Abstract
Introduction
Dementia has been associated with COVID‐19 prevalence, but whether this reflects higher infection, older age of patients, or disease severity remains unclear.
Methods
We investigated a cohort of 12,863 UK Biobank community‐dwelling individuals > 65 years old (1814 individuals ≥ 80 years old) tested for COVID‐19. Individuals were stratified by age to account for age as a confounder. Risk factors were analyzed for COVID‐19–positive diagnosis, hospitalization, and death.
Results
All‐cause dementia, Alzheimer's disease (AD), and Parkinson's disease (PD) were associated with COVID‐19‐positive diagnosis, and all‐cause dementia and AD remained associated in individuals ≥ 80 years old. All‐cause dementia, AD, or PD were not risk factors for overall hospitalization, but increased the risk of hospitalization of COVID‐19 patients. All‐cause dementia and AD increased the risk of COVID‐19–related death, and all‐cause dementia was uniquely associated with increased death in ≥ 80‐year‐old patients.
Discussion
All‐cause dementia and AD are age‐independent risk factors for disease severity and death in COVID‐19.
Keywords: age, all‐cause dementia, Alzheimer's disease, COVID‐19, Parkinson's disease, prospective cohort study, risk factors, UK Biobank
1. INTRODUCTION
Caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the COVID‐19 pandemic has swept the world for more than 1 year, with more than 79 million confirmed cases and more than 1.7 million deaths worldwide as of December 29, 2020. 1 Male sex, African ethnicity, and age, among other sociodemographic and clinical factors, have been established as risk factors for poor COVID‐19 outcomes. 2 , 3 , 4 Older individuals have been found to be particularly vulnerable to COVID‐19: for example, in the UK 90% of COVID‐related deaths have been among people more than 60 years old, and individuals aged 80 or over have been found to be at a 20‐fold higher risk than 50‐ to 59‐year‐olds. 3
Dementia has been identified as a risk factor for COVID‐19, in addition to other chronic comorbidities, including cardiovascular and respiratory diseases, hypertension, diabetes, obesity, and cancer. 3 , 5 However, the causes for this association remain unclear. It has been suggested that increased prevalence of COVID‐19 in individuals with dementia may be, at least in part, accounted for by living in care or nursing homes, by depending on external caregivers, and/or by lack of independence and ability to maintain hygiene/preventative health measures in isolation, 6 , 7 all of which increase the risk of infection. Moreover, age is the major risk factor for development of dementia (notably Alzheimer's disease [AD]), raising the possibility that the association between COVID‐19 and all‐cause dementia or AD might be explained by dementia patients being older on average than patients without dementia. Finally, it is still unclear whether AD and Parkinson's disease (PD), the two most prevalent neurodegenerative disorders in the elderly, are specifically associated with COVID‐19 infection or its outcomes, or whether the association between COVID‐19 and all‐cause dementia results from contributions from other forms of dementia (non‐AD, non‐PD).
To address these questions, we investigated the prevalence of COVID‐19 in a community‐living cohort from the UK Biobank (UKB). An advantage of using data from the UKB is that detailed clinical data are available for infection (COVID‐19 positivity), hospitalization (used as a proxy of disease severity), and deaths, thus allowing independent assessment of risk factors associated with infection and disease development. Between March 16 and August 24, 2020, a total of 12,863 individuals above 65 years old (1814 aged 80 years old and older) were tested for COVID‐19 in our studied cohort, with 1167 positive. This allowed us to examine the associations of several risk factors including all‐cause dementia, and AD and PD in particular, with COVID‐19 positivity, severity (hospitalization), and deaths. To account for age as a risk factor, we stratified our cohort into three groups comprising individuals 66 to 74, 75 to 79, and those aged 80 years old and older. Results indicate that all‐cause dementia, and AD in particular, are associated with higher risk of COVID‐19–positive diagnosis and hospitalization independent of age, and are strong risk factors for severity and death among hospitalized patients even in individuals 80 years old and older.
RESEARCH IN CONTEXT
Systematic review: We searched PubMed for papers (original articles, reviews, editorials, commentaries) published between January 1 and November 30, 2020, using search terms “COVID‐19″ AND [“dementia” OR “Alzheimer” OR “Parkinson”]. Available evidence indicates that patients with dementia or Alzheimer's disease (AD) are more susceptible to COVID‐19. However, whether this reflects higher infection rates, older age, or increased disease severity in such patients is not clear.
Interpretation: Among several other demographic characteristics and comorbidities in a community‐dwelling cohort, only all‐cause dementia and AD remained as risk factors for COVID‐19 diagnosis in individuals ≥ 80 years old. All‐cause dementia was further associated with age‐independent higher risk of COVID‐19–related hospitalization and death, and was the only risk factor for COVID‐19–related death in the oldest individuals (≥ 80 years old).
Future direction: These findings point to all‐cause dementia or AD as targets for stringent preventive measures, higher surveillance, and early intervention in COVID‐19.
HIGHLIGHTS
All‐cause dementia and Alzheimer's disease (AD) are risk factors for COVID‐19 diagnosis in individuals ≥ 80 years old.
All‐cause dementia and AD are age‐independent risk factors for COVID‐19–related hospitalization.
All‐cause dementia is a risk factor for COVID‐19–related death in inpatients ≥ 80 years old.
2. METHODS
2.1. UK Biobank cohort
We accessed the UKB database under application ID 64777. The UKB is a large prospective cohort comprising extensive phenotypic and genotypic data from approximately 500,000 individuals in the UK. 8 International Classification of Diseases (ICD) versions 9 and 10 (ICD 9/10) codes for AD, PD and other forms of dementia (e.g., vascular dementia, Lewy body disease, Pick's disease) were retrieved from the electronic health records of patients, based on prior examination or on evaluation by a research nurse. In some cases, but not all, this may reflect prior imaging or biomarker results. Definitions used here are largely clinical and do not incorporate the recently proposed National Institute on Aging–Alzheimer's Association research framework for biological classification of AD on the basis of objective biomarkers. 9 Participants were recruited from 2006 to 2010, and their current ages range from 49 to 86 years. Detailed information is available on sociodemographic characteristics, lifestyle, and health care of participants. Data from individuals > 65 years of age (n = 297,854) who were tested for COVID‐19 between March 16 and August 24, 2020 (n = 12,863 tested individuals) were retrieved for the current analysis. COVID‐19–positive individuals in the analysis (n = 1167) corresponded to patients with at least one positive test under diagnosis reference code “U07.1″ according to ICD‐10, 10 representing COVID‐19 virus detection confirmed by reverse transcription quantitative polymerase chain reaction. Samples were collected at various times in relation to the course of SARS‐CoV‐2 infection. Out of the 12,863 samples analyzed, 12,274 (95%) were collected from the upper respiratory tract (e.g., nasal or nasal/oropharyngeal swabs). Additional samples included serum (2%) and other types (3%). COVID‐19 tests in the UK from March 16, 2020 onward were initially largely restricted to those with symptoms in hospital, that is, swab testing in Public Health England (PHE) labs and National Health Service (NHS) hospitals for those with a clinical need. As of March 29, 2020, testing capacity was increased to include swab testing for the wider population, which included asymptomatic people. Test samples were recorded in UKB as being from a hospitalized inpatient if marked as originating from an acute (emergency) care provider, an Accident & Emergency department, an inpatient location, or from health care–associated infection; some individuals may not have been hospitalized after being seen at emergency facilities. Tests marked as being from “Healthcare Worker Testing” were not recorded as inpatient samples.
