Abstract
Background
There are some data showing that repurposed drugs used for the Coronavirus disease‐19 (COVID‐19) have potential to increase the risk of QTc prolongation and torsade de pointes (TdP), and these arrhythmic side effects have not been adequately addressed in COVID‐19 patients treated with these repurposed medications.
Methods
This is the prospective study of 2403 patients hospitalised at 13 hospitals within the COVID‐19 epicentres of the Iran. These patients were treated with chloroquine, hydroxychloroquine, lopinavir/ritonavir, atazanavir/ritonavir, oseltamivir, favipiravir and remdesivir alone or in combination with azithromycin. The primary outcome of the study was incidence of critical QTc prolongation, and secondary outcomes were incidences of TdP and death.
Results
Of the 2403 patients, 2365 met inclusion criteria. The primary outcome of QTc ≥ 500 ms and ∆QTc ≥ 60 ms was observed in 11.2% and 17.6% of the patients, respectively. The secondary outcomes of TdP and death were reported in 0.38% and 9.8% of the patients, respectively. The risk of critical QT prolongation increased in the presence of female gender, history of heart failure, treatment with hydroxychloroquine, azithromycin combination therapy, simultaneous furosemide or beta‐blocker therapy and acute renal or hepatic dysfunction. However, the risk of TdP was predicted by treatment with lopinavir‐ritonavir, simultaneous amiodarone or furosemide administration and hypokalaemia during treatment.
Conclusion
This cohort showed significant QTc prolongation with all COVID‐19 medications studied, however, life‐threatening arrhythmia of TdP occurred rarely. Among the repurposed drugs studied, hydroxychloroquine or lopinavir‐ritonavir alone or in combination with azithromycin clearly demonstrated to increase the risk of critical QT prolongation and/or TdP.
What’s known
Hydroxychloroquine/chloroquine alone or in combination with azithromycin clearly demonstrated to increase the risk of critical QT prolongation or induction of Torsades de Pointes (TdP).
What’s new
This study clearly showed that other COVID‐19 repurposed medications including lopinavir/ritonavir, atazanavir/ritonavir, oseltamivir, favipiravir, and remdesivir alone or in combination with azithromycin have potential to increase the risk of significant QTc prolongation.
Although all the studied COVID‐19 medications has potential for the significant QT prolongation, life‐threatening arrhythmia of TdP occurred rarely.
Among the repurposed drugs studied, hydroxychloroquine or lopinavir‐ritonavir alone or in combination with azithromycin clearly demonstrated to increase the risk of critical QT prolongation or induction of TdP.
1. INTRODUCTION
As the coronavirus disease 2019 (COVID‐19) global pandemic spreads across the world, the use of off‐label repurposed drugs, such as chloroquine/hydroxychloroquine with and without azithromycin, have gained attraction, appearing in international and domestic therapeutic guidelines. The presumed efficacies of these drugs mainly originated from in vitro investigations 1 , 2 , 3 and small non‐randomised studies. 4 , 5 However, subsequent randomised studies have failed to confirm these findings. 6 , 7 , 8 , 9 Simultaneously, a group of antiviral agents including lopinavir/ritonavir, atazanavir/ritonavir, oseltamivir, favipiravir and remdesivir have been tested in prospective observational and randomised clinical trials in different countries. 10 , 11 , 12
Although these repurposed drugs are generally well‐tolerated in clinical practice, some of these drugs such as chloroquine, hydroxychloroquine, azithromycin and lopinavir/ritonavir have been clearly shown to increase the risk of QT interval prolongation and torsade de pointes (TdP). 13 There are no adequate clinical data on QT prolonging effect and torsadogenic potentials of other drugs such as atazanavir/ritonavir, oseltamivir, favipiravir and remdesivir. This risk of arrhythmic death could be further amplified if multiple medications are used in combination. Although there are some studies evaluating QT prolongation and TdP risk in those treated with chloroquine, hydroxychloroquine with and without azithromycin in COVID‐19 patients, 14 , 15 , 16 the present study evaluated eight repurposed drugs in a large of number of the patients with COVID‐19.
Our country is seriously involved with the covid‐19 outbreak and many patients received these drugs or are going to receive in the future. Therefore, we designed this study to evaluate the risk of QT interval prolongation, TdP and death among the hospitalised COVID‐19 patients treated with chloroquine, hydroxychloroquine, lopinavir/ritonavir, atazanavir/ritonavir, oseltamivir, favipiravir and remdesivir alone or in combination with azithromycin.
2. METHODS
2.1. Study population
In this multicentre national survey, we prospectively collected data on pharmacotherapy of patients with COVID‐19. Between June 2020 and October 2020, a total of consecutive 2403 patients were enrolled from 13 hospitals within the COVID‐19 epicentres of the Iran. For the purpose of this study, we included all patients who are 18 years of age and older, have positive polymerase chain reaction (PCR) for SARS‐CoV‐2 in nasopharyngeal swab and/or typical chest‐CT findings and treated with chloroquine, hydroxychloroquine, lopinavir/ritonavir, atazanavir/ritonavir, tocilizumab, oseltamivir, favipiravir and remdesivir as monotherapy or in combination with azithromycin. In addition, patients should have an interpretable baseline ECG and at least one ECG recorded on second day of therapy or later. Baseline ECGs otherwise unsuitable for accurate QT interval measurement were excluded. Patients without 12‐lead ECG or rhythm strip recordings on day 2 of drug therapy or later were also excluded. This study was approved by the Research Ethics Committee of National Institute for Medical Research Development (Approval ID: IR.NIMAD.REC.1399.055) and all patients gave written informed consent for participation in the study.
