Summary
Evidence on the evolution of graft function in kidney transplant recipients recovering from coronavirus disease‐2019 (COVID‐19) is lacking. This multicenter observational study evaluated the short‐term clinical outcomes in recipients with acute kidney injury (AKI) secondary to COVID‐19. Out of 452 recipients following up at five centers, 50 had AKI secondary to COVID‐19. 42 recipients with at least 3‐month follow‐up were included. Median follow‐up was 5.23 months [IQR 4.09–6.99]. Severe COVID‐19 was seen in 21 (50%), and 12 (28.6%) had KDIGO stage 3 AKI. Complete recovery of graft function at 3 months was seen in 17 (40.5%) patients. Worsening of proteinuria was seen in 15 (37.5%) patients, and 4 (9.5%) patients had new onset proteinuria. Graft failure was seen in 6 (14.3%) patients. Kidney biopsy revealed acute tubular injury (9/11 patients), thrombotic microangiopathy (2/11), acute cellular rejection (2/11), and chronic active antibody‐mediated rejection (3/11). Patients with incomplete recovery were likely to have lower eGFR and proteinuria at baseline, historical allograft rejection, higher admission SOFA score, orthostatic hypotension, and KDIGO stage 3 AKI. Baseline proteinuria and the presence of orthostatic hypotension independently predicted incomplete graft recovery. This shows that graft recovery may remain incomplete after AKI secondary to COVID‐19.
Keywords: acute kidney injury, COVID‐19, graft function, kidney transplantation, outcomes, recovery
Introduction
Emerging evidence suggests that kidney transplant recipients are at an increased risk of severe coronavirus disease‐2019 (COVID‐19) [1], with a higher rate of hospital admissions [2], mortality [2, 3], and acute kidney injury [4]. However, data on clinical outcomes of transplant recipients recovering from COVID‐19 are lacking.
The in‐hospital mortality is largely related to the severity of COVID‐19, but various factors affect the graft function in the long term—underlying (pre‐COVID‐19) graft function, hemodynamic or septic insults, and use of nephrotoxic drugs. Also, recipients with COVID‐19 are at higher immunological risk because of reduction in immunosuppression, transfusion of blood products, and virus‐related immunomodulation [5]. In this study, we included a cohort of recipients who had AKI secondary to COVID‐19 with at least 3‐month follow‐up, with an aim to identify the evolution of clinical outcomes after recovery from COVID‐19 and to recognize the predictive factors for adverse clinical outcomes.
Patients and methods
Study design and participants
This cohort study included all adult (>18 years) kidney transplant recipients at five tertiary referral centers in western Maharashtra, who were diagnosed with COVID‐19 between March 22, 2020, and September 22, 2020, (6 months) and had acute kidney injury during their course of admission. Patients with a presumed and suspected diagnosis of COVID‐19, those with an estimated glomerular filtration rate (eGFR) <15 ml/min/1.73 m2 before admission, and who did not complete at least 3 months of follow‐up were excluded. The study was approved by the Institutional ethics committee (EC/OA‐112/2020) with a waiver of consent. It was registered with Clinical Trials Registry – India (CTRI/2020/06/026000). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Data collection
We obtained data on demographics, comorbidities, past medical history, concomitant medication use, clinical presentation, hospital course, laboratory parameters, treatment, and outcomes. All the patients were admitted to the renal high dependency unit managed by nephrologists. After discharge, patients were followed up weekly in the transplant clinic till the graft function stabilized, and monthly thereafter. During these visits, patients underwent thorough physical examination, investigations (complete blood count, serum creatinine, serum electrolytes, liver function tests, urine routine, and urine protein quantification), and outcomes were recorded.
Clinical outcome assessment and definitions
The primary outcome was complete recovery of graft function at 3 months. It was defined as serum creatinine and proteinuria reaching the baseline value. Baseline Serum creatinine and proteinuria were calculated by taking an average of three values before the diagnosis of COVID‐19. Proteinuria more than 300 mg above the baseline was considered as raised above the baseline. The secondary outcomes were patient survival, acute graft rejections, transient worsening of proteinuria (<3 months). Severe COVID‐19 was defined as oxygen saturation (SpO2) ≤94% at room air or acute respiratory distress syndrome [Partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) < 300]. Baseline graft function was assessed by estimated glomerular filtration rate (eGFR) using CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) formula. The presence of proteinuria at baseline was defined as protein excretion of more than 300 mg in a day. Acute kidney injury was classified as per Kidney Diseases Improving Global Outcomes (KDIGO) staging. The Sequential Organ Failure Assessment (SOFA) score was used to the assess severity of organ dysfunctions [6]. Anemia was defined at hemoglobin <12 g/dl, and severe anemia was defined as hemoglobin <9 g/dl. Tacrolimus trough levels more than 7 ng/ml after the first year post‐transplant were considered supratherapeutic.