We used data on hospitalization (n = 932 COVID‐19–positive hospitalized patients) as a proxy of disease severity, by consolidating hospitalization information from two UKB registries, namely “covid19_result.txt” and hospital episodes statistics (HES) “hesin.txt.” For hospital inpatient data records, the admission date was March 16, 2020 or later. Deaths registered in UKB with diagnosis “U07.1″ as primary or secondary cause were considered positive cases of death caused by COVID‐19 (n = 397). To control for a possible bias associated with younger individuals experiencing milder (or no) symptoms after infection and never getting tested, we excluded younger individuals (aged 49–65) from the analyses, and only included individuals 66 years old and older tested for COVID‐19. When age stratification was performed, we considered three groups, one aged between 66 and 74 years (n = 6182), another aged between 75 and 79 years (n = 4867), and a third aged between 80 and 86 years (n = 1814).
2.2. Statistical analyses
Statistical analyses were performed using R version 4.0.2. 11 A binary logistic regression was performed in a univariable model using sociodemographic and clinical variables, including sex, age, ethnic group (self‐declared), blood type, body mass index (BMI), high blood pressure, diabetes, asthma, heart attack, cardiovascular disease, chronic obstructive pulmonary disease (COPD), reported cancer event, all‐cause dementia, AD, and PD. In this model, age was initially considered a numerical continuous variable, and subsequently stratified into three groups as described above (and see Results).
Binary logistic regression was also performed using a multivariable model stepwise regression strategy with the stepAIC function of the MASS package from R. 12 In the multivariable stepwise regression, results are only shown for variables that composed the full model with the lowest Akaike information criterion (AIC) score. The AIC allows testing how well a model fits the data set without overfitting it: the model with the lowest AIC score is expected to strike an optimal balance between its ability to fit the data set while at the same time avoiding overfitting. When dementia was analyzed together with AD and PD in a multivariable stepwise regression model, we excluded AD and PD individuals from the “all‐cause dementia” dataset and created an “Other dementia” group with the remaining individuals to have only independent variables in the model.
3. RESULTS
3.1. All‐cause dementia is associated with higher risk of COVID‐19 diagnosis
To investigate the impact of sociodemographic characteristics and clinical comorbidities on the diagnosis of COVID‐19, we analyzed the entire cohort of tested individuals > 65 years old (n = 12,863), comprising 1167 COVID‐19–positive and 11,696 negative individuals. This initial analysis showed that age, male sex, African ethnicity, BMI, high blood pressure, diabetes, angina, stroke, all‐cause dementia, AD, and PD were associated with a higher risk of COVID‐19 diagnosis, while asthma and cancer were inversely associated with risk of COVID‐19 diagnosis (Table 1; Figure 1A). Several of these risk factors remained significant in a multivariable stepwise regression model, notably age (odds ratio [OR] = 1.019, 95% confidence interval [CI] = 1.004–1.034, P = .011), AD (OR = 5.700, CI = 3.709–8.762, P < .001), PD (OR = 2.242, CI = 1.511–3.328, P < .001) and other forms of dementia (OR = 3.412, CI = 2.234–5.213, P < .001; Figure S1, Table S1 in supporting information), indicating that older individuals and those with dementia, notably AD and PD, are at a higher risk of positive diagnosis of COVID‐19.
TABLE 1.
Univariable analysis of risk factors for COVID‐19 positivity
| Variables | COVID‐19– positive a | Negative a | OR [95% CI] | P b | |
|---|---|---|---|---|---|
| Age | 74.86 (4.46) | 74.45 (4.33) | 1.022 [1.008–1.037] | .002 | |
| Sex | Female | 484 (41.47) | 5787 (49.48) | ||
| Male | 683 (58.53) | 5909 (50.52) | 1.382 [1.224–1.562] | <.001 | |
| Ethnicity | White | 1059 (90.75) | 11176 (95.55) | ||
| African | 42 (3.6) | 142 (1.21) | 3.121 [2.175–4.388] | <.001 | |
| Asian | 37 (3.17) | 218 (1.86) | 1.791 [1.238–2.52] | .001 | |
| Others | 29 (2.49) | 160 (1.37) | 1.913 [1.257–2.809] | .002 | |
| Blood type | A | 497 (44.57) | 4986 (44.03) | ||
| AB | 39 (3.5) | 399 (3.52) | 0.981 [0.687–1.362] | .910 | |
| B | 113 (10.13) | 1078 (9.52) | 1.052 [0.845–1.298] | .646 | |
| O | 466 (41.79) | 4861 (42.93) | 0.962 [0.842–1.098] | .564 | |
| BMI | 29.02 (5.24) | 28.28 (5.02) | 1.028 [1.016–1.04] | <.001 | |
| Diabetes | No | 1010 (86.55) | 10518 (89.93) | ||
| Yes | 157 (13.45) | 1178 (10.07) | 1.388 [1.157–1.655] | <.001 | |
| Heart attack | No | 1096 (93.92) | 11106 (94.96) | ||
| Yes | 71 (6.08) | 590 (5.04) | 1.219 [0.939–1.561] | .126 | |
| Angina | No | 1059 (90.75) | 10819 (92.5) | ||
| Yes | 108 (9.25) | 877 (7.5) | 1.258 [1.015–1.545] | .032 | |
| Stroke | No | 1117 (95.72) | 11321 (96.79) | ||
| Yes | 50 (4.28) | 375 (3.21) | 1.351 [0.988–1.809] | .050 | |
| High blood pressure | No | 633 (54.24) | 7003 (59.88) | ||
| Yes | 534 (45.76) | 4693 (40.12) | 1.259 [1.115–1.42] | <.001 | |
| Cancer event | No | 908 (77.81) | 8672 (74.15) | ||
| Yes | 259 (22.19) | 3024 (25.85) | 0.818 [0.707–0.943] | .006 | |
| Pulmonary embolism | No | 1149 (98.46) | 11487 (98.21) | ||
| Yes | 18 (1.54) | 209 (1.79) | 0.861 [0.512–1.358] | .546 | |
| Asthma | No | 1152 (98.71) | 11352 (97.06) | ||
| Yes | 15 (1.29) | 344 (2.94) | 0.43 [0.244–0.697] | .001 | |
| COPD | No | 1160 (99.4) | 11602 (99.2) | ||
| Yes | 7 (0.6) | 94 (0.8) | 0.745 [0.313–1.495] | .453 | |
| All‐cause dementia | No | 1099 (94.17) | 11532 (98.6) | ||
| Yes | 68 (5.83) | 164 (1.4) | 4.351 [3.239–5.783] | <.001 | |
| Alzheimer's disease | No | 1133 (97.09) | 11632 (99.45) | ||
| Yes | 34 (2.91) | 64 (0.55) | 5.454 [3.546–8.245] | <.001 | |
| Parkinson's disease | No | 1132 (97) | 11549 (98.74) | ||
| Yes | 35 (3) | 147 (1.26) | 2.429 [1.647–3.486] | <.001 | |
Results of univariable analysis of risk factors for positive diagnosis of COVID‐19 in tested individuals (n = 12,863 individuals, of whom 1167 were positive for COVID‐19 and 11,696 negative).