2.2. Data collection
Data were collected by patient interview and review of medical records. Collected data were entered into web‐based electronic database (Regitory, Tehran, Iran: https://regitory5.rhc.ac.ir). All information was kept confidential and password protected. We gathered all data related to patient demographics, associated comorbidities (ie heart disease, heart failure, renal failure, liver disease), laboratory data (ie electrolyte levels, renal function test and liver function tests), drug history (including COVID‐19 drugs and other QT prolonging drugs), electrocardiographic findings at baseline ECG and after drug intake, and all important events during admission or follow‐up (TdP, sudden death and mortality).
2.3. Drug therapy protocols
The decision to treat with above‐mentioned drugs was based on the clinical decision of the treating physician and national guidelines. The treatment regimens were as follows: (1) Chloroquine 500 mg by mouth twice daily for 1 day followed by 250 mg by mouth twice daily for 5‐7 days; (2) Hydroxychloroquine 400 mg by mouth twice daily for 1 day followed by 200 mg by mouth twice daily for 5‐7 days; (3) Azithromycin 500 mg by mouth daily for 1 day and followed by 250 mg daily for 5 days; (4) Lopinavir/ritonavir 200/50 mg twice daily for 5 days; (5) Atazanavir/ritonavir 300/100 mg daily for 5 days; (6) Oseltamivir 75 mg twice daily for 5 days; (7) Favipiravir 1600 mg twice daily for 1 day and then 600‐800 mg twice daily for 5 days; (8) Remdesivir 200 mg daily for first day and then 100 mg daily for 5‐7 days.
All decisions on patient care were taken by the hospital clinicians, and no attempt was made by research team to influence their decisions. Furthermore, decisions regarding situations such as critical QT prolongation and or TdP were at the sole discretion of the physicians responsible for patient care.
2.4. QT measurements
A baseline QTc was measured from a 12‐lead ECG before treatment. If no baseline ECGs were available, ECGs recorded within 30 days of drug initiation were used for the baseline measures. On‐treatment QT measurements were done using 12‐lead ECGs or single‐lead rhythm strip of lead II.
QT measurements were independently reviewed and validated by 13 expert cardiologists who were blinded to other patient data. The QT intervals were calculated manually from either lead II or V5 using the tangent method 17 and QT corrections were done using Bazett's formula. For patients with intraventricular conduction delays (paced rhythms or bundle branch block), a modified QTc was calculated using the formula: modified QTc = (QT‐(QRS‐120))/√RR. 18 Using ECGs recorded before and during treatment, we also assessed the change from baseline in QTc (∆QTc).
2.5. Study outcomes
The primary outcome of the study was incidence of critical QTc prolongation, defined as maximum on‐therapy QTc ≥ 500 ms (if QRS < 120 ms) or QTc ≥ 550 ms (if QRS ≥ 120 ms) and ∆QTc of ≥60 ms Secondary outcomes were incidences of documented TdP and all‐cause mortality. Cause of death was adjudicated by review of the resuscitation records from all patients with attempted resuscitation, and the reviewers of these data were blinded to the QTc data. TdP should be clearly documented by a single‐lead ECG tracing.
2.6. Statistical analysis
Fitness of interval variables with normal distribution was assessed by one‐sample Kolmogorov‐Smirnov test. Data are presented as mean ± SD for continuous and frequency (percentage) for categorical variables. Comparisons of characteristics were made using Pearson's chi‐square or Fisher's exact test for categorical variables and unpaired Student t test for continuous variables. The ECG characteristics before versus during drug therapy were compared using paired t test. Independent predictors for prolonged QTc were identified by logistic regression models. P value < .05 was considered as statistically significant. Statistical analyses were performed using IBM SPSS Statistics 22 for Windows (IBM Corp, Armonk, NY).
3. RESULTS
3.1. Patient characteristics
Clinical characteristics of the study population were presented in Table 1. Of the 2403 patients initially enrolled in the study, 2365 met inclusion criteria and 38 were excluded because of non‐interpretable baseline ECG or incomplete clinical data. Of 2365 patients, 1311 (54.6%) were men and the mean age was 59.6 ± 16.4 years (range, 18‐99 years). The most common comorbidities were hypertension (35.8%), diabetes mellitus (31%), non‐ischemic structural heart disease (14.9%) and coronary artery disease (12.9%).
TABLE 1.
Baseline clinical characteristics
| Characteristic (n = 2365) | Value |
|---|---|
| Age (y) | 59.6 ± 16.4 |
| Male gender | 1303 (54.6) |
| Nasopharyngeal PCR test | 2059 (86) |
| Positive PCR result | 1586 (77) |
| Lung CT scan | 778 (33) |
| COVID‐19 pneumonia in lung CT scan | 766 (98.5) |
| Coronary artery disease | 308 (13) |
| Non‐ischemic structural heart disease | 355 (15) |
| Heart failure | 186 (7.8) |
| Chronic kidney disease | 174 (7.3) |
| Chronic hepatic disease | 16 (0.7) |
| Hypertension | 855 (35.8) |
| Diabetes | 742 (31) |
| Creatinine level (mg/dL) | 1.4 ± 1.0 |
| Potassium level (mEq/L) | 4.3 ± 2.0 |
| Magnesium level (mEq/L) | 2.1 ± 0.74 |
| Aspartate Aminotransferase level (µ/L) | 52 ± 90 |
| Alanine Aminotransferase level (µ/L) | 41 ± 55 |
| Bilirubin level | 1.3 ± 1.4 |
Continuous variables were presented as mean ± SD. Categorical variables are presented as n (%).
Abbreviations: COVID‐19, coronavirus disease 2019; CT, computed tomography; PCR, polymerase chain reaction.
Hydroxychloroquine was prescribed to treat COVID‐19 infection in 1430 (60.5%) of the patients. A minority of the patients (n = 9, 0.4%) received chloroquine. Azithromycin was added to 1080 (75.5%) patients in hydroxychloroquine group and 3 (33%) in chloroquine group. Lopinavir/ritonavir and atazanavir/ritonavir were administered to 689 (29%) and 16 (0.7%) of the patients, respectively. Azithromycin was added to 206 (30%) patients in lopinavir/ritonavir group and 4 (25%) in atazanavir/ritonavir group. One‐hundred twenty‐one patients (5%) were treated with oseltamivir, including 103 monotherapy and 18 combination therapy with azithromycin. Other antiviral agents favipiravir and remdesivir were also employed in 33 (1.4%) and 67 (2.8%) patients, respectively.
