Abstract
Despite high levels of CXCR3 ligands in mechanically ventilated COVID‐19 patients, BALF CD8 T cells were not enriched in CXCR3+ cells but rather CCR6+, likely due to high CCL20 levels in BALF, and had very high PD‐1 expression. In mechanically ventilated, but not ward, patients Th‐1 immunity is impaired.
Keywords: COVID‐19, T cells, CCR6, CCL20
In COVID‐19, a dysregulated immune response amplifies tissue and organ damage [1]. T cells, key antiviral effector cells and orchestrators of the immune response, appear strongly affected in patients that develop severe disease, with signs of apoptosis and exhaustion [1], but our knowledge remains limited regarding the impact of COVID‐19 on T cells in the lungs. In a proportion of critically ill patients with COVID‐19, T cells are activated in blood but not in bronchoalveolar lavage fluid (BALF) [2], which may indicate a failure to migrate to the lungs. We hypothesized that T cells from patients hospitalized with severe COVID‐19, particularly those admitted to the intensive care unit (ICU), have a disturbed expression pattern of chemokine receptors that hampers the migration of relevant effector T cells from the blood to the lungs.
To test our hypothesis, we measured chemokine levels and chemokine receptor expression in blood samples from 22 COVID‐19 patients admitted to the ward, and in paired blood and BALF samples of nine COVID‐19 patients mechanically ventilated in the ICU (see Supporting Information for methods and gating strategy). Ward patients had a median age of 62.5 (range 46–93) years, 12 of 22 were male, and samples were taken at a median of 3 (range 1–15) days after admission. ICU patients had a median age of 71 (range 49–74) years, were all male, and samples were taken at a median of 27 (range 20–43) days after hospital admission. Around time of sampling, ward patients had a median Modified Early Warning Score of 2 (range 1–8); ICU patients had a median SOFA of 8 (range 5–11).
Most patients had low absolute T‐cell counts at time of sampling, with no difference between groups (Fig. 1A). In ICU patients, the proportion of CD8+ T cells was higher in BALF compared with blood, while in most patients the percentage of CD4+ T cells was lower in BALF, suggesting a preferential CD8+ T‐cell migration to the lung (Fig. 1B). Mucosa‐associated invariant T (MAIT) cells were recently reported to be decreased in blood during critical COVID‐19, but increased in endotracheal aspirates [3]. Consistently, if sufficient MAIT events could be analyzed in BALF (three out of nine cases), proportions of MAITs in BALF were higher as compared to blood (Fig. 1B).
Figure 1.
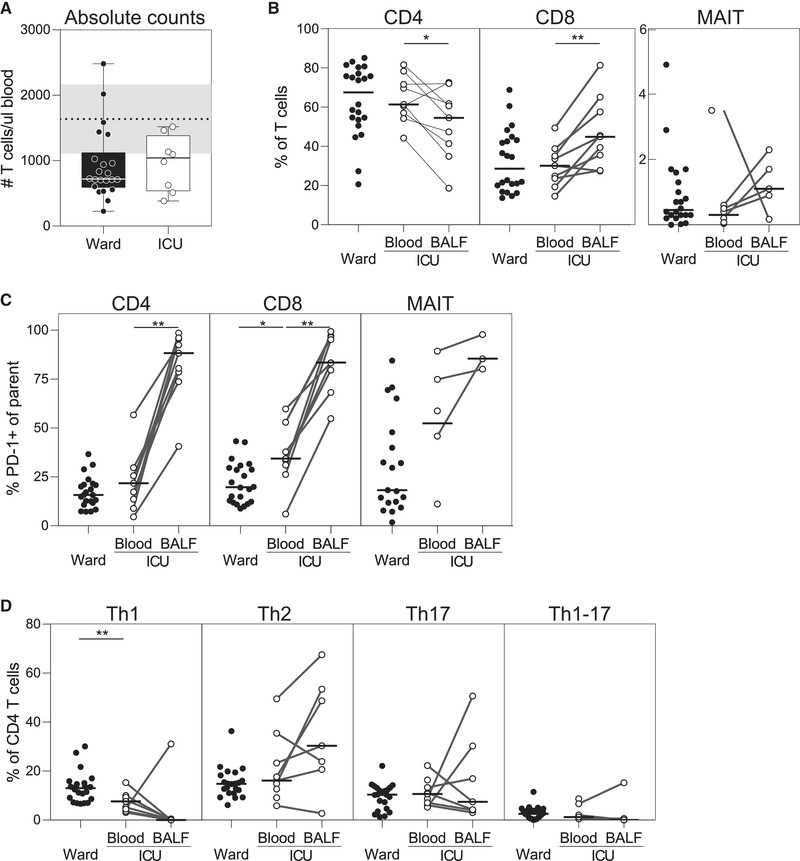
High PD‐1 expression and defective Th‐1 skewing of T cells in mechanically ventilated COVID‐19 patients. Absolute T‐cell counts (median ± interquartile range) were determined in blood of COVID‐19 patients that were admitted to either the ward (n = 22) or the ICU (n = 9), with dotted line representing normal/reference counts (A). Proportions of CD4+, CD8+ and mucosa‐associated invariant T (MAIT) cells (B), PD‐1 expression on these cells (C), as well as T‐helper subsets (D); Th1 cells (CXCR3+CCR4‐CCR6‐), Th2 cells (CXCR3‐CCR4+CCR6‐), Th17 cells (CXCR3‐CCR4+CCR6+), and Th1/Th17 cells (CXCR3+CCR4‐CCR6+) were determined in blood or paired BALF using flow cytometry (gating strategy presented in the Supporting Information). Horizontal lines indicate medians. Differences were tested using Wilcoxon rank‐sum test (blood ward vs. blood ICU) or Wilcoxon signed‐rank test (blood vs. BALF). *p < 0.05, **p < 0.01. Data were collected from consecutive enrolled patients with 1–4 patients per experiment.