Bold P‐values are significant. Table shows absolute numbers of individuals (% in brackets).
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
FIGURE 1.
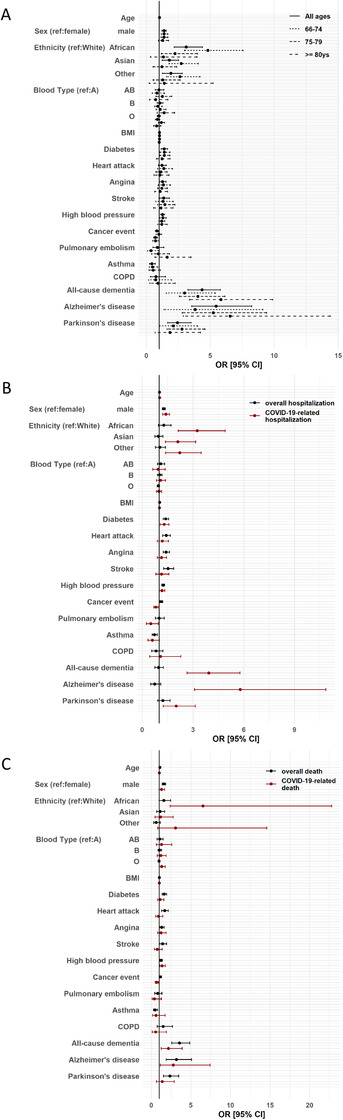
Forest plots showing associations between demographic/clinical variables and COVID‐19–related outcomes. Points represent odds ratio (OR) and horizontal lines represent 95% confidence intervals (CI). A, Risk factors for positive diagnosis of COVID‐19; solid lines are for the analysis using the entire dataset (n = 12,863 individuals), dashed lines represent the groups of individuals 66 to 74 years old (n = 6182), 75 to 79 years old (n = 4867), and ≥ 80 years old (n = 1814), as indicated in the figure. B, Risk factors for COVID‐19–related hospitalization; black symbols/lines correspond to overall hospitalization (n = 12,863) and red corresponds to COVID‐19–related hospitalization (n = 6232). C, Risk factors for COVID‐19–related death; black symbols/lines indicate overall deaths (n = 12,863) and red lines represent COVID‐19–related deaths (n = 976). In all panels, variables with large 95% CIs (> 30) are not included for clarity. BMI, body mass index; COPD, chronic obstructive pulmonary disease
3.2. Age as a confounding factor
Age is the main risk factor for AD, and is also a risk factor for PD. Indeed, we confirmed that all groups of individuals with dementia (all‐cause dementia, AD, and PD) in our cohort were on average older than non‐demented individuals (Figure S2 in supporting information), with AD individuals exhibiting the highest median age (78 years [interquartile range (IQR) = 75–80] for AD vs. 75 years [IQR = 71–78] for controls, P < .001), followed by all‐cause dementia (77 years [IQR = 74–80] vs. 75 years for controls, P < .001) and PD (76 years [IQR = 73–79] for PD vs. 75 years for controls, P < .001). This indicates that, as expected, all‐cause dementia and age are not independent variables in our analysis.
To evaluate possible interactions between the risk factors of our interest and age, we built three different interaction multivariable models using all‐cause dementia, AD, or PD each as independent categorical variables and age as continuous numerical independent variables, and COVID‐19–positive diagnosis as dependent variable (Table S2). A significant interaction was observed between all‐cause dementia and age for COVID‐19–positive diagnosis (OR = 1.16, CI = 1.03–1.22, P = .010). No significant interactions with age were observed for AD (P = .13) and PD (P = .80; Table S2).
To reduce the impact of age as a confounder, we stratified the study cohort by age into three groups with age intervals of 66 to 74, 75 to 79, and 80 to 86 years old. In the univariable model, all‐cause dementia and AD remained associated with a higher risk of positive diagnosis of COVID‐19 in all age groups, while PD was a risk factor only in the two younger age groups (Table 2). It is noteworthy that, among all comorbidities examined, only all‐cause dementia (OR = 5.837, CI = 3.386–9.880, P < .001) and AD (OR = 6.551, CI = 2.892–14.398, P < .001) were associated with higher risk of COVID‐19–positive diagnosis in the elderly group (80 years old and older; Table 2).
TABLE 2.
Univariable analysis of risk factors for COVID‐19 positivity stratified by age group
| 66–74 y (n = 6182) | 75–79 y (n = 4867) | 80–86 y (n = 1814) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | COVID‐19 postive | Negative | OR [95% CI] | P‐value a | COVID‐19 postive | Negative | OR [95% CI] | P‐value a | COVID‐19 postive | Negative | OR [95% CI] | P‐value a | |
| Sex | Female | 217 (43.23) | 2925 (51.5) | 189 (39.87) | 2100 (47.8) | 78 (40.84) | 762 (46.95) | ||||||
| Male | 285 (56.77) | 2755 (48.5) | 1.394 [1.161–1.678] | <.001 | 285 (60.13) | 2293 (52.2) | 1.381 [1.139–1.677] | .001 | 113 (59.16) | 861 (53.05) | 1.282 [0.947–1.743] | .110 | |
| Ethnicity | White | 427 (85.06) | 5382 (94.75) | 446 (94.09) | 4223 (96.13) | 186 (97.38) | 1571 (96.8) | ||||||
| African | 26 (5.18) | 68 (1.2) | 4.819 [2.985–7.558] | <.001 | 13 (2.74) | 55 (1.25) | 2.238 [1.163–3.999] | .010 | 3 (1.57) | 19 (1.17) | 1.334 [0.311–3.96] | .646 | |
| Asian | 29 (5.78) | 134 (2.36) | 2.728 [1.772–4.063] | <.001 | 8 (1.69) | 63 (1.43) | 1.202 [0.529–2.379] | .626 | 0 (0) | 21 (1.29) | 0 [0–5.05e+06] | .978 | |
| Others | 20 (3.98) | 96 (1.69) | 2.626 [1.563–4.201] | <.001 | 7 (1.48) | 52 (1.18) | 1.275 [0.525–2.64] | .550 | 2 (1.05) | 12 (0.74) | 1.408 [0.218–5.214] | .656 | |
| Blood Type | A | 226 (47.88) | 2436 (44.2) | 186 (40.61) | 1879 (44.1) | 85 (45.95) | 671 (43.23) | ||||||
| AB | 14 (2.97) | 182 (3.3) | 0.829 [0.453–1.401] | .512 | 20 (4.37) | 162 (3.8) | 1.247 [0.744–1.987] | .375 | 5 (2.7) | 55 (3.54) | 0.718 [0.245–1.681] | .490 | |
| B | 44 (9.32) | 531 (9.64) | 0.893 [0.631–1.238] | .510 | 43 (9.39) | 399 (9.36) | 1.089 [0.76–1.529] | .633 | 26 (14.05) | 148 (9.54) | 1.387 [0.85–2.2] | .176 | |