3.2. Electrocardiographic characteristics
A 12‐lead ECG was obtained from all patients before treatment. ECG characteristics were summarised in Table 2. The mean baseline QTc interval was 399.5 ± 42.5 ms and 48 patients (2.0%) had a baseline QTc ≥ 500 ms On‐treatment measurements showed significant increase in QTc interval (432.5 ± 53.8 ms, P < .001). All COVID‐19 drug regimens were associated with significant increase in on‐treatment QTc (all P < .05). Maximal increases in on‐treatment QTc were observed following combination of azithromycin with either chloroquine, lopinavir/ritonavir or hydroxychloroquine (Table 3). Compared with monotherapy, combination therapy led to significantly more increase in on‐treatment QTc (∆QTc: 26.4 ms vs 37.6 ms, P < .001) and higher number of the patients with QTc ≥ 500 ms (8.0% vs 13.5%, P < .001) and ∆QTc ≥ 60 ms (12% vs 212.6%, P < .001).
TABLE 2.
Electrocardiographic characteristics
| ECG parameter | Baseline measurement | On‐therapy measurement | P value |
|---|---|---|---|
| Heart rate (bpm) | 90.5 ± 28.0 | 90.2 ± 20.0 | .51 |
| PR interval (ms) | 163.0 ± 33.0 | 163.0 ± 39.0 | <.001 |
| QRS width (ms) | 84.5 ± 22.0 | 86.5 ± 25.0 | <.001 |
| QT interval (ms) | 340.5 ± 41.7 | 356.3 ± 52.4 | <.001 |
| QTc interval (ms) | 399.5 ± 42.5 | 432.5 ± 54.0 | <.001 |
Variables were presented as mean ± SD.
TABLE 3.
QT change with different COVID‐19 pharmacotherapy
| COVID drug | Baseline QTc | On‐treatment QTc | ∆QTc | P value |
|---|---|---|---|---|
| Chloroquine (n = 9) | 389.8 ± 33.0 | 447.6 ± 35.0 | +57.8 | .002 |
| Chloroquine monotherapy (n = 6) | 394.0 ± 32.0 | 432.0 ± 31.0 | +38 | .002 |
| Chloroquine + Azithromycin (n = 3) | 381.6 ± 40.0 | 479.3 ± 17.0 | +97.7 | .002 |
| Hydroxychloroquine (n = 1430) | 399.0 ± 45.0 | 436.0 ± 57.0 | +37 | <.001 |
| Hydroxychloroquine monotherapy (n = 350) | 394.4 ± 44.0 | 428.4 ± 59.0 | +34 | <.001 |
| Hydroxychloroquine + Azithromycin (n = 1080) | 400.5 ± 45.0 | 438.9 ± 56.5 | +38.4 | <.001 |
| Lopinavir/ritonavir (n = 689) | 395.5 ± 38.5 | 422.5 ± 49.0 | +27 | <.001 |
| Lopinavir/ritonavir monotherapy (n = 483) | 388 ± 36.8 | 409.3 ± 36.8 | +21.3 | <.001 |
| Lopinavir/ritonavir + Azithromycin (n = 206) | 413.9 ± 36.6 | 453.5 ± 39.3 | +39.6 | <.001 |
| Atazanavir/ritonavir (n = 16) | 427.0 ± 28.5 | 456.5 ± 32.3 | +29.6 | <.001 |
| Atazanavir/ritonavir monotherapy (n = 12) | 423.6 ± 29.7 | 453.5 ± 34.0 | +30 | <.001 |
| Atazanavir/ritonavir + Azithromycin (n = 4) | 437.2 ± 38.2 | 465.7 ± 28.6 | +28.5 | <.001 |
| Oseltamivir (n = 121) | 410.0 ± 38.3 | 435.0 ± 44.3 | +25 | <.001 |
| Oseltamivir monotherapy (n = 18) | 413.2 ± 35.9 | 432.7 ± 31.6 | +19.5 | <.001 |
| Oseltamivir + Azithromycin (n = 103) | 409.5 ± 38.8 | 435.6 ± 46.3 | +26.1 | <.001 |
| Favipiravir (n = 33) | 414.4 ± 22.9 | 433.5 ± 32.0 | +19.0 | <.001 |
| Remdesivir (n = 67) | 418.3 ± 32.0 | 442.7 ± 37.3 | +24.4 | <.001 |
Continuous variables were presented as mean ± SD. Categorical variables are presented as n (%).
3.3. Outcome analysis
Primary and secondary outcome for different drug regimens were summarised in Table 4. After receiving COVDI‐19 medications, QTc ≥ 500 ms and ∆QTc ≥ 60 ms were detected in 11.2% (n = 266) and 17.6% (n = 417) of the patients respectively.
TABLE 4.