Statistical analysis
We presented categorical variables as count and percentages. We calculated mean and standard deviation for continuous variables which were normally distributed (Shapiro Test) and median and interquartile range (IQR) for those which were not. For comparison of categorical variables, we have used the Fisher's exact test when observations were <5, and the Pearson’s chi‐square test when they were not. For continuous variables that were normally distributed, we have used t‐test and for not‐normally distributed data, we have used the Mann–Whitney test. Predictors of the primary outcome were calculated using multivariable binary logistic regression. The significance level was fixed at P < 0.05, and all tests were two tailed.
Results
Out of 452 transplant recipients on regular follow‐up, 60 transplant recipients were hospitalized with COVID‐19 from March 22, 2020, to September 22, 2020. Of these, 50 (83.3%) recipients had acute kidney injury, of which eight patients succumbed. The remaining 42 had a follow‐up of a minimum of three months and were included in this analysis (Fig. 1). The median follow‐up was 5.23 months [IQR 4.09–6.99 months]. Thirty‐two (76.2%) were males, and the mean age was 41.33 ± 11.5 years (Table 1). Hypertension was the most common co‐morbid condition (71.4%). The median duration from transplant to the diagnosis of COVID‐19 was 5.37 years (IQR 27–87 months). Baseline eGFR was 57.96 ± 20.21 ml/min/1.73 m2. Twelve (28.6%) patients had received treatment for allograft rejection in the past, these patients had a lower eGFR at baseline (47.90 ± 23.86 ml/min/1.73 m2 vs. 65.34 ± 18.23 ml/min/1.73 m2; P = 0.014). Their tacrolimus trough level before COVID‐19 was not significantly different from those without rejection (5.73 ± 2.26 ng/ml vs. 5.46 ± 1.49 ng/ml; P = 0.731) l/min/1.73 m2. 16 (38.1%) had proteinuria (>300 mg/day) at baseline.
Figure 1.
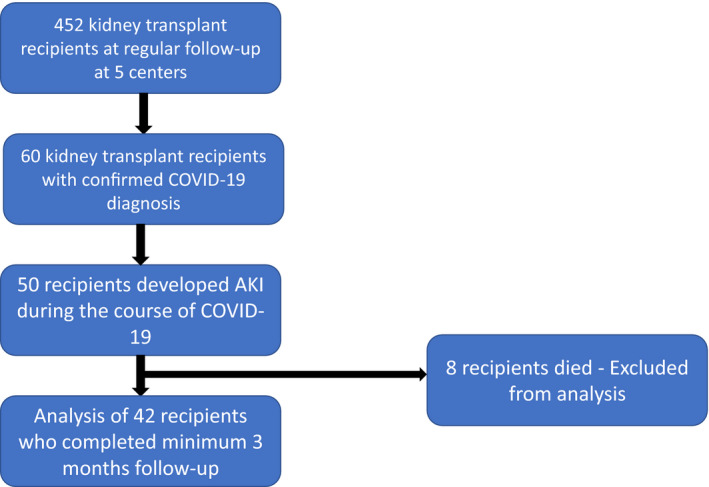
Flowchart of study population selection.
Table 1.