| O | 188 (39.83) | 2362 (42.86) | 0.858 [0.701–1.049] | .136 | 209 (45.63) | 1821 (42.74) | 1.159 [0.942–1.428] | .163 | 69 (37.3) | 678 (43.69) | 0.803 [0.573–1.122] | .200 | |
| BMI | 29.25 (5.51) | 28.32 (5.22) | 1.032 [1.015–1.049] | <.001 | 29.12 (5.19) | 28.28 (4.92) | 1.033 [1.014–1.052] | <.001 | 28.2 (4.55) | 28.11 (4.57) | 1.004 [0.971–1.037] | .793 | |
| Diabetes | No | 441 (87.85) | 5171 (91.04) | 406 (85.65) | 3924 (89.32) | 163 (85.34) | 1423 (87.68) | ||||||
| Yes | 61 (12.15) | 509 (8.96) | 1.405 [1.05–1.851] | .018 | 68 (14.35) | 469 (10.68) | 1.401 [1.058–1.832] | .016 | 28 (14.66) | 200 (12.32) | 1.222 [0.783–1.846] | .357 | |
| Heart attack | No | 477 (95.02) | 5469 (96.29) | 444 (93.67) | 4143 (94.31) | 175 (91.62) | 1494 (92.05) | ||||||
| Yes | 25 (4.98) | 211 (3.71) | 1.358 [0.868–2.037] | .158 | 30 (6.33) | 250 (5.69) | 1.12 [0.743–1.629] | .571 | 16 (8.38) | 129 (7.95) | 1.059 [0.594–1.77] | .836 | |
| Angina | No | 466 (92.83) | 5369 (94.52) | 425 (89.66) | 4012 (91.33) | 168 (87.96) | 1438 (88.6) | ||||||
| Yes | 36 (7.17) | 311 (5.48) | 1.334 [0.918–1.882] | .115 | 49 (10.34) | 381 (8.67) | 1.214 [0.878–1.646] | .226 | 23 (12.04) | 185 (11.4) | 1.064 [0.655–1.657] | .792 | |
| Stroke | No | 485 (96.61) | 5531 (97.38) | 452 (95.36) | 4250 (96.74) | 180 (94.24) | 1540 (94.89) | ||||||
| Yes | 17 (3.39) | 149 (2.62) | 1.301 [0.754–2.105] | .312 | 22 (4.64) | 143 (3.26) | 1.447 [0.891–2.241] | .115 | 11 (5.76) | 83 (5.11) | 1.134 [0.562–2.078] | .704 | |
| High blood pressure | No | 294 (58.57) | 3666 (64.54) | 247 (52.11) | 2481 (56.48) | 92 (48.17) | 856 (52.74) | ||||||
| Yes | 208 (41.43) | 2014 (35.46) | 1.288 [1.069–1.55] | .008 | 227 (47.89) | 1912 (43.52) | 1.193 [0.986–1.442] | .069 | 99 (51.83) | 767 (47.26) | 1.201 [0.89–1.623] | .232 | |
| Cancer event | No | 395 (78.69) | 4434 (78.06) | 368 (77.64) | 3118 (70.98) | 145 (75.92) | 1120 (69.01) | ||||||
| Yes | 107 (21.31) | 1246 (21.94) | 0.964 [0.768–1.2] | .747 | 106 (22.36) | 1275 (29.02) | 0.704 [0.56–0.879] | .002 | 46 (24.08) | 503 (30.99) | 0.706 [0.494–0.993] | .050 | |
| Pulmonary embolism | No | 499 (99.4) | 5588 (98.38) | 466 (98.31) | 4313 (98.18) | 184 (96.34) | 1586 (97.72) | ||||||
| Yes | 3 (0.6) | 92 (1.62) | 0.365 [0.089–0.976] | .087 | 8 (1.69) | 80 (1.82) | 0.926 [0.41–1.811] | .836 | 7 (3.66) | 37 (2.28) | 1.631 [0.658–3.494] | .244 | |
| Asthma | No | 493 (98.21) | 5472 (96.34) | 468 (98.73) | 4288 (97.61) | 191 (100) | 1592 (98.09) | ||||||
| Yes | 9 (1.79) | 208 (3.66) | 0.48 [0.227–0.887] | .033 | 6 (1.27) | 105 (2.39) | 0.524 [0.204–1.1] | .126 | 0 (0) | 31 (1.91) | 0 [0–838.588] | .973 | |
| COPD | No | 499 (99.4) | 5633 (99.17) | 470 (99.16) | 4352 (99.07) | 191 (100) | 1617 (99.63) | ||||||
| Yes | 3 (0.6) | 47 (0.83) | 0.721 [0.175–1.974] | .583 | 4 (0.84) | 41 (0.93) | 0.903 [0.271–2.249] | .847 | 0 (0) | 6 (0.37) | 0 [0–4.09e+09] | .972 | |
| All–cause dementia | No | 489 (97.41) | 5630 (99.12) | 443 (93.46) | 4318 (98.29) | 167 (87.43) | 1584 (97.6) | ||||||
| Yes | 13 (2.59) | 50 (0.88) | 2.993 [1.55–5.381] | <.001 | 31 (6.54) | 75 (1.71) | 4.029 [2.587–6.131] | <.001 | 24 (12.57) | 39 (2.4) | 5.837 [3.386–9.88] | <.001 | |
| Alzheimer's disease | No | 496 (98.8) | 5662 (99.68) | 457 (96.41) | 4362 (99.29) | 180 (94.24) | 1608 (99.08) | ||||||
| Yes | 6 (1.2) | 18 (0.32) | 3.805 [1.374–9.121] | .005 | 17 (3.59) | 31 (0.71) | 5.234 [2.815–9.413] | <.001 | 11 (5.76) | 15 (0.92) | 6.551 [2.892–14.398] | <.001 | |
| Parkinson's disease | No | 492 (98.01) | 5626 (99.05) | 455 (95.99) | 4328 (98.52) | 185 (96.86) | 1595 (98.27) | ||||||
| Yes | 10 (1.99) | 54 (0.95) | 2.118 [1.01–4.002] | .031 | 19 (4.01) | 65 (1.48) | 2.78 [1.61–4.584] | <.001 | 6 (3.14) | 28 (1.73) | 1.847 [0.684–4.228] | .179 | |
Bold P‐values are significant. Table shows absolute numbers of individuals (% in brackets).
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
3.3. All‐cause dementia is associated with higher risk of COVID‐19–related hospitalization, but not with overall causes of hospitalization
To identify factors associated with higher risk of hospitalization (a proxy for disease severity), we analyzed the cohort of 12,863 tested individuals according to a univariable model using hospitalization as dependent variable. Within this cohort, 6232 individuals were hospitalized and 6631 were not hospitalized between March 16 and August 24, 2020. This analysis showed that age, male sex, BMI, and chronic disorders, including high blood pressure, diabetes, cancer, and cardiovascular diseases, were associated with higher overall risk of hospitalization (Figure 1B, Table 3). However, no association with overall hospitalization was observed for all‐cause dementia, AD, or PD. Similar results were obtained in a stepwise multivariable analysis (Table S3 in supporting information).