Primary and secondary outcomes with different drug regimens
| COVID drug | QTc ≥ 500 ms | ∆QTc ≥ 60 ms | TdP |
|---|---|---|---|
| Chloroquine (n = 9) | 0 | 3 (33.0) | 0 |
| Chloroquine (n = 6) | 0 | 2 (33.0) | 0 |
| Chloroquine + Azithromycin (n = 3) | 0 | 1 (33.0) | 0 |
| Hydroxychloroquine (n = 1430) | 196 (13.7) | 300 (21.0) | 4 (0.3) |
| Hydroxychloroquine monotherapy (n = 350) | 41 (11.7) | 63 (18.0) | 0 |
| Hydroxychloroquine + Azithromycin (n = 1080) | 155 (14.4) | 237 (22.0) | 4 (0.4) |
| Lopinavir/ritonavir (n = 689) | 52 (7.5) | 90 (13.0) | 5 (0.78) |
| Lopinavir/ritonavir (n = 483) | 27 (5.6) | 38 (8.0) | 0 |
| Lopinavir/ritonavir + Azithromycin (n = 206) | 25 (12.0) | 52 (25.0) | 5 (2.4) |
| Atazanavir/ritonavir (n = 16) | 3 (18.7) | 2 (12.5) | 0 |
| Atazanavir/ritonavir (n = 12) | 2 (16.7) | 1 (8.3) | 0 |
| Atazanavir/ritonavir + Azithromycin (n = 4) | 1 (25) | 1 (25) | 0 |
| Oseltamivir (n = 121) | 8 (6.6) | 13 (10.7) | 0 |
| Oseltamivir (n = 18) | 0 | 3 (16.7) | 0 |
| Oseltamivir + Azithromycin (n = 103) | 8 (7.8) | 10 (9.7) | 0 |
| Favipiravir (n = 33) | 1 (3.0) | 3 (9.0) | 0 |
| Remdesivir (n = 67) | 6 (9.0) | 6 (9.0) | 0 |
Variables are presented as n (%).
Compared with those with QTc < 500 ms, the patients with QTc ≥ 500 ms were more likely to have history of heart failure and chronic kidney disease (CKD), more likely to receive hydroxychloroquine, lopinavir‐ritonavir, azithromycin, diuretics, beta‐blockers and calcium antagonists and more likely to develop acute renal or hepatic dysfunction during treatment. However, female gender, history of heart failure, acute hepatic dysfunction, treatment with hydroxychloroquine, azithromycin combination therapy and simultaneous furosemide therapy remained as independent predictors of QTc ≥ 500 ms in multivariate analysis (Table 5).
TABLE 5.
Predictors of QTc ≥ 500 ms
| Risk factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | |
| Female gender | .167 | 0.83 | 0.65‐1.08 | .012 | 0.71 | 0.55‐0.93 |
| Coronary artery disease | .096 | 1.35 | 0.95‐1.90 | |||
| Heart failure | <.001 | 2.90 | 2.00‐4.16 | <.001 | 2.17 | 1.40‐3.30 |
| Chronic kidney disease | .017 | 1.66 | 1.09‐2.50 | |||
| Acute renal dysfunction | <.001 | 1.85 | 1.40‐2.40 | |||
| Acute liver dysfunction | <.001 | 2.20 | 1.64‐2.97 | <.001 | 1.96 | 1.43‐2.70 |
| Hydroxychloroquine | <.001 | 1.96 | 1.47‐2.60 | <.001 | 1.78 | 1.29‐2.44 |
| Lopinavir‐ritonavir | <.001 | 0.56 | 0.40‐0.77 | |||
| Oseltamivir | .098 | 0.54 | 0.26‐1.13 | |||
| Azithromycin coadministration | <.001 | 1.80 | 1.37‐2.40 | .023 | 1.40 | 1.05‐1.95 |
| Furosemide | <.001 | 2.70 | 1.97‐3.70 | .004 | 1.70 | 1.19‐2.47 |
| Hydrochlorothiazide | .013 | 2.68 | 1.19‐6.03 | |||
| Betablocker | <.001 | 2.28 | 1.75‐2.98 | |||
| Calcium blocker | .003 | 1.68 | 1.2‐2.40 | |||
| Digoxin | .096 | 1.85 | 0.88‐3.86 | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
In comparison with those who had ∆QTc < 60 ms, patients with ∆QTc ≥ 60 ms were more likely to have history of CKD, more likely to develop acute renal or hepatic dysfunction during treatment, and more likely to receive hydroxychloroquine, lopinavir‐ritonavir, oseltamivir, azithromycin combination therapy, furosemide and beta‐blockers. In multivariate analysis, simultaneous beta‐blocker therapy and acute renal failure remained as independent predictors for ∆QTc ≥ 60 ms (Table 6).
TABLE 6.
Predictors of ∆QT ≥ 60 ms
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | |
| Heart failure | .078 | 1.39 | 0.96‐1.99 | |||
| Chronic kidney disease | <.001 | 1.84 | 1.29‐2.60 | |||
| Acute renal dysfunction | <.001 | 1.92 | 1.54‐2.40 | <.001 | 2.40 | 1.70‐3.40 |
| Acute hepatic dysfunction | .002 | 1.53 | 1.17‐2.00 | |||
| Hydroxychloroquine | <.001 | 1.85 | 1.47‐2.34 | .086 | 1.50 | 0.94‐2.40 |
| Lopinavir‐ritonavir | <.001 | 0.62 | 0.48‐0.79 | |||
| Azithromycin coadministration | <.001 | 2.02 | 1.60‐2.55 | |||
| Oseltamivir | .041 | 0.55 | 0.30‐0.98 | |||
| Remdesivir | .059 | 0.45 | 0.19‐1.05 | |||
| Furosemide | <.001 | 2.00 | 1.50‐2.66 | |||
| Betablocker | <.001 | 1.60 | 1.27‐2.02 | .023 | 1.50 | 1.05‐2.10 |
Abbreviations: CI, confidence interval; OR, odds ratio.
During the study period, a total of 9 patients (0.38%) experienced TdP and required emergent defibrillation. Of the 9 patients who had TdP, 4 were treated with combination of hydroxychloroquine and azithromycin and 5 with combination of lopinavir/ritonavir and azithromycin. Patients who developed TdP were more likely to have history of non‐ischemic structural heart disease, history of heart failure, history of CKD, history of chronic liver disease, more likely to receive lopinavir‐ritonavir, azithromycin combination therapy, furosemide, metolazone, amiodarone and other QT prolonging drugs, more likely to have QTc ≥ 500 ms and ∆QTc ≥ 60 ms during treatment, and more likely to develop electrolyte abnormalities (hypokalaemia, hypocalcaemia) and acute renal dysfunction. However, treatment with lopinavir‐ritonavir, simultaneous amiodarone or furosemide administration and hypokalaemia during treatment remained as independent predictors of TdP (Table 7).