Baseline demographics and medication use.
| Variable | Total (n = 42) | Graft function recovered (n = 17) | Graft function not recovered (n = 25) | P value |
|---|---|---|---|---|
| Age (years) | 41.33 ± 11.5 | 44.25 ± 13.47 | 39.36 ± 9.78 | 0.183 |
| Male | 32 (76.2%) | 13 (76.4%) | 19 (76.0%) | 0.999 |
| Time since transplant to SARS‐CoV‐2 positivity (months) | 49.5 [25.5–87] | 34 [13.5–89] | 59 [38–87] | 0.093 |
| Time from SARS‐CoV‐2 positivity until last follow‐up (months) | 5.23 [4.09–6.99] | 5.62 [4.40–6.57] | 4.86 [3.84–7.13] | – |
| Comorbidities | ||||
| Diabetes mellitus (pre‐ or post‐transplant) | 12 (28.6%) | 6 (35.3%) | 6 (24.5%) | 0.498 |
| Hypertension | 30 (71.4%) | 11 (64.7%) | 19 (76.0%) | 0.498 |
| Heart disease | 1 (2.4%) | 0 | 1 (4%) | 1.0 |
| Lung disease | 5 (11.7%) | 2 (11.8%) | 3 (12%) | 0.982 |
| Tuberculosis (active or historical) | 8 (19%) | 2 (11.8%) | 6 (24%) | 0.439 |
| Type of transplant (LRDT vs. DDT) | 35 (83.3%) | 14 (82.4%) | 21 (84%) | 1 |
| Kidney from marginal donors* | 4 (9.5%) | 1 (5.9%) | 3 (12%) | 0.789 |
| Etiology of kidney disease | ||||
| Diabetes | 1 (2.4%) | 0 | 1 (4%) | |
| Glomerular | 5 (11.9%) | 2 (11.7%) | 3 (12%) | |
| Polycystic kidney disease | 1 (2.4%) | 1 (5.8%) | 0 | – |
| CAKUT | 6 (14.2%) | 1 (5.8%) | 5 (20%) | |
| Unknown | 29 (69%) | 13 (76.4%) | 16 (64%) | |
| BMI (kg/m2) | 25 + 5.7 | 25 + 7.13 | 25 ± 4.40 | 0.971 |
| Medications | ||||
| Antibody induction (anti‐thymocyte globulin/basiliximab/garfalon) | 25 (59.5%) | 13 (76.5%) | 12 (48%) | 0.137 |
| Calcineurin inhibitor | 40 (95.3%) | 17 (100%) | 23 (92%) | 0.507 |
| Mycophenolate mofetil | 33 (78.6%) | 14 (82.5%) | 19 (76%) | 0.713 |
| Azathioprine | 6 (14.3%) | 2 (11.7%) | 4 (16%) | 0.979 |
| mTOR inhibitor | 2 (4.8%) | 1 (5.8%) | 1 (4%) | 1 |
| Steroids | 42 (100%) | 17 (100%) | 25 (100%) | – |
| ACEi/ARB | 7 (16.7%) | 2 (11.8%) | 5 (20%) | 0.685 |
| Tacrolimus trough levels prior to COVID‐19 | 5.54 ± 1.72 | 5.06 ± 1.22 | 5.87 ± 1.95 | 0.178 |
| Baseline eGFR [CKD‐EPI] ml/min/1.73 m2 | 60.36 ± 21.25 | 68.6 ± 21.17 | 54.76 ± 19.79 | 0.037 |
| Baseline proteinuria (>300 mg/day) | 16 (38.1%) | 2 (11.8%) | 14 (56%) | 0.004 |
| Historical allograft rejection | 12 (28.6%) | 1 (5.9%) | 11 (44%) | 0.013 |
CAKUT, congenital anomaly of kidney and urinary tract; CKD‐EPI, chronic kidney disease epidemiology collaboration; DDT, ecease donor transplant; LRDT, Live related donor transplant.
Data not available for marginal donors (n = 5), antibody induction (n = 1).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Clinical presentation and management
Twenty‐one (50%) patients developed severe COVID‐19 (Table 2). The median [IQR] SOFA score on admission was 3 [1–4.25]. Diarrhea as a presenting feature was seen in 23 (54.8%) patients. Orthostatic hypotension was present in 13 (31%) of patients and two developed shock requiring inotropes. Twelve (28.6%) patients developed KDIGO stage 3 AKI and three required hemodialysis. Antimetabolite was withdrawn in 84.6% of patients, and calcineurin inhibitors (CNI) were decreased or stopped in 25/40 patients (62.5%). Twenty (47.6%) patients with severe COVID‐19 received intravenous dexamethasone 6 mg or methylprednisolone 40 mg for a period of 7 or 10 days as per clinical condition, in other patients, the steroid dose was maintained the same. Remdesivir was given to 13 (31%) patients with severe COVID‐19. The dose administered was 600 mg over 5 days for 12 patients, and one patient received 1100 mg over 10 days. On recovery, the steroid was tapered to the original dose, and antimetabolite was reintroduced at reduced doses in the first week after discharge. CNI dosing was guided by trough concentrations.