TABLE 3.
Univariable analysis of risk factors for overall hospitalization versus COVID‐19‐related hospitalization
| Total individuals n = 12,863, of whom 6232 hospitalized inpatients and 6631 not hospitalized | Total hospitalized inpatients n = 6232, of whom 932 COVID‐19 inpatients and 5,300 other inpatients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Inpatient | Not in Hospital | OR [95% CI] | P value a | COVID‐19 inpatients | Other inpatients | OR [95% CI] | P value a | |
| Age | 74.64 (4.34) | 74.35 (4.35) | 1.016 [1.008–1.024] | <.001 | 75.07 (4.37) | 74.57 (4.33) | 1.027 [1.01–1.044] | .001 | |
| Sex | Female | 2848 (45.7) | 3423 (51.62) | 362 (38.84) | 2486 (46.91) | ||||
| Male | 3384 (54.3) | 3208 (48.38) | 1.268 [1.183–1.359] | <.001 | 570 (61.16) | 2814 (53.09) | 1.391 [1.207–1.605] | <.001 | |
| Ethnicity | White | 5919 (94.98) | 6316 (95.25) | 841 (90.24) | 5078 (95.81) | ||||
| African | 100 (1.6) | 84 (1.27) | 1.27 [0.949–1.704] | .109 | 35 (3.76) | 65 (1.23) | 3.251 [2.121–4.902] | <.001 | |
| Asian | 120 (1.93) | 135 (2.04) | 0.949 [0.739–1.216] | .677 | 31 (3.33) | 89 (1.68) | 2.103 [1.368–3.149] | <.001 | |
| Others | 93 (1.49) | 96 (1.45) | 1.034 [0.775–1.378] | .821 | 25 (2.68) | 68 (1.28) | 2.22 [1.371–3.483] | <.001 | |
| Blood type | A | 2676 (44.52) | 2807 (43.67) | 397 (44.66) | 2279 (44.49) | ||||
| AB | 223 (3.71) | 215 (3.34) | 1.088 [0.896–1.322] | .396 | 31 (3.49) | 192 (3.75) | 0.927 [0.614–1.354] | .706 | |
| B | 588 (9.78) | 603 (9.38) | 1.023 [0.902–1.159] | .724 | 93 (10.46) | 495 (9.66) | 1.079 [0.84–1.374] | .547 | |
| O | 2524 (41.99) | 2803 (43.61) | 0.945 [0.876–1.019] | .138 | 368 (41.39) | 2156 (42.09) | 0.98 [0.84–1.142] | .795 | |
| BMI | 28.64 (5.19) | 28.06 (4.89) | 1.023 [1.016–1.03] | <.001 | 29.09 (5.36) | 28.57 (5.15) | 1.019 [1.005–1.032] | .005 | |
| Diabetes | No | 5489 (88.08) | 6039 (91.07) | 798 (85.62) | 4691 (88.51) | ||||
| Yes | 743 (11.92) | 592 (8.93) | 1.381 [1.232–1.548] | <.001 | 134 (14.38) | 609 (11.49) | 1.293 [1.054–1.578] | .012 | |
| Heart attack | No | 5858 (94) | 6344 (95.67) | 868 (93.13) | 4990 (94.15) | ||||
| Yes | 374 (6) | 287 (4.33) | 1.411 [1.206–1.653] | <.001 | 64 (6.87) | 310 (5.85) | 1.187 [0.891–1.557] | .228 | |
| Angina | No | 5677 (91.09) | 6201 (93.52) | 841 (90.24) | 4836 (91.25) | ||||
| Yes | 555 (8.91) | 430 (6.48) | 1.41 [1.237–1.608] | <.001 | 91 (9.76) | 464 (8.75) | 1.128 [0.886–1.422] | .319 | |
| Stroke | No | 5983 (96) | 6455 (97.35) | 891 (95.6) | 5092 (96.08) | ||||
| Yes | 249 (4) | 176 (2.65) | 1.526 [1.256–1.859] | <.001 | 41 (4.4) | 208 (3.92) | 1.127 [0.79–1.569] | .495 | |
| High blood pressure | No | 3537 (56.76) | 4099 (61.82) | 500 (53.65) | 3037 (57.3) | ||||
| Yes | 2695 (43.24) | 2532 (38.18) | 1.233 [1.15–1.324] | <.001 | 432 (46.35) | 2263 (42.7) | 1.16 [1.008–1.333] | .038 | |
| Cancer event | No | 4575 (73.41) | 5005 (75.48) | 718 (77.04) | 3857 (72.77) | ||||
| Yes | 1657 (26.59) | 1626 (24.52) | 1.115 [1.03–1.207] | .007 | 214 (22.96) | 1443 (27.23) | 0.797 [0.675–0.937] | .007 | |
| Pulmonary embolism | No | 6122 (98.23) | 6514 (98.24) | 923 (99.03) | 5199 (98.09) | ||||
| Yes | 110 (1.77) | 117 (1.76) | 1 [0.769–1.301] | .998 | 9 (0.97) | 101 (1.91) | 0.502 [0.235–0.941] | .049 | |
| Asthma | No | 6086 (97.66) | 6418 (96.79) | 918 (98.5) | 5168 (97.51) | ||||
| Yes | 146 (2.34) | 213 (3.21) | 0.723 [0.583–0.894] | .003 | 14 (1.5) | 132 (2.49) | 0.597 [0.328–1.003] | .069 | |
| COPD | No | 6188 (99.29) | 6574 (99.14) | 925 (99.25) | 5263 (99.3) | ||||
| Yes | 44 (0.71) | 57 (0.86) | 0.82 [0.55–1.215] | .325 | 7 (0.75) | 37 (0.7) | 1.076 [0.438–2.276] | .859 | |
| All–cause dementia | No | 6122 (98.23) | 6509 (98.16) | 888 (95.28) | 5234 (98.75) | ||||
| Yes | 110 (1.77) | 122 (1.84) | 0.959 [0.738–1.243] | .750 | 44 (4.72) | 66 (1.25) | 3.929 [2.65–5.772] | <.001 | |
| Alzheimer's disease | No | 6192 (99.36) | 6573 (99.13) | 912 (97.85) | 5280 (99.62) | ||||
| Yes | 40 (0.64) | 58 (0.87) | 0.732 [0.486–1.093] | .131 | 20 (2.15) | 20 (0.38) | 5.789 [3.088–10.856] | <.001 | |
| Parkinson's disease | No | 6135 (98.44) | 6546 (98.72) | 907 (97.32) | 5228 (98.64) | ||||
| Yes | 97 (1.56) | 85 (1.28) | 1.218 [0.909–1.635] | .188 | 25 (2.68) | 72 (1.36) | 2.001 [1.24–3.127] | .003 | |
Bold P‐values are significant. Table shows absolute numbers of individuals (% in brackets).