Several studies have reported increased PD‐1 expression (programmed death 1) in severe COVID‐19, which may indicate T‐cell exhaustion [1] or reflect recent activation of antigen‐specific polyfunctional T cells [4]. In line with previous reports [3], PD‐1 expression was much higher on T cells in BALF as compared with blood (Fig. 1C).
Little is known about T helper (Th)‐subset distribution in COVID‐19, but ex vivo stimulation of T cells predominantly results in production of Th1‐type cytokines, despite a tendency toward lower expression of CXCR3 and Th1‐related transcription factor T‐bet [5]. Th1 cells are predominantly involved in defense against viruses and key in mounting an adequate cytotoxic CD8+ response. Interestingly, we show a lower proportion of circulating Th1 cells (CXCR3+CCR4‐CCR6‐) in ICU patients when compared with ward patients, and in eight out of nine patients an even lower proportion in BALF (Fig. 1D). The low number of Th1 cells may be caused by apoptosis, migration out of the circulation (e.g., to lymphoid organs or other affected organs), or a failure to mount a Th1 response in ICU patients during the course of the disease.
To uncover which chemokine receptors are involved in T‐cell migration to the lungs during COVID‐19, we analyzed the overall expression patterns of CXCR3, CCR4, and CCR6, and the levels of their respective ligands. CXCR3 and its ligands CXCL9, CXCL10, and CXCL11 are induced by IFN‐driven local inflammation [6]. The proportion of CD8 T cells expressing CXCR3—considered important in the response against viral pathogens [6]—tended to be higher in blood of ICU patients than ward patients, but was significantly reduced in BALF (Fig. 2A), despite higher levels of CXCL11 (Fig. 2B). Although CXCR3 expression can be downregulated due to ligand interaction, our CXCR3+ gate includes cells up to a 75% downregulation (Supporting Information Fig. S1). The apparent failure to recruit CXCR3+ CD8+ T cells to the lungs could result from high peripheral levels of CXCL9 and CXCL10 (Fig. 2B) and may point toward an overactive peripheral IFN response.
Figure 2.
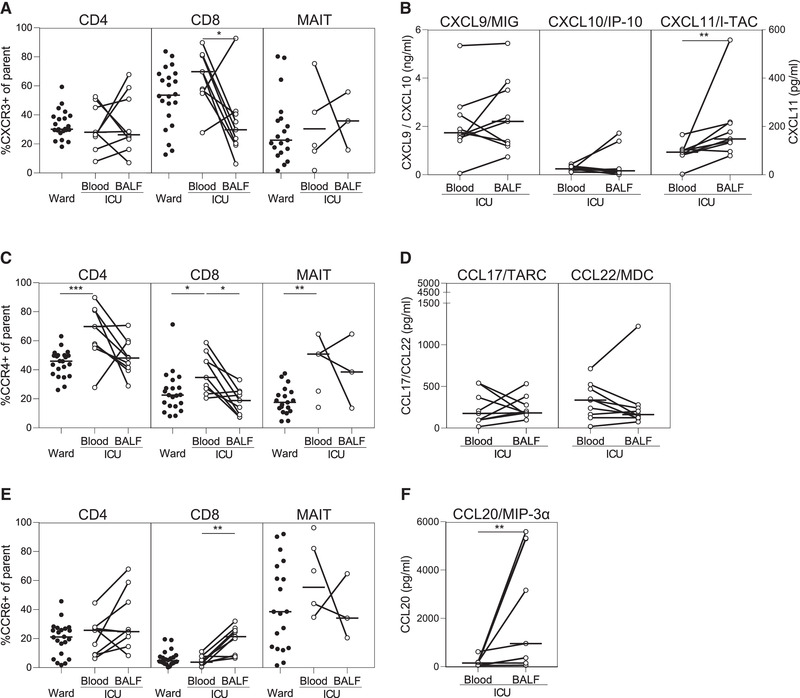
CCR6+ CD8 T cells enriched in BALF of mechanically ventilated COVID‐19 patients. Chemokine receptor expression (CXCR3 (A), CCR4 (C), CCR6 (E)) on T cells, and levels of chemokines that bind these receptors (ligands of CXCR3 (B), ligands of CCR4 (D), ligands of CCR6 (F)) in patients with COVID‐19 admitted to the ward (n = 22) or ICU (n = 9) were determined using flow cytometry or Luminex, respectively. Horizontal lines indicate medians. Differences were tested using Wilcoxon rank‐sum test (blood ward vs. blood ICU) or Wilcoxon‐signed rank test (blood vs. BALF). *p < 0.05, **p < 0.01, ***p < 0.001. Data were collected from consecutive enrolled patients with 1–4 patients per experiment.