Table 2.
Clinical presentation, laboratory parameters, and management strategies.
| Variable | Total (n = 42) | Graft function recovered (n = 17) | Graft function not recovered (n = 25) | P value |
|---|---|---|---|---|
| Presence of severe COVID‐19 | 21 (50%) | 8 (47.1%) | 13 (52%) | 0.999 |
| Presence of diarrhea | 23 (54.8%) | 7 (41.2%) | 16 (64%) | 0.209 |
| Hypotension requiring inotropes | 2 (4.8%) | 1 (5.8%) | 1 (4%) | 1 |
| Presence of orthostatic hypotension* | 13 (31%) | 2 (11.8%) | 11 (44%) | 0.041 |
|
SOFA score on admission Median [IQR] |
3 [1–4.25] | 2 [1–3.5] | 3 [2.5–5] | 0.011 |
| Severe anemia | 12 (28.6%) | 2 (11.8%) | 10 (40%) | 0.081 |
| Drop in hemoglobin from baseline (g/dl) | 2.17 ± 1.18 | 1.75 ± 0.95 | 2.45 ± 1.25 | 0.059 |
| Leukopenia (WBC <4000/µl) | 24 (57.1%) | 8 (47.1%) | 16 (64%) | 0.348 |
| Peak serum creatinine (mg/dl) | 2.40 [1.77–3.70] | 1.80 [1.60–2.45] | 2.80 [2.35–5.70] | 0.002 |
| Acute kidney injury | ||||
| KDIGO stage 1 | 24 (57.1%) | 15 (88.2%) | 9 (36%) | 0.004 |
| KDIGO stage 2 | 6 (14.3%) | 0 | 6 (24%) | |
| KDIGO stage 3 | 12 (28.6%) | 2 (11.8%) | 10 (40%) | |
| Raised AST/ALT | 8 (19%) | 4 (23.5%) | 4 (16%) | 0.694 |
| Diffuse (>50%) lung involvement on imaging | 10 (24.4%) | 3 (17.7%) | 7 (29.2%) | 0.480 |
| C‐reactive protein (mg/l) | 38.8 [15.22–68] | 44 [8.9–68] | 33.6 [17.68–64] | 0.756 |
| Lactate dehydrogenase, U/l | 600 [409–855] | 601 [373–778] | 599 [432–1317] | 0.458 |
| D‐dimer (µg/ml)* | 0.89 [0.59–1.98] | 0.87 [0.50–1.67] | 0.89 [0.79–2.23] | 0.745 |
| Serum ferritin (µg/l)* | 931 [317–1201] | 531 [215–4201] | 1002 [931–1202] | 0.116 |
| IL‐6 (pg/ml)* | 29.5 [9.25–166.1] | 97.4 [9.50–292.0] | 19.8 [3.49–41.8] | 0.372 |
| Presence of supratherapeutic tacrolimus trough levels [C 0]* | 10 (23.8%) | 3 (17.6%) | 7 (28%) | 0.490 |
| C 0 level* | 6.69 ± 4.73 | 7.41 ± 6.36 | 6.26 ± 3.56 | 0.516 |
| Management strategies | ||||
| MMF/AZA withdrawal † | 33/39 (84.6%) | 14 (87.5%) | 19 (82.6%) | 1 |
| CNI withdrawal or decrease ‡ | 25/40 (62.5%) | 11 (64.7%) | 14 (60.9%) | 1 |
| Increased steroids | 20 (47.6%) | 9 (52.9%) | 11 (44%) | 0.754 |
| Remdesivir | 13 (31%) | 7 (41.2%) | 6 (24%) | 0.314 |
| Days of oxygen supplementation mean ± SD; Median [IQR] |
3.33 ± 5.12 0 [0–6.25] |
2.76 ± 3.68 0 [0–7.5] |
3.72 ± 5.95 0 [0–6] |
0.999 |
| Hemodialysis | 3 (7.1%) | 0 | 3 (12%) | 0.260 |
AZA, azathioprine; KDIGO, kidney diseases improving global outcomes; MMF, mycophenolate mofetil; NIPPV, noninvasive positive pressure ventilation; SOFA, sequential organ failure assessment.