TABLE 7.
Predictors of torsade de pointes
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | |
| Non‐ischemic heart disease | .012 | 4.66 | 1.24‐17.40 | |||
| Coronary artery disease | .068 | 3.38 | 0.84‐13.6 | |||
| Heart failure | .004 | 6.00 | 1.50‐24.4 | |||
| Chronic kidney disease | .003 | 6.40 | 1.60‐26.0 | |||
| Chronic liver disease | <.001 | 19.5 | 2.30‐165.8 | |||
| Lopinavir‐ritonavir | .080 | 3.05 | 0.82‐11.40 | .006 | 8.2 | 1.84‐36.0 |
| Azithromycin coadministration | .012 | 0.58 | 0.57‐0.60 | |||
| Furosemide | <.001 | 9.08 | 2.40‐34.0 | .030 | 4.83 | 1.16‐20.0 |
| Metolazone | <.001 | 36.6 | 4.10‐328.4 | .028 | 34.7 | 1.46‐826 |
| QT prolonging drugs | .021 | 4.47 | 1.11‐18.0 | |||
| Amiodarone | <.001 | 31.3 | 7.54‐130.0 | .002 | 14.5 | 2.74‐77.0 |
| Hypokalaemia | <.001 | 8.40 | 2.10‐34.0 | .006 | 8.50 | 1.84‐39.3 |
| Hypocalcaemia | .025 | 4.03 | 1.07‐15.0 | |||
| Acute renal dysfunction | .040 | 3.60 | 0.97‐13.6 | |||
| QTc ≥ 500 ms | .036 | 3.97 | 0.99‐16.0 | |||
| ∆QT ≥ 60 ms | <.001 | 9.46 | 2.36‐38.0 | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
Of the 2365 patients in the whole study cohort, 231 (9.8%) died during the study period. In comparison with those who survived, the patients who died were older, more likely to be men, more likely to have coronary artery disease, heart failure, CKD, diabetes, hypertension and more likely to receive hydroxychloroquine, lopinavir‐ritonavir, combination therapy, furosemide, amiodarone, beta‐blockers and digoxin, and more likely to experience hypokalaemia, acute renal or liver dysfunction, QTc ≥ 500 ms and ∆QTc ≥ 60 ms during treatment. However, age, azithromycin combination therapy, greater amiodarone exposure, furosemide therapy and acute renal dysfunction remained as independent predictors of mortality in multivariate analysis (Table 8). Survival analysis of the eight drug regimens showed clear increase in mortality among the patients who received hydroxychloroquine or lopinavir‐ritonavir with and without azithromycin combination therapy (Figure 1).
TABLE 8.
Predictors of mortality
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | |
| Age | <.001 | 10 | 9.0‐13.0 | <.001 | 1.03 | 1.02‐1.04 |
| Male gender | .014 | 1.40 | 1.07‐1.88 | |||
| Coronary artery disease | <.001 | 2.00 | 1.50‐2.90 | |||
| Heart failure | <.001 | 2.40 | 1.60‐3.56 | |||
| Chronic kidney disease | <.001 | 2.90 | 1.98‐4.30 | |||
| Diabetes mellitus | .012 | 1.40 | 1.08‐1.89 | |||
| Hypertension | .002 | 1.55 | 1.18‐2.04 | |||
| Hydroxychloroquine | .003 | 1.57 | 1.17‐2.10 | |||
| Lopinavir/ritonavir | .008 | 0.65 | 0.47‐0.89 | |||
| Azithromycin coadministration | <.001 | 2.33 | 1.70‐3.20 | <.001 | 2.23 | 1.60‐3.10 |
| Furosemide | <.001 | 5.09 | 3.75‐6.90 | <.001 | 2.60 | 1.84‐3.65 |
| Amiodarone | <.001 | 4.65 | 2.37‐9.15 | .033 | 2.37 | 1.07‐5.27 |
| Beta‐blocker | <.001 | 2.46 | 1.86‐3.25 | |||
| Digoxin | <.001 | 4.88 | 2.64‐9.05 | |||
| Hypokalaemia | .002 | 1.60 | 1.18‐2.20 | |||
| On‐treatment QTc ≥ 500 ms | <.001 | 2.84 | 2.03‐3.97 | |||
| ∆QT ≥ 60 ms | <.001 | 2.40 | 1.78‐3.24 | |||
| Acute renal dysfunction | <.001 | 5.70 | 4.30‐7.58 | <.001 | 3.70 | 2.73‐5.02 |
| Acute hepatic dysfunction | <.001 | 1.77 | 1.27‐2.45 | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
FIGURE 1.