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
In contrast, all‐cause dementia (OR = 3.929, CI = 2.650–5.772, P < .001), AD (OR = 5.789, CI = 3.088–10.856, P < .001), and PD (OR = 2.001, CI = 1.240–3.127, P = .003) were associated with higher risk of COVID‐19–related hospitalization when the 932 hospitalized inpatients with positive COVID‐19 diagnosis were compared to 5300 inpatients without COVID‐19 diagnosis in univariable analysis (Table 3). In stepwise multivariable analysis, AD, PD, and other forms of dementia remained associated with higher risk of hospitalization due to COVID‐19, together with age, male sex, African, Asian, other ethnicities, and BMI (Table S4 in supporting information). No interaction between age and all‐cause dementia, AD, or PD was observed in multivariable interaction models for risk of hospitalization in the COVID‐19–positive cohort.
Univariable analysis of the hospitalized cohort stratified by age into the three age groups described above showed that all‐cause dementia was associated with higher risk of hospitalization of COVID‐19–positive inpatients in all three age groups, while AD was associated with the two older groups and PD was only associated with the intermediate age group (Table S5 in supporting information). Notably, in the oldest group (80 years old and older) the only risk factors associated with higher risk of hospitalization of COVID‐19–positive individuals were all‐cause dementia and AD (Table S5). These findings point to all‐cause dementia as a risk factor for severe outcomes in elderly patients infected with COVID‐19.
3.4. Individuals with all‐cause dementia are at higher risk of COVID‐19–related death independent of age
To determine risk factors associated with COVID‐19–related death, we first performed a univariable analysis using death as dependent variable for the entire cohort of tested individuals (n = 12,863; 976 deaths). As expected, age, male sex, and several clinical variables, including BMI, diabetes, cardiovascular diseases, high blood pressure, all‐cause dementia, AD, and PD, were associated with higher overall risk of death (Figure 1C; Table 4). Interestingly, however, when risk of death specifically due to COVID‐19 was analyzed, several variables, notably age, ceased to be associated, whereas all‐cause dementia (OR = 2.172, CI = 1.231–3.900, P = .008) and AD (OR = 2.766, CI = 1.123–7.420, P = .032) remained associated with higher risk of COVID‐19–related death (Figure 1C; Table 4).
TABLE 4.
Univariable analysis of risk factors for overall death versus COVID‐19‐related death
| Total individuals n = 12,863, of whom 976 dead and 11,887 alive | Total deaths n = 976 individuals, of whom 397 COVID‐19‐related deaths, 579 other death causes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Dead | Alive | OR [95% CI] | P‐value a | COVID‐19 deaths | Other deaths | OR [95% CI] | P‐value a | |
| Age | 75.85 (4.09) | 74.38 (4.35) | 1.085 [1.068–1.103] | <.001 | 76.02 (4.06) | 75.73 (4.12) | 1.018 [0.987–1.05] | .269 | |
| Sex | Female | 375 (38.42) | 5896 (49.6) | 139 (35.01) | 236 (40.76) | ||||
| Male | 601 (61.58) | 5991 (50.4) | 1.577 [1.38–1.804] | <.001 | 258 (64.99) | 343 (59.24) | 1.277 [0.981–1.666] | .070 | |
| Ethnicity | White | 925 (94.77) | 11310 (95.15) | 365 (91.94) | 560 (96.72) | ||||
| African | 21 (2.15) | 163 (1.37) | 1.575 [0.967–2.435] | .052 | 17 (4.28) | 4 (0.69) | 6.521 [2.391–22.799] | <.001 | |
| Asian | 21 (2.15) | 234 (1.97) | 1.097 [0.678–1.681] | .687 | 9 (2.27) | 12 (2.07) | 1.151 [0.465–2.746] | .753 | |
| Others | 9 (0.92) | 180 (1.51) | 0.611 [0.289–1.129] | .152 | 6 (1.51) | 3 (0.52) | 3.068 [0.804–14.612] | .114 | |
| Blood type | A | 418 (44.42) | 5065 (44.05) | 154 (40.63) | 264 (46.98) | ||||
| AB | 35 (3.72) | 403 (3.5) | 1.052 [0.723–1.486] | .781 | 15 (3.96) | 20 (3.56) | 1.286 [0.63–2.574] | .481 | |
| B | 95 (10.1) | 1096 (9.53) | 1.05 [0.829–1.319] | .679 | 39 (10.29) | 56 (9.96) | 1.194 [0.754–1.876] | .445 | |
| O | 393 (41.76) | 4934 (42.91) | 0.965 [0.836–1.114] | .627 | 171 (45.12) | 222 (39.5) | 1.32 [0.997–1.751] | .053 | |
| BMI | 28.99 (5.52) | 28.29 (5) | 1.026 [1.014–1.039] | <.001 | 29.27 (5.5) | 28.8 (5.53) | 1.015 [0.992–1.039] | .196 | |
| Diabetes | No | 827 (84.73) | 10701 (90.02) | 333 (83.88) | 494 (85.32) | ||||
| Yes | 149 (15.27) | 1186 (9.98) | 1.626 [1.347–1.949] | <.001 | 64 (16.12) | 85 (14.68) | 1.117 [0.783–1.587] | .539 | |
| Heart attack | No | 898 (92.01) | 11304 (95.1) | 367 (92.44) | 531 (91.71) | ||||
| Yes | 78 (7.99) | 583 (4.9) | 1.684 [1.308–2.14] | <.001 | 30 (7.56) | 48 (8.29) | 0.904 [0.557–1.446] | .678 | |
| Angina | No | 883 (90.47) | 10995 (92.5) | 355 (89.42) | 528 (91.19) | ||||
| Yes | 93 (9.53) | 892 (7.5) | 1.298 [1.031–1.616] | .023 | 42 (10.58) | 51 (8.81) | 1.225 [0.794–1.881] | .355 | |
| Stroke | No | 932 (95.49) | 11506 (96.79) | 382 (96.22) | 550 (94.99) | ||||
| Yes | 44 (4.51) | 381 (3.21) | 1.426 [1.023–1.939] | .029 | 15 (3.78) | 29 (5.01) | 0.745 [0.384–1.387] | .364 | |
| High blood pressure | No | 536 (54.92) | 7100 (59.73) | 200 (50.38) | 336 (58.03) | ||||
| Yes | 440 (45.08) | 4787 (40.27) | 1.218 [1.067–1.388] | .003 | 197 (49.62) | 243 (41.97) | 1.362 [1.054–1.761] | .018 | |
| Cancer event | No | 707 (72.44) | 8873 (74.64) | 306 (77.08) | 401 (69.26) | ||||
| Yes | 269 (27.56) | 3014 (25.36) | 1.12 [0.966–1.295] | .129 | 91 (22.92) | 178 (30.74) | 0.67 [0.498–0.896] | .007 | |
| Pulmonary embolism | No | 962 (98.57) | 11674 (98.21) | 394 (99.24) | 568 (98.1) | ||||
| Yes | 14 (1.43) | 213 (1.79) | 0.798 [0.442–1.324] | .416 | 3 (0.76) | 11 (1.9) | 0.393 [0.089–1.269] | .154 | |
| Asthma | No | 962 (98.57) | 11542 (97.1) | 393 (98.99) | 569 (98.27) | ||||
| Yes | 14 (1.43) | 345 (2.9) | 0.487 [0.271–0.802] | .009 | 4 (1.01) | 10 (1.73) | 0.579 [0.158–1.745] | .359 | |
| COPD | No | 965 (98.87) | 11797 (99.24) | 394 (99.24) | 571 (98.62) | ||||
| Yes | 11 (1.13) | 90 (0.76) | 1.494 [0.752–2.681] | .211 | 3 (0.76) | 8 (1.38) | 0.543 [0.118–1.892] | .370 | |
| All–cause dementia | No | 925 (94.77) | 11706 (98.48) | 367 (92.44) | 558 (96.37) | ||||
| Yes | 51 (5.23) | 181 (1.52) | 3.566 [2.571–4.861] | <.001 | 30 (7.56) | 21 (3.63) | 2.172 [1.231–3.9] | .008 | |
| Alzheimer's disease | No | 956 (97.95) | 11809 (99.34) | 384 (96.73) | 572 (98.79) | ||||
| Yes | 20 (2.05) | 78 (0.66) | 3.167 [1.879–5.092] | <.001 | 13 (3.27) | 7 (1.21) | 2.766 [1.123–7.42] | .032 | |
| Parkinson's disease | No | 947 (97.03) | 11734 (98.71) | 383 (96.47) | 564 (97.41) | ||||
| Yes | 29 (2.97) | 153 (1.29) | 2.349 [1.541–3.457] | <.001 | 14 (3.53) | 15 (2.59) | 1.374 [0.649–2.894] | .399 | |
Bold P‐values are significant. Table shows absolute numbers of individuals (% in brackets).