Data missing for lung involvement (n = 1); D‐dimer (n = 40); C‐reactive protein (n = 12); lactate dehydrogenase (n = 14); serum ferritin (n = 26); interleukin 6 (n = 30); tacrolimus trough levels C 0 (n = 5).
Not on antimetabolite: n = 3; decreased AZA but not stopped for n = 1.
Not on CNI (calcineurin Inhibitor) n = 2.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Clinical outcomes
Complete recovery of graft function at 3 months was seen in 17 (40.5%) patients. Median [IQR] days for AKI to recover was 21 [14.5–39]. Median [IQR] eGFR at 3 months was 49.9 ml/min/1.73 m2 [32.1–62.75 ml/min/1.73 m2]. Worsening of pre‐existing proteinuria (>300 mg above the baseline) was seen in 15 (37.5%) patients with a median [IQR] of 1675 mg/day [957.5–2550 mg/day]. In 3 of these patients, proteinuria recovered to baseline within 3 months. New onset proteinuria was present in 4 (9.5%) patients. As compared to patients with complete recovery those with incomplete recovery had significantly lower eGFR and higher proteinuria at baseline, on admission, at 3‐month follow‐up, and at last follow‐up (Fig. 2, Table 3). There was no significant difference in tacrolimus trough concentrations between these two groups at these time points. Six (14.3%) patients had progressive worsening of graft function and remained on maintenance hemodialysis at 3 months. Of these, three patients had required hemodialysis during COVID‐19 and three patients were initiated on dialysis subsequently on follow‐up visits. One patient having persistent graft dysfunction (eGFR 57.4 ml/min/1.73 m2 at 3 months) developed sudden onset chest discomfort at home and succumbed after 4 months of COVID‐19. The cause of death could not be identified.
Figure 2.
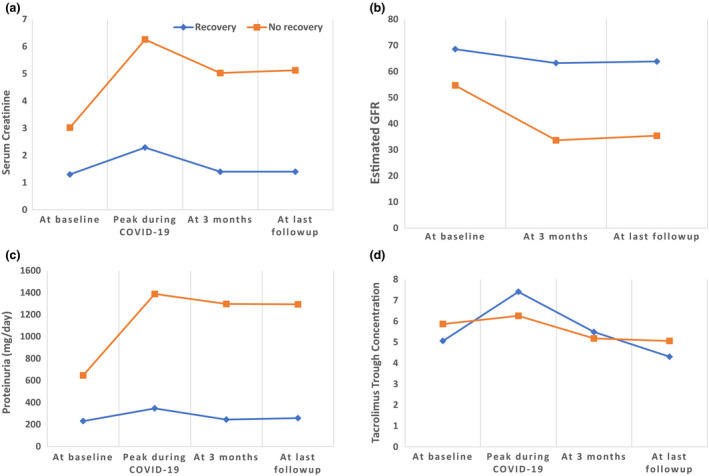
Evolution of graft function (a. serum creatinine, b. estimated GFR, c. proteinuria and d. tacrolimus trough levels) with time before and after COVID‐19 infection.
Table 3.
Evolution of graft function and CNI levels with time in patients with recovery vs. no recovery.