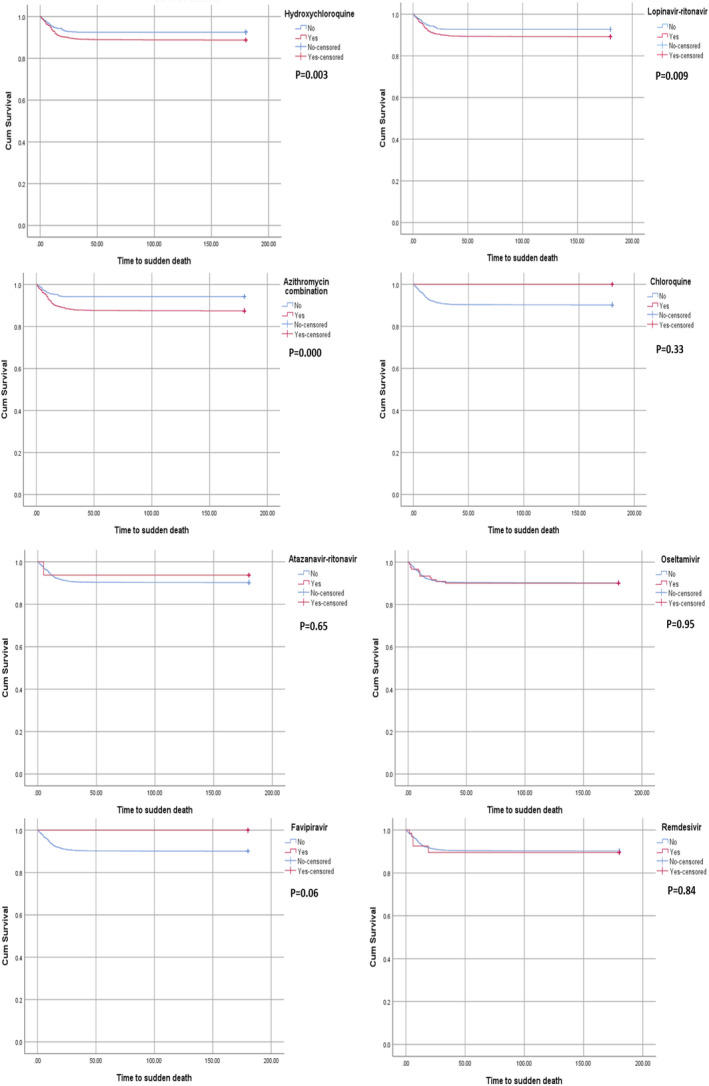
Survival function estimated by the Kaplan‐Meier analysis among the eight different drug regimens used to treat COVID‐19. Log‐rank P‐value was significant among the patients who received hydroxychloroquine or lopinavir‐ritonavir with and without azithromycin combination therapy
4. DISCUSSION
In present cohort of 2365 patients with COVID‐19, primary outcome of critical QT prolongation defined as QTc ≥ 500 ms or ∆QTc ≥ 60 ms was observed in 11.2% and 17.6% of the patients, respectively. There were 9 people (0.38%) of the secondary outcome of TdP in entire cohort.
To the best of our knowledge, this is the largest multicentre study reporting on QT prolongation and arrhythmic complications of treatment with multiple repurposed drugs in patients with COVID‐19. Although there is some information on cardiac safety of chloroquine and hydroxychloroquine with and without azithromycin in patients with COVID‐19, 14 , 15 , 16 our current knowledge on cardiac safety of other repurposed medications for the COVID‐19 treatment is limited. Present cohort included a large dataset on cardiac safety of hydroxychloroquine, lopinavir‐ritonavir and oseltamivir with and without azithromycin. This study, for the first time, systematically investigated the QT prolongation and arrhythmogenicity in patients with COVID‐19 who were treated with atazanavir‐ritonavir, favipiravir and remdesivir. We showed that all eight COVID‐19 medications were associated with some degree of QTc prolongation. Maximal QTc interval prolongation observed in chloroquine combination with azithromycin and favipiravir monotherapy was associated with minimum QT prolongation. Combination therapy with azithromycin led to a significantly greater increase in the QTc interval when compared with monotherapy. Hydroxychloroquine use and combination therapy with azithromycin were predictors for having critical QT prolongation during treatment. All cases of the TdP occurred in combination therapy group. Although critical QT prolongation was associated with higher risk of TdP, only treatment with lopinavir‐ritonavir, simultaneous administration of amiodarone or furosemide and hypokalaemia could predict the occurrence of TdP during therapy. Of the COVID‐19 medications evaluated in this study, azithromycin combination therapy also predicted a higher risk of mortality.
Effect of chloroquine/hydroxychloroquine with and without azithromycin on QTc interval and TdP has been studied in several cohorts of COVID‐19 patients. 14 , 15 , 16 In the present study, 1430 patients who were treated with hydroxychloroquine with or without azithromycin developed QTc ≥ 500 ms in 14%, ∆QTc ≥ 60 ms in 21% and TdP in 0.3% of the patients. Female gender, history of heart failure, acute hepatic dysfunction, treatment with hydroxychloroquine, azithromycin combination therapy and simultaneous furosemide therapy were significant predictors of QTc ≥ 500 ms In largest published study, 415 patients with COVID‐19 from 3 hospitals who were treated with hydroxychloroquine/azithromycin were prospectively included. 15 Critical QTc prolongation ≥500 ms was detected in 21% of patients and no instance of TdP was reported. Age, body mass index <30 kg/m2, heart failure, elevated creatinine and peak troponin >0.04 mg/ml were significant predictors of QTc ≥ 500 ms Second important study reported the retrospective analysis of 251 patients with COVID‐19 from 2 centres who were treated with combination of hydroxychloroquine and azithromycin. 16 On‐treatment QTc ≥ 500 ms had developed in 23% of the patients, ∆QTc ≥ 60 ms in 22% was seen in 20% of the patients, and one patient experienced TdP (0.4%). Baseline QTc interval and simultaneous amiodarone therapy were predictors for QTc ≥ 500 ms and baseline creatinine level and simultaneous amiodarone therapy were predictors of simultaneous amiodarone therapy.
In addition to chloroquine/hydroxychloroquine with and without azithromycin, we studied the effect of five other antiviral repurposed drugs on QT interval prolongation and arrhythmic events. Lopinavir‐ritonavir and atazanavir‐ritonavir, two fixed‐dose combination antiretroviral medications, repurposed for COVID‐19 treatment in the light of some efficacy in the SARS‐CoV and the Middle‐East Respiratory Syndrome Coronavirus (MERS‐CoV). 19 Lopinavir/ritonavir and atazanavir/ritonavir were used 29% and 0.7% of our patients, respectively. Azithromycin was added to 30% of the patients in lopinavir/ritonavir group and 25% in atazanavir/ritonavir group. Treatment with lopinavir‐ritonavir increased the risk of TdP by more than 8 times; five cases of TdP occurred in patients who are receiving lopinavir‐ritonavir and azithromycin combination, however, there was no report of TdP in atazanavir‐ritonavir group. Latter finding may be related to small number (n = 19) of the patients who were treated with atazanavir‐ritonavir.
