; Haplo: haploidentical; HCQ: hydroxychloroquine; HL: Hodgkin lymphoma; HTN: arterial hypertension; ISD: immunosuppressant drugs (not specified); Lop/r: lopinavir/ritonavir; Lym: lymphoma; M: male; MAC: myeloablative regimen; MDS: myelodysplastic syndrome; MF: myelofibrosis; MM: multiple myeloma; MMF: mycophenolate mofetil; MPD: myeloproliferative disorder; MRD: matched related donor; mTOR: mTOR inhibitors; MUD: matched unrelated donor; MisMUD: mismatched unrelated donor; MTX: methotrexate; N/A: not applicable; Nov: November; NR: not reported; Pt: patient; PTCy: post‐transplant cyclophosphamide; Prog: progression; Oct: October; Ref: reference; Remd: remdesivir; Rib, ribavirin; RIC: reduced intensity conditioning regimen; Ritu: rituximab; Rux: ruxolitinib; Tacro: tacrolimus; Toci: tocilizumab; UCB: umbilical cord blood.
Comorbidities include hypertension, congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus, HIV, and chronic kidney disease.
To date, the only COVID‐19 case reported in the pre‐engraftment period was diagnosed at day +6 post HCT, and a detailed description of the clinical course during the engraftment phase was not provided. 4 Our two cases illustrate the impact of COVID‐19 on HCT recipients during the pre‐engraftment period and at the time of immune reconstitution. Although our autologous HCT recipient (Case 1) recovered without antiviral therapy, he received one dose of tocilizumab and a 4‐day course of dexamethasone at the time of engraftment to abrogate the potential cytokine release and engraftment syndrome. Our allogeneic HCT recipient (Case 2) developed severe COVID‐19 pneumonia and respiratory failure despite antiviral and immunomodulatory medications and corticosteroids. Of importance, there are similarities in immune recovery after allogeneic and autologous HCT; the timing of immune recovery can be affected by many factors, such as the source of stem cells, the degree of human leukocyte antigen match, and the use of immunosuppressant drugs, including corticosteroids, that could affect the immune reconstitution. Recovery of the immune system occurs gradually over the post‐transplantation period. 16 The first immune cells to engraft are monocytes, followed by granulocytes, platelets, and natural killer cells, which together constitute the innate immunity. In contrast, adaptive immunity, consisting of B and T lymphocytes, may take 1‐2 y to full recover. 16 Also, engraftment syndrome is more prevalent among allogeneic HCT recipients and occurs at the time of neutrophil recovery; it can be associated with poor prognosis. 17 , 18 It is possible that our second patient developed a concurrent engraftment syndrome with COVID‐19 pneumonia that led to respiratory failure and ARDS. Therefore, it is crucial to identify high‐risk patients who may benefit from early preemptive treatment strategies such as antiviral therapy. It is noteworthy that two reported patients were receiving ruxolitinib for chronic GvHD treatment at the time of COVID‐19 infection; one patient had an attenuated course of infection, and the other patient had ARDS but after resuming ruxolitinib, remarkable improvement, and recovery occurred. 3 , 5 Given this observation, the role of ruxolitinib has gained attention since it is characterized not only by immunosuppressive activity but also by anti‐inflammatory effects with the potential role of dampening the cytokine release syndrome; importantly, a clinical trial evaluating ruxolitinib as novel therapy for COVID‐19 pneumonia is ongoing (NCT04338958). 19
On the other hand, a cohort study by Kuderer et al reported a 30‐day all‐cause mortality of 13% in patients with cancer and COVID‐19, including patients with hematologic malignancies, which was higher than the mortality rate reported in the general population. 20 , 21 However, based on our current review, the mortality rate in HCT recipients is quite high (17%). Therefore, preventive measures remain the best approach for reducing the burden of COVID‐19 infections in HCT recipients, and strict adherence to infection control measures and increased awareness among patients, caregivers, and health‐care personnel are critical. Another important clinical aspect to be considered is that the immune recovery during engraftment may lead to severe COVID‐19 pneumonia secondary to exuberant inflammatory response that may require early therapy such as remdesivir and/or steroids before decompensation. Considering the substantial comorbidities and mortality among cancer patients with COVID‐19, notably in HCT recipients, novel therapeutic and preventive strategies are of utmost importance.