| Variable | Graft function recovered (n = 17) | Graft function not recovered (n = 25) | P value |
|---|---|---|---|
| Serum creatinine (mg/dl) | |||
| At baseline | 1.30 ± 0.38 | 1.72 ± 0.89 | 0.045 |
| Peak during COVID‐19 | 2.29 ± 1.23 | 3.97 ± 2.64 | 0.009 |
| At 3‐month follow‐up | 1.40 ± 0.36 | 3.63 ± 3.24 | 0.008 |
| At last follow‐up | 1.42 ± 0.54 | 3.73 ± 3.25 | 0.002 |
| Estimated GFR CKD‐EPI (ml/min/1.73 m2) | |||
| At baseline | 68.60 ± 21.17 | 54.70 ± 19.79 | 0.037 |
| At 3‐month follow‐up | 63.27 ± 19.7 | 33.67 ± 18.18 | <0.001 |
| At last follow‐up | 63.87 ± 19.90 | 35.42 ± 20.45 | <0.001 |
| Proteinuria(mg/day) | |||
| At baseline | 100 [65–216] | 400 [110–1200] | 0.015 |
| Peak during COVID‐19 | 120 [64–305] | 890 [135–2400] | 0.002 |
| At 3‐month follow‐up | 110 [65–330] | 960 [120–2250] | <0.001 |
| At last follow‐up | 100 [70–405] | 1100 [119.5–2400] | 0.001 |
| Tacrolimus trough concentration (ng/ml) | |||
| At baseline* | 5.06 ± 1.22 | 5.87 ± 1.95 | 0.178 |
| Peak during COVID‐19† | 7.41 ± 6.36 | 6.26 ± 3.56 | 0.516 |
| At 3‐month follow‐up‡ | 5.49 ± 1.82 | 5.18 ± 2.72 | 0.742 |
| At last follow‐up§ | 4.31 ± 2.04 | 5.06 ± 1.82 | 0.509 |
Missing data for * N = 8, † N = 10, ‡ n = 12, § n = 28.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Evaluation and management of graft dysfunction
Pending biopsy report, 5 (12%) patients received injectable methylprednisolone, and oral prednisone dose was increased in 6 (14.3%) patients. Antimetabolite was increased in 7 (16.7%) patients. Ten (23.8%) patients had supratherapeutic tacrolimus trough levels and required a dose reduction. One (2.4%) patient developed de novo donor‐specific antibody (DSAs) (Antibody against HLA‐DQA1*02:01 with background corrected MFI = 14 819). His DSAs were negative one year back when he was evaluated for borderline acute cellular rejection and was treated with anti‐rejection therapy.
Kidney histopathology
Eleven patients underwent kidney biopsy for evaluation of graft dysfunction (Tables S1 and S2). Nine patients had features of acute tubular injury in the form of denudation of tubular cells and loss of brush borders. Three patients had chronic active antibody‐mediated rejection. One patient had a borderline acute cellular rejection with chronic antibody‐mediated rejection which was not present in the previous biopsy done 2.5 years back. One patient had borderline acute cellular rejection. Two patients had biopsy features of acute thrombotic microangiopathy along with acute tubular injury, one of them also had chronic active antibody‐mediated rejection. Both these patients were treated with intravenous immunoglobulin (IVIG) and plasmapheresis, one patient lost the graft and the other patient’s eGFR at 3 months was 18.6 ml/min/1.73 m2. A focal segmental glomerulosclerosis pattern of injury (non‐collapsing) was seen in one patient. All the patients had interstitial fibrosis and tubular atrophy, which was worsened in 4 of them as compared with the previous biopsy. Two patients had features of chronic CNI toxicity. Six patients had mesangial matrix expansion, and 3 of them had de novo mesangial deposition of IgA (+3) and C3 (+).
Risk factors associated with incomplete recovery of kidney function
In univariate analysis, patients with incomplete recovery were more likely to have lower baseline eGFR (P = 0.043), proteinuria at baseline (P = 0.004), and a history of allograft rejection (P = 0.013; Table 1). They had a higher SOFA score at admission (P = 0.011), larger drop in hemoglobin from baseline (P = 0.059), and were more likely to have orthostatic hypotension (P = 0.041) and KDIGO stage 3 AKI (P = 0.004). There was no difference in the groups with respect to the severity of COVID‐19, hepatic dysfunction, inflammatory markers, and treatment strategies including Remdesivir. On multivariable binary logistic regression (Table S3), the presence of proteinuria at baseline (P = 0.009) and orthostatic hypotension during the course of COVID‐19 (P = 0.042) independently predicted incomplete recovery of graft at 3 months.
Discussion
We report short‐term (median 5 months) outcomes of 42 kidney transplant recipients who had AKI because of COVID‐19. In 25 (59.5%) patients, the graft function did not recover to baseline at 3 months. 15 (37.5%) patients had worsening of pre‐existing proteinuria, and 4 (9.5%) patients had new onset proteinuria. Six (14.2%) patients had progressive graft failure and were initiated on maintenance hemodialysis. Graft rejection was seen in 4 (9.5%) patients. Patients with incomplete recovery of graft function were more likely to have lower baseline eGFR, proteinuria at baseline, historical graft rejection, higher SOFA score at admission, orthostatic hypotension, larger drop in hemoglobin from baseline, and KDIGO stage 3 AKI during COVID‐19 admission.