Oseltamivir, an antiviral to treat influenza, was used in the early days of the COVID‐19 pandemic because there was some evidence that the active site of the spike protein of SARS‐COV2 virus is similar to that of influenza virus neuraminidase, suggesting that neuraminidase inhibitors (eg oseltamivir) may be useful to treat COVID‐19. In the present study, it was used in 5% of the patients mainly as combination therapy with azithromycin. Oseltamivir use significantly increased baseline QT and led to critical QT prolongation as QTc ≥ 500 ms in 6.6% and ∆QTc ≥ 60 ms in 11% of the patients. However, there were no instances of oseltamivir‐related TdP in entire cohort. In a small study, Celik et al 20 critical QTc prolongation (QTc ≥ 500 ms or ∆QTc ≥ 60 ms) was detected in 12% of the population. The use of oseltamivir in combination with hydroxychloroquine and azithromycin was found to cause critical QTc prolongation more than five times. Both studies indicate that we should be more careful when prescribing the combination of oseltamivir with COVI‐19 medications during influenza season.
Favipiravir is an oral drug that was approved for influenza and Ebola‐virus infection. In our study, favipiravir monotherapy was safer than other COVID‐19 mediations in terms of QTc prolongation. Of the 33 patients on favipiravir therapy, there were one patient (3%) of QTc ≥ 500 ms and 3 patients (9%) of ∆QTc ≥ 60 ms, however, no TdP was observed. In a case report, QTc prolongation was observed on the 9th day of treatment in a patient who was using favipiravir for Ebola virus infection. 21 However, small study by Cap et al in COVID‐19 patients showed no significant increase in QTc interval. 22 Together, available data indicate that favipiravir is better tolerated in terms of cardiac arrhythmia in COVID‐19 patients.
Remdesivir originally tested in patients with Ebola, now the FDA approved an Emergency Use Authorisation to allow treatment of COVID‐19 patients hospitalised with severe disease. 11 Currently, there is little data on QT prolonging effect of this drug. In our series of 67 patients on remdesivir, there was significant increase in QTc interval. In addition, we observed 6 cases (9%) of QTc ≥ 500 ms and 6 cases (9%) of ∆QTc ≥ 60 ms, however, no instance of TdP was reported. There is only one case report of critical QT prolongation (555 ms) in COVID‐19 patient following third dose of a 5‐day treatment with remdesivir. 23 It is important to note that this patient was on azithromycin while receiving remdesivir which is well known to prolong the QT interval. It is possible that it contributed to the prolongation of the QT interval in this patient. However, in our study, all patients received remdesivir monotherapy. It appears that remdesivir monotherapy, among the COVID‐19 medications studied, has a low‐risk profile in terms of QT prolongation and TdP induction.
Repurposed drugs used in COVID‐19 treatment cause QT prolongation by blocking the voltage‐gated ion channel that mediates the rapid component of the delayed rectifier potassium current, IKr, resulting in lengthening of the ventricular action potential. 24 A timely early Afterdepolarisation, in the presence of a prolonged QT interval, may result in TdP. This risk increases in the presence of concomitant use of QT prolonging agents, loop diuretic, female gender, elderly people, structural heart disease, heart failure, myocardial infarction, electrolyte disturbances, bradycardia, renal disease and hepatic disease. 25 In addition, concomitant cardiac injury from SARS‐CoV‐2 infection may increase the risk of adverse events from generally safe drugs. 26 We similarly showed that female gender, history of heart failure, treatment with hydroxychloroquine, azithromycin combination therapy, simultaneous furosemide or beta‐blocker therapy, and acute renal or hepatic dysfunction were independent increased risk of the critical QT prolongation, however, the risk of TdP increased with lopinavir‐ritonavir use, amiodarone or loop diuretic coadministration and hypokalaemia during treatment. Drug‐induced bradycardia is the common mechanism for the QT prolongation following amiodarone, beta‐blocker and digoxin use, 25 however, amiodarone has also a known risk of prolonging QTc secondary to action potential prolongation. 27 We also observed that the risk of mortality increased independently by azithromycin combination therapy, simultaneous amiodarone or loop diuretic therapy, and acute renal dysfunction.
Importance of ECG monitoring has been studied in a recent multicentre study in 6476 hospitalised patients with COVID‐19 who were treated with hydroxychloroquine with or without azithromycin. 28 Using a simplified approach to monitoring for QT prolongation and arrhythmia, TdP was observed in 1 (0.015%) patient and 67 (1.03%) patients had hydroxychloroquine with and without azithromycin held or discontinued as a result of excessive QT prolongation.
Practical recommendations for healthcare providers: (1) Considering the limited efficacy and important safety concerns, we think that chloroquine/hydroxychloroquine, fixed‐dose combination antiretroviral medications with and without azithromycin should be avoided in COVID‐19 treatment. (2) Although monotherapy with oseltamivir, favipiravir and remdesivir are better tolerated in terms of the critical QT prolongation and TdP, treatment with these drugs still needs close ECG monitoring for QT prolongation. (3) Optimisation of renal/hepatic functions and electrolyte status is highly recommended. (4) Simultaneous use of antiarrhythmic drugs such as amiodarone, beta‐blockers, etc is discouraged.
4.1. Limitations
Results of the present study should be interpreted in the light of certain limitations: first, patients without a baseline or postmedication ECGs were excluded from the analysis, which can represent a bias. We tried to minimise this bias by the consecutive inclusion of patients meeting study criteria. Second, the present cohort was consisted of hospitalised patients, and the results may not apply to outpatient setting or prophylactic treatments. Third, although a specific dosing schedule was recommended by national COVID‐19 guidelines for all physicians, the decisions when and how to prescribe these drugs were deferred to the prescribing physicians.