When compared with ward patients, CCR4 expression was higher on circulating T cells from ICU patients. CCR4 preferentially drives migration to the skin, and CCR4+ CD8+ T cells have been reported to express neither granzymes nor perforin [7]—both crucial cytotoxic effector molecules—rendering them unlikely to combat SARS‐CoV‐2 effectively. The proportion of CCR4+ T cells was lower in BALF than in blood, while levels of CCL17 and CCL22 in BALF were similar to blood samples, suggesting that CCR4 is not associated with CD4+ or CD8+ T cell migration to the lungs during prolonged mechanical ventilation in patients with COVID‐19 (Fig. 2C and D).
In contrast to CXCR3 and CCR4, the expression of CCR6 on CD8+ T cells was significantly elevated in BALF when compared with blood (Fig. 2E), corroborated by increased levels of CCL20 (Fig. 2F). It was recently suggested that production of CCL20 by SARS‐CoV‐2‐infected alveolar macrophages or inflammatory macrophages drives T‐cell recruitment to the lungs and thereby plays a part in sustained alveolar inflammation [8]. In blood of ward patients, PD‐1 expression was higher on CCR6+ CD8+ T cells when compared with CXCR3+ CD8+ T cells, which suggests increased activation of the former subset in blood (Supporting Information Fig. S2). In contrast, PD‐1 expression was higher on CXCR3+ CD4+ T cells when compared with CCR6+ CD4+ T cells. Functional studies have shown that CCR6+ CD8+ T cells have characteristics of inflammatory effector memory T cells that lack granzyme B expression [9]. Granzyme B, however, is important for both killing of virus‐infected cells and inhibiting viral protein production [10], thus we speculate that CCR6+ CD8+ T cells may be less effective in viral clearance but rather may contribute to detrimental local inflammation.
In conclusion, we describe differential expression patterns of chemokine receptors and their corresponding ligands—between the lungs and blood of mechanically ventilated patients with COVID‐19 in the ICU, and between patients of differing disease severity (ward vs. ICU). Reduced Th1 cells in blood and BALF of mechanically ventilated patients, combined with an enrichment of CCR6+ but not CXCR3+ CD8+ T cells in the lungs of mechanically ventilated patients, suggests a failed or ineffective cytotoxic antiviral response. A limitation of our study is the gender distribution and the difference in time of sampling between ICU and ward patients. A sensitivity analysis including only male patients, however, did not affect any of the reported associations. Furthermore, we could not compare our data to chemokine receptor expression in lungs under noninfectious circumstances. Future functional studies can help elucidating the mechanisms of cellular migration during COVID‐19 and what role ineffective or inappropriate T‐cell responses play in the prolongation of critical illness.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202049046.
Supporting information
Supporting Information
Acknowledgements
We would like to thank all those involved in the ArtDECO and COUNTER‐COVID studies. Furthermore we like to thank Barbara Dierdorp, Tamara Dekker, and René Lutter for their contributions in performing the Luminex assays. AS and TDYR were funded in part by Dutch research council (NWO)(NACTAR, grant number 16447) and JWD was supported by a personal grant of NWO (VENI, grant number 016.186.046).
Data availability statement
Data are available on request from the authors.
References
- 1. Chen, Z. and John Wherry, E. , Nat. Rev. Immunol. 2020. 20: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saris A. et al., Thorax 2021. 10.1136/thoraxjnl-2020-216256. [DOI] [Google Scholar]
- 3. Jouan Y. et al., J. Exp. Med.. 2020. 217: e20200872.32886755 [Google Scholar]
- 4. Rha, M.‐S. et al., Immunity 2020. 54: 44–52.e3.33338412 [Google Scholar]
- 5. De Biasi, S. et al., Nat. Commun. 2020. 11: 1–17.31911652 [Google Scholar]
- 6. Kuo, P. T. et al., Front. Med. 2018. 5: 1–10. [Google Scholar]
- 7. Kondo, T. and Takiguchi, M. , Int. Immunol. 2009. 21: 523–532. [DOI] [PubMed] [Google Scholar]
- 8. Grant, R. A. et al., Nature 2021. 590: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kondo, T. et al., Eur. J. Immunol. 2007. 37: 54–65. [DOI] [PubMed] [Google Scholar]
- 10. Marcet‐Palacios, M. et al., PLoS Pathog 2011. 7: e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data are available on request from the authors.