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
In addition, in stepwise multivariable analysis, AD (OR = 2.814, CI = 1.095–7.233, P = .032) was associated with higher risk of death of COVID‐19–positive inpatients, together with African ethnicity, while cancer events were associated with lower risk (Table S6 in supporting information). No significant interactions were observed between age and all‐cause dementia, AD, or PD. Analysis in age‐stratified groups showed that all‐cause dementia remained specifically associated with COVID‐19–related death in the oldest group (80 years old and older) in univariable analysis (Table S7 in supporting information). These findings indicate that all‐cause dementia is a specific risk factor for death in COVID‐19 patients, especially in the older (80 years old and older) population.
4. DISCUSSION
With approximately 80 million individuals infected globally, the COVID‐19 pandemic is now in its second wave, with nearly half a million new cases daily worldwide. Although the number of cases in younger individuals appears to be increasing, older people are at higher risk of severe complications and poor outcomes of the disease, and the number of COVID‐19–related deaths is disproportionately high in the elderly. 3 In particular, residents in senior homes and in long‐term care/nursing facilities have been severely impacted by COVID‐19, with high rates of infection and death. 6 , 7
COVID‐19 has been associated with neurological complications, with manifestations ranging from headaches and anosmia to strokes and confusion, 13 and the possibility of long‐term cognitive impairment in COVID‐19 patients has been raised. 14 Conversely, patients with all‐cause dementia have been found to be especially vulnerable to infection by SARS‐CoV‐2 and to development of COVID‐19, 3 , 5 , 15 , 16 , 17 and a recent analysis of electronic health records of 61.9 million patients (aged ≥ 18 years) in the United States found that patients with dementia were at significantly increased risk for COVID‐19. 18 The reasons for this vulnerability are not completely clear. In part, this may be due to living conditions in care/nursing homes, where major outbreaks have occurred, to the need for intensive caregiver assistance, and to the inability to self‐isolate and manage preventative health measures. However, it is possible that inherent clinical characteristics of patients with all‐cause dementia increase their susceptibility to infection and to disease complications. Identification of the relative risks of individuals with all‐cause dementia to infection and to development of severe disease is central to the implementation of public health plans to mitigate the impact of the second wave of COVID‐19 in this vulnerable patient group.
Here, we have separately examined the relative risks of patients with all‐cause dementia to infection by SARS‐CoV‐2 (i.e., positive COVID‐19 diagnosis), to the development of severe health conditions requiring hospitalization, and to COVID‐19–related deaths. Our study used sociodemographic and clinical data from a community‐dwelling cohort of individuals > 65 years old registered in the UKB. Because individuals in this cohort live in the community, this removes the potential bias introduced by investigating patients living in care/nursing facilities. Moreover, availability of detailed information on the clinical history of participants allows for comparative analysis of the association of various variables or comorbidities with disease risk. Noteworthy, under the general umbrella of “all‐cause dementia,” we were further able to assess the risk contributions of AD and PD, the two most prevalent forms of dementia in the elderly.
While our analysis confirmed that several factors, including age, male sex, African ethnicity, BMI, comorbidities, and all‐cause dementia all increased the risk of COVID‐19–positive diagnosis in the total cohort of tested individuals > 65 years old, we found that only all‐cause dementia and, in particular, AD, were associated with higher risk of COVID‐19–positive diagnosis in individuals 80 years old and older. Thus, in the oldest individuals, it appears that aging supersedes the contributions of all other comorbidities except in those individuals with AD or all‐cause dementia. It is possible, nonetheless, that the specific risk of infection in the oldest patients with dementia reflects a more advanced stage of dementia and, thus, a greater requirement of caregivers that may act as a conduit of infection.
All‐cause dementia, and in particular AD and PD, were significantly associated with higher risk of COVID‐19–related hospitalization, but not with overall risk of hospitalization during the study period. In this regard, we note it is possible that patients with dementia but with disease conditions other than COVID‐19 may have refrained from seeking medical attention or may have been refused hospital admission during the pandemic due to the institutional risk of SARS‐CoV‐2 infection (increased indirect mortality during the pandemic). Interestingly, among a number of sociodemographic variables and clinical conditions examined, all‐cause dementia was the only factor that showed an association with higher risk of COVID‐19–related hospitalization in individuals 80 years old and older. This suggests that dementia is a determinant of severity of disease leading to hospitalization of COVID‐19–positive patients, and especially so in the oldest individuals.
Remarkably, whereas several factors were found to be associated with increased overall risk of death in the studied cohort, only few variables, including all‐cause dementia (and AD in particular), were found to be specifically associated with higher risk of COVID‐19–related death. Of note, we further found that all‐cause dementia remained significantly associated with increased death risk in the oldest (80 years old and older) individuals positive for SARS‐CoV‐2.
While BMI was found to be a significant disease risk factor in our cohort of COVID‐19–tested individuals, the odds ratio for this (OR = 1.028, CI = 1.016–1.040; P‐value = < .001) was lower than the risk identified in other studies. 19 , 20 This may reflect our use of BMI as a numerical variable in our analysis, rather than the categorical analyses used in other studies. It is also noteworthy that we did not find significant associations between COVID–19‐related hospitalization and death with certain medical conditions or comorbidities that have been associated with COVID‐19 severity in other studies. These include, for example, pulmonary diseases (asthma, COPD) 21 and cardiovascular diseases, 3 , 22 which have been reported to be significant risk factors for COVID‐19. A possible explanation for this may be that, compared to cohorts investigated in other studies, our cohort comprises older individuals (> 65 years old, with a significant proportion of individuals 80 years old or older). Our analyses indeed suggest that aging supersedes many other risk factors for COVID‐19–related death. Nonetheless, even controlling for age by stratifying our cohort, we still found a significant association between COVID‐19–related death and all‐cause dementia.