Acute kidney injury in COVID‐19 has been attributed to various factors including direct injury because of SARS‐CoV‐2, hemodynamic insults, cytokine‐related injury, and coagulation dysfunction [7]. Histological lesions described in the native kidneys are acute tubular injury, pigmented tubular casts, focal segmental glomerulosclerosis (collapsing variant), and segmental glomerular fibrin thrombi, and “Viral‐like” particles in the glomerulus and tubules [8]. The kidney allograft is at an additional risk of AKI because of the above COVID‐19‐related factors and transplant‐related factors like graft rejection and CNI toxicity. Histological data from allograft biopsies are lacking. Features of acute tubular injury were seen in all of our patients in varying degrees. One patient had focal segmental glomerulosclerosis but not the collapsing variant. Thrombotic microangiopathy has been reported in native kidneys in COVID‐19 [9] and is linked to the hypercoagulable state. In our cohort, two patients had features of thrombotic microangiopathy but it was found after recovery from COVID‐19 and was also associated with other features of graft rejection (Table 3). Evidence of graft rejection episodes in recipients with COVID‐19 is lacking with only two studies have reported an incidence of 1% [10] and 8% [11] (in recipients within 60 days after transplant). Graft rejection as seen in 9.5% of our patients and the presence of de novo donor‐specific antibodies in one patient can be attributed to the reduction of immunosuppression made during the period of COVID‐19 and also because of immunological dysregulation associated with COVID‐19.
There is evidence suggesting that kidney function may not completely recover after COVID‐19 in the general population. In a cohort of 1655 US veterans with AKI because of COVID‐19, 47% did not recover to baseline by discharge [12]. In the STOP‐COVID study of 637 critically ill adults with COVID‐19 who developed AKI, 34% of those who survived remained dialysis dependent at discharge and 18% remained dialysis dependent 60 days after ICU admission [13]. Similar data of long‐term kidney outcomes of COVID‐19 in transplant recipients are lacking. Our data are in contrast with Paula et al. [14], who reported 3‐month outcome of 26 kidney transplant recipients with AKI during COVID‐19, with all patients recovering to baseline at the end of follow‐up. There were no rejection episodes of graft rejection, and none of the patients developed de novo donor‐specific antibodies. These differences can be attributed higher prevalence of stage 3 AKI in our cohort (12/42 vs. 2/26).
In our study, recovery of kidney function was not associated with baseline comorbidities, use of renin‐angiotensin inhibitors, baseline immunosuppression, the severity of COVID‐19, and presence of diarrhea. More patients treated with remdesivir had complete recovery (41% vs. 24%). Though this was not statistically significant, it points toward the safety of remdesivir in this population as we have previously reported [15]. More patients with incomplete recovery had supratherapeutic tacrolimus trough levels (28% vs. 18%), but this was not significant. The presence of proteinuria at the baseline was an independent predictor of incomplete graft recovery. This highlights the need for close monitoring of graft function in such patients. Similarly, clinical parameters like orthostatic hypotension and a larger drop in hemoglobin from baseline portended poor kidney recovery. These can serve as red flags in transplant recipients with COVID‐19 and warrant prompt treatment and frequent monitoring.
Viral infections like CMV, BKV, and adenovirus have been associated with increased risk of rejection [5, 16, 17, 18]. Though the exact mechanism is not clear, it has been attributed to viral cross‐reactivity with HLA class I leading to T‐cell activation, direct endothelial cell damage, and release of proinflammatory cytokines. Whether similar association is seen with COVID‐19 needs to be confirmed in future studies. Four patients in our cohort required blood transfusion for symptomatic anemia. This can lead to development of de novo donor‐specific antibodies augmenting the graft injury. We found de novo mesangial deposits of IgA and C3 in 3/9 patients which warrant further evaluation in future studies.
Comprehensive evaluation and meticulous follow‐up of 100% of patients are the notable merits of our study. To our knowledge, this is the largest longitudinal cohort reporting the outcome of AKI because of COVID‐19 in transplant recipients. Still, small sample size averts the establishment of the association and makes our study hypothesis generating, the findings of which need to be confirmed in future larger studies.
In conclusion, we found that more than half of patients who develop AKI because of COVID‐19 have incomplete recovery of graft function. They are at risk for rejection, the appearance of de novo DSAs, and graft loss. They constitute a high‐risk group that warrant more frequent follow‐up in the transplant clinic than 2–3 months as suggested by guidelines (KDIGO 2009). They should get serial measurements of eGFR and proteinuria and should have a low threshold for screening for DSAs and for graft biopsy.