CONFLICTS OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
AEM, JAA, VEM, JS, IIR, KM, GTS, UD, FK, and RFC wrote the mansucript draft and revised the manuscript; JAA, VEM, KM, GT, and UD were involved in patients treatment. All the authors approved the final version of the manuscript.
Malek AE, Adachi JA, Mulanovich VE, et al. Immune reconstitution and severity of COVID‐19 among hematopoietic cell transplant recipients. Transpl Infect Dis. 2021;23:e13606. 10.1111/tid.13606
Funding information
None.
Contributor Information
Alexandre E. Malek, Email: alex.e.malek@gmail.com.
Roy F. Chemaly, Email: alex.e.malek@gmail.com.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated.
REFERENCES
- 1. World Health Organization. Novel coronavirus‐China . January 12, 2020. Available online: https://covid19.who.int/. Accessed December 31st, 2020.
- 2. Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foss FM, Rubinowitz A, Landry ML, et al. Attenuated Novel SARS Coronavirus 2 Infection in an Allogeneic Hematopoietic Stem Cell Transplant Patient on Ruxolitinib. Clin Lymphoma Myeloma Leuk. 2020;20(11):720‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanellopoulos A, Ahmed MZ, Kishore B, et al. COVID‐19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020;190(2):e67‐e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saraceni F, Scortechini I, Mancini G, et al. Severe COVID‐19 in a patient with chronic graft‐versus‐host disease after hematopoietic stem cell transplant successfully treated with ruxolitinib. Transpl Infect Dis. 2020;23(1):e13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niu A, McDougal A, Ning BO, et al. COVID‐19 in allogeneic stem cell transplant: high false‐negative probability and role of CRISPR and convalescent plasma. Bone Marrow Transplant. 2020;55(12):2354‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malard F, Genthon A, Brissot E, et al. COVID‐19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55(11):2180‐2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varma A, Kosuri S, Ustun C, et al. COVID‐19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34(10):2809‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hatzl S, Eisner F, Schilcher G, et al. Response to “COVID‐19 in persons with haematological cancers”. Leukemia. 2020;34(8):2265‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah GL, DeWolf S, Lee YJ, et al. Favorable outcomes of COVID‐19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130(12):6656‐6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coll E, Fernández‐Ruiz M, Sánchez‐Álvarez JE, et al. COVID‐19 in transplant recipients: The Spanish experience. Am J Transplant. 2020. 10.1111/ajt.16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altuntas F, Ata N, Yigenoglu TN, et al. COVID‐19 in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2020. 10.1038/s41409-020-01084-x [DOI] [PubMed] [Google Scholar]
- 13. Sultan AM, Mahmoud HK, Fathy GM, Abdelfattah NM. The outcome of hematopoietic stem cell transplantation patients with COVID‐19 infection. Bone Marrow Transplant. 2020. 10.1038/s41409-020-01094-9 [DOI] [PubMed] [Google Scholar]
- 14. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30(4):425‐437. [DOI] [PubMed] [Google Scholar]
- 17. Spitzer TR. Engraftment syndrome: Double‐edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50(4):469‐475. [DOI] [PubMed] [Google Scholar]
- 18. Chang L, Frame D, Braun T, et al. Engraftment syndrome after allogeneic hematopoietic cell transplantation predicts poor outcomes. Biol Blood Marrow Transplant. 2014;20(9):1407‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID‐19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805‐1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baud D, Qi X, Nielsen‐Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis. 2020;20(7):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated.