Our study has certain limitations. The UKB comprises clinical and sociodemographic variables from a community of registered individuals, and thus does not represent the UK population. 23 We further note that the UKB cohort studied here comprised essentially White individuals (95.1%), with a small proportion of individuals of African (1.4%) or Asian (1.8%) (self‐declared) ethnicities. Moreover, the total number of COVID‐19 cases in the studied cohort is limited, reducing the power of our analysis. We acknowledge that statistical power could have been increased by comparing COVID‐19–positive individuals to the entire cohort of > 500,000 individuals in the UKB. However, we have restricted our analysis to those 12,863 individuals tested for COVID‐19 as a more rigorous approach for determination of statistically robust risk factors. Finally, although individuals in our studied cohort lived in the community, we do not know to what extent individuals required external caregivers, which might increase the chance of exposure to infection.
Our findings reveal that all‐cause dementia, including AD, is strongly associated with higher risk of COVID‐19 severity and death, but they do not highlight the mechanisms underlying this association. One possibility is that the overall frailty of patients with dementia predisposes them to poor disease outcomes. Chronic inflammatory conditions or defective immune responses (immunosenescence) in patients with dementia may increase their vulnerability to infection or reduce their ability to mount effective responses to infection. Of note, an interesting study has shown that SARS‐CoV‐2 may invade the central nervous system (CNS) through the olfactory mucosa and that presence of the virus in the CNS results in a local innate immune/inflammatory response mediated by microglia. 24 The same study found the presence of SARS‐CoV‐2 in the brainstem, which comprises the primary cardiovascular and respiratory control center, raising the possibility that CNS infection may mediate or aggravate respiratory/cardiovascular problems in COVID‐19 patients. Patients with dementia (notably, AD) exhibit a chronic state of hyperactivation of the brain innate immunity 25 and may also present alterations in blood‐brain barrier permeability (another possible pathway of CNS infection by SARS‐CoV‐2), suggesting a possible link between all‐cause dementia and vulnerability in COVID‐19. Finally, because dementia is a known risk factor for delirium in hospitalized older patients, 26 we examined the possibility that an increased prevalence of delirium in COVID‐19 patients with dementia might underlie increased hospitalization or death. However, no association was found between delirium episodes and COVID‐19–positive diagnosis, hospitalization, or deaths in the studied cohort.
In conclusion, our findings highlight the prominent role of all‐cause dementia, and in particular AD, over correlated risk factors for disease severity and death in COVID‐19 patients, providing a target for early intervention. Genome‐wide analyses of individuals in the UKB may reveal some of the molecular players leading to increased risk in patients with all‐cause dementia, as recently reported for the apolipoprotein E ε4 allele. 27 While shielding these patients from infection may prove challenging due to their inherent need of caregivers and difficulty in adhering to strict personal health‐care measures, these results suggest that patients with all‐cause dementia should receive special attention upon hospitalization to prevent evolution of the disease to a potentially irreversible condition.
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
ETHICS APPROVAL
UKB received ethical approval from the North West Multi‐centre Research Ethics Committee (REC reference: 16/NW/0274). All participants gave written informed consent before enrolment in the study, which was conducted in accordance with the principles of the Declaration of Helsinki. Direct dissemination of the results to participants is not possible/applicable. This study was performed under UKB application number 64777.
AUTHOR CONTRIBUTIONS
STF conceived the work; SVA and STF obtained funding; ACT performed the statistical analyses; ACT and SVA verified the underlying data; ACT, SVA, and STF interpreted the results; ACT and STF wrote the original draft; ACT, SVA, and STF revised the manuscript and approved the final version.
DATA SHARING STATEMENT
UKB data used in the present work can be requested by bona fide researchers for approved projects, through https://www.ukbiobank.ac.uk/
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Dr. Ronir Raggio Luiz, Institute for Studies of Collective Health, Federal University of Rio de Janeiro (IESC, UFRJ) for suggestions and for critical appraisal of this work. This work has been supported by grants from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, #210.257/2020, to STF), National Institute for Translational Neuroscience (to STF), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, #2018/23693‐5, to SVA), Fundação Butantan (to SVA), and Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq, #306646/2019‐6 to SVA and #308638/2013‐1 to STF).
Tahira AC, Verjovski‐Almeida S, Ferreira ST. Dementia is an age‐independent risk factor for severity and death in COVID‐19 inpatients. Alzheimer's Dement. 2021;17:1818–1831. 10.1002/alz.12352
Sergio Verjovski‐Almeida and Sergio T. Ferreira contributed equally to this study.
Contributor Information
Sergio Verjovski‐Almeida, Email: verjo@iq.usp.br.
Sergio T. Ferreira, Email: ferreira@bioqmed.ufrj.br.
REFERENCES
- 1. WHO . World Health Organization Situation Reports: WHO; 2020. [Google Scholar]
- 2. Ho FK, Celis‐Morales CA, Gray SR, et al. Modifiable and non‐modifiable risk factors for COVID‐19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10:e040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mok VCT, Pendlebury S, Wong A, et al. Tackling challenges in care of Alzheimer's disease and other dementias amid the COVID‐19 pandemic, now and in the future. Alzheimer's Dement. 2020;16:1571‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in king County, Washington. N Engl J Med. 2020;382:2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisman DN, Bogoch I, Lapointe‐Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with Coronavirus Disease 2019 (COVID‐19) in long‐term care facilities in Ontario, Canada. JAMA Network Open. 2020;3:e2015957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu P, Gifford A, Meng X, et al. Mapping ICD‐10 and ICD‐10‐CM codes to phecodes: workflow development and initial evaluation. JMIR Med Inform. 2019;7:e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. R_Core_Team . The R Project for Statistical Computing. R_Core_Team; 2015. [Google Scholar]
- 12. Venables WN, Ripley BD, Venables WN. Modern Applied Statistics with S. 4th ed.. New York: Springer; 2002. [Google Scholar]
- 13. Iadecola C, Anrather J, Kamel H. Effects of COVID‐19 on the nervous system. Cell. 2020;183:16‐27. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Felice FG, Tovar‐Moll F, Moll J, Munoz DP, Ferreira ST. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the central nervous system. Trends Neurosci. 2020;43:355‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting COVID‐19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou J, Liu C, Sun Y, Huang W, Ye K. Cognitive disorders associated with hospitalization of COVID‐19: results from an observational cohort study. Brain Behav Immun. 2021;91:383‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrison SL, Fazio‐Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID‐19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17:e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Davis PB, Gurney ME, Xu R. COVID‐19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimer's Dement. 2021:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID‐19. Nat Rev Endocrinol. 2020;16:341‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID‐19: results from an integrated health care organization. Ann Intern Med. 2020;173:773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID‐19‐related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Network Open. 2020;3:e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health‐related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci. 2020;24(2):168‐175. [DOI] [PubMed] [Google Scholar]
- 25. Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu Rev Med. 2018;69:437‐449. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta‐analysis. Age Ageing. 2014;43:326‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuo CL, Pilling LC, Atkins JL, et al. ApoE e4e4 genotype and mortality with COVID‐19 in UK Biobank. J Gerontol A Biol Sci Med Sci. 2020;75:1801‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