5. CONCLUSIONS
Hospitalised COVID‐19 patients treated with chloroquine, hydroxychloroquine, lopinavir/ritonavir, atazanavir/ritonavir, oseltamivir, favipiravir and remdesivir alone or in combination with azithromycin had a significant increase in QTc during drug therapy. Several risk factors identified patients at risk of critical QTc prolongation. Despite this finding, life‐threatening arrhythmia of TdP occurred rarely. Among the repurposed drugs studied, hydroxychloroquine or lopinavir‐ritonavir alone or in combination with azithromycin clearly demonstrated to increase the risk of critical QT prolongation or induction of TdP.
DISCLOSURES
Authors contributing to this project have no conflict to disclose.
Haghjoo M, Golipra R, Kheirkhah J, et al. Effect of COVID‐19 medications on corrected QT interval and induction of torsade de pointes: Results of a multicenter national survey. Int J Clin Pract. 2021;75:e14182. 10.1111/ijcp.14182
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding information
This study was sponsored by Rajaie Cardiovascular Medical and Research Center (grant no. 9962)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71:732‐739. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fintelman‐Rodrigues N, Sacramento CQ, Ribeiro Lima C, et al. Atazanavir, alone or in combination with ritonavir, inhibits SARS‐CoV‐2 replication and proinflammatory cytokine production. Antimicrob Agents Chemother. 2020. Sep 21;64:e00825‐20. 10.1128/AAC.00825-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020. Jul;56:105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagier J‐C, Million M, Gautret P, et al. Outcomes of 3,737 COVID‐19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis. 2020. Jul‐Aug;36:101791. 10.1016/j.tmaid.2020.101791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omrani AS, Pathan SA, Thomas SA, et al. Randomized double‐blinded placebo‐controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non‐severe Covid‐19. EClinicalMedicine. 2020. Dec;29:100645. 10.1016/j.eclinm.2020.100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324:2165‐2176. 10.1001/jama.2020.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID‐19: a randomized clinical trial. JAMA. 2020. Dec 1;324:2165‐2176. 10.1001/jama.2020.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in mild‐to‐moderate Covid‐19. N Engl J Med. 2020. Nov 19;383:2041‐2052. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir‐Ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020. May 7;382:1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—Final report. N Engl J Med. 2020. Nov 5;383:1813‐1826. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Solidarity Trial Consortium . Repurposed antiviral drugs for Covid‐19‐interim WHO Solidarity trial results. N Engl J Med. 2021;384:497‐511. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID‐19 treatment. Circulation. 2020. Jun 16;141:e906‐e907. 10.1161/CIRCULATIONAHA.120.047521 [DOI] [PubMed] [Google Scholar]
- 14. Saleh M, Gabriels J, Chang D, et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS‐CoV‐2 infection. Circ Arrhythm Electrophysiol. 2020. Jun;13:e008662. 10.1161/CIRCEP.120.008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Connell TF, Bradley CJ, Abbas AE, et al. Hydroxychloroquine/Azithromycin therapy and QT prolongation in hospitalized patients with COVID‐19. JACC Clin Electrophysiol. 2021;7:16–25. 10.1016/j.jacep.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID‐19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020. Sep;17:1425‐1433. 10.1016/j.hrthm.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Postema PG, Wilde AA. The measurement of the QT interval. Curr Cardiol Rev. 2014. Aug;10:287‐294. 10.2174/1573403x10666140514103612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel P, Borovskiy Y, Deo R. A novel method for correcting QT interval for QRS duration, predicts all‐cause mortality. J Am Coll Cardiol. 2015;65:A336. [Google Scholar]
- 19. Nukoolkarn V, Lee VS, Malaisree M, Aruksakulwong O, Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS‐CoV 3CL(pro) inhibitors. J Theor Biol. 2008;254:861e7. 10.1016/j.jtbi.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Çelik HG, Keske Ş, Şener Ü, et al. Why we should be more careful using hydroxychloroquine in influenza season during COVID‐19 pandemic? Int J Infect Dis. 2021. Jan;102:389‐391. 10.1016/j.ijid.2020.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chinello P, Petrosillo N, Pittalis S, Biava G, Ippolito G, Nicastri E. QTc interval prolongation during favipiravir therapy in an Ebolavirus‐infected patient. PLOS Negl Trop Dis. 2017;11:e0006034. 10.1371/journal.pntd.0006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Çap M, Bilge Ö, Işık F, et al. The effect of favipiravir on QTc interval in patients hospitalized with coronavirus disease 2019. J Electrocardiol. 2020;63:115‐119. 10.1016/j.jelectrocard.2020.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta AK, Parker BM, Priyadarshi V, Parker J. Cardiac adverse events with remdesivir in COVID‐19 infection. Cureus. 2020;12:e11132. 10.7759/cureus.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michaud V, Dow P, Al Rihani SB, et al. Risk assessment of drug‐induced long QT syndrome for some COVID‐19 repurposed drugs. Clin Transl Sci. 2021. Jan;14(1):20‐28. 10.1111/cts.12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487. 10.1161/CIRCOUTCOMES.113.000152. Epub 2013 May 28. Erratum in: Circ Cardiovasc Qual Outcomes. 2013 Nov;6(6):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of off‐label COVID‐19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. 2020. Apr;9:215‐221. 10.1177/2048872620922784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torres V, Tepper D, Flowers D, et al. QT prolongation and the antiarrhythmic efficacy of amiodarone. J Am Coll Cardiol. 1986. Jan;7:142‐147. 10.1016/s0735-1097(86)80272-8 [DOI] [PubMed] [Google Scholar]
- 28. Saleh M, Gabriels J, Chang D, et al. Safely administering potential QTc prolonging therapy across a large health care system in the COVID‐19 era. Circ Arrhythm Electrophysiol. 2020. Nov;13:e008937. 10.1161/CIRCEP.120.008937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