Authorship
All the authors contributed toward the study and read and approved final manuscript. DB and TEJ: designed the study and analyzed the data. SD, SB, CG, TM, AK, NS, AP, ST, AEP, AH, VK, SJ and AS: involved in the data collection and performance of the study. DB, TEJ and VK: prepared the manuscript.
Funding
The authors have declared no funding.
Conflict of interests
The authors have declared no conflicts of interests.
Supporting information
Table S1. Graft biopsy positive findings of transplant recipients.
Table S2. Summary of kidney histopathology findings.
Table S3. Multivariable binary logistic regression for predictor of non recovery of graft function.
Acknowledgements
We acknowledge the support from Dr Hemant Deshmukh (Dean, Seth GSMC & KEM H, Mumbai) and Dr M.Y. Nadkar (Head, Department of medicine, Seth GSMC & KEM H) for setting up and maintaining a COVID‐19 high dependency kidney unit free of charge to the patients. We acknowledge the help from Dr Pratap Jadhav in statistical analysis. We acknowledge the help of Mr Kishore Wardhan in data collection for this study.
Clinical trials of India CTRI registration no. – CTRI/2020/06/026000.
References
- 1. Centers for Disease Control and Prevention . Coronavirus Disease 2019 – People of Any Age with Underlying Medical Conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html [Google Scholar]
- 2. Raja MA, Mendoza MA, Villavicencio A, et al. COVID‐19 in solid organ transplant recipients: a systematic review and meta‐analysis of current literature [published online ahead of print, 2020 Nov 14]. Transplant Rev 2021; 35: 100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elias M, Pievani D, Randoux C, et al. COVID‐19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol 2020; 31: 2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molnar MZ, Bhalla A, Azhar A, et al. Outcomes of critically ill solid organ transplant patients with COVID‐19 in the United States. Am J Transplant 2020; 20: 3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanichanan J, Udomkarnjananun S, Avihingsanon Y, Jutivorakool K. Common viral infections in kidney transplant recipients. Kidney Res Clin Pract 2018; 37: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vincent J‐L, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis‐related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26: 1793. [DOI] [PubMed] [Google Scholar]
- 7. Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID‐19 and the kidney: what we think we know so far and what we don't. J Nephrol 2020; 33: 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int 2020; 98: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jhaveri KD, Meir LR, Chang BSF, et al. Thrombotic microangiopathy in a patient with COVID‐19. Kidney Int 2020; 98: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez‐Cubillo B, Higuera MAM, Lucena R, et al. Should cyclosporine be useful in renal transplant recipients affected by SARS‐CoV‐2. Am J Transplant 2020; 20: 3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pascual J, Melilli E, Jiménez‐Martín C, et al. COVID‐19 – related mortality during the first 60 days after kidney transplantation. Eur Urol 2020; 78: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al‐Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID‐19. Clin J Am Soc Nephrol 2020; 16: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta S, Coca SG, Chan L, et al. AKI treated with renal replacement therapy in critically ill patients with COVID‐19. J Am Soc Nephrol 2021; 32: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anton Pampols P, Trujillo H, Melilli E, et al. Immunosuppression minimization in kidney transplant recipients hospitalized for COVID‐19. Clin Kidney J 2021; 14: 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thakare S, Gandhi C, Modi T, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep 2021; 6: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopez C, Simmons RL, Mauer SM, Najarian JS, Good RA, Gentry S. Association of renal allograft rejection with virus infections. Am J Med 1974; 56: 280. [DOI] [PubMed] [Google Scholar]
- 17. Cainelli F, Vento S. Infections and solid organ transplant rejection: a cause‐and‐effect relationship? [published correction appears in Lancet Infect Dis 2002 Oct; 2(10):645]. Lancet Infect Dis 2002; 2: 539. [DOI] [PubMed] [Google Scholar]
- 18. Storsley L, Gibson IW. Adenovirus interstitial nephritis and rejection in an allograft. J Am Soc Nephrol 2011; 22: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Graft biopsy positive findings of transplant recipients.
Table S2. Summary of kidney histopathology findings.
Table S3. Multivariable binary logistic regression for predictor of non recovery of graft function.


