Abstract
Background
Studies report hypercoagulability in coronavirus disease 2019 (COVID‐19), leading many institutions to escalate anticoagulation intensity for thrombosis prophylaxis.
Objective
To determine the bleeding risk with various intensities of anticoagulation in critically ill patients with COVID‐19 compared with other respiratory viral illnesses (ORVI).
Patients/Methods
This retrospective cohort study compared the incidence of major bleeding in patients admitted to an intensive care unit (ICU) within a single health system with COVID‐19 versus ORVI. In the COVID‐19 cohort, we assessed the effect of anticoagulation intensity received on ICU admission on bleeding risk. We performed a secondary analysis with anticoagulation intensity as a time‐varying covariate to reflect dose changes after ICU admission.
Results
Four hundred and forty‐three and 387 patients were included in the COVID‐19 and ORVI cohorts, respectively. The hazard ratio of major bleeding for the COVID‐19 cohort relative to the ORVI cohort was 1.26 (95% confidence interval [CI]: 0.86–1.86). In COVID‐19 patients, an inverse‐probability treatment weighted model found therapeutic‐intensity anticoagulation on ICU admission had an adjusted hazard ratio of bleeding of 1.55 (95% CI: 0.88–2.73) compared with standard prophylactic‐intensity anticoagulation. However, when anticoagulation was assessed as a time‐varying covariate and adjusted for other risk factors for bleeding, the adjusted hazard ratio for bleeding on therapeutic‐intensity anticoagulation compared with standard thromboprophylaxis was 2.59 (95% CI: 1.20–5.57).
Conclusions
Critically ill patients with COVID‐19 had a similar bleeding risk as ORVI patients. When accounting for changes in anticoagulation that occurred in COVID‐19 patients, therapeutic‐intensity anticoagulation was associated with a greater risk of major bleeding compared with standard thromboprophylaxis.
Keywords: anticoagulants, COVID‐19, critical illness, hemorrhage, thrombosis
Essentials
-
•
In patients with COVID‐19, the bleeding risk associated with increased intensity of anticoagulation for thromboprophylaxis is not well‐studied.
-
•
We performed a retrospective cohort study of critically ill patients admitted within a single health system with COVID‐19 or other respiratory viral illnesses
-
•
Critically ill patients with COVID‐19 had a similar risk of bleeding as critically ill patients with other respiratory viral illnesses.
-
•
In COVID‐19 patients, therapeutic anticoagulation was associated with an increased risk of bleeding compared to standard thromboprophylaxis.
Alt-text: Unlabelled Box
1. INTRODUCTION
Patients with coronavirus disease 2019 (COVID‐19) can develop critical illness and respiratory failure requiring admission to an intensive care unit (ICU).1 Early reports suggest microvascular thrombosis and venous thromboembolism (VTE) contribute to morbidity and mortality in COVID‐19.2., 3. Critical illness is a well‐described risk factor for VTE. ICU patients typically receive prophylactic‐intensity anticoagulation to prevent this complication.4 Studies before the COVID‐19 pandemic reported approximately 8% to 10% of ICU patients develop VTE despite receiving VTE prophylaxis.4., 5. Initial reports in patients with COVID‐19 suggested a much higher rate of VTE, ranging from 17% to 69%.6., 7., 8., 9., 10., 11. This prompted some clinicians to increase the intensity of anticoagulation used for VTE prophylaxis in patients with COVID‐19 from standard to intermediate or even therapeutic intensity.12., 13. Potentially because of increased awareness of the thrombotic risks of COVID‐19 or use of increased intensity of anticoagulation, more recent data from institutions in the United States reported a lower incidence of thrombosis.14., 15. Expert guidance on VTE prophylaxis in patients with COVID‐19 has varied because of a lack of randomized controlled trials.16., 17., 18. Fortunately, at the time of publication of this study, there are a number of ongoing trials comparing anticoagulation strategies in COVID‐19 (NCT04362085, NCT04406389, NCT04359277).
While we eagerly await the final results of these trials, clinicians need real‐time guidance regarding the risks and benefits of different intensities of anticoagulation. The risk of major bleeding with varying intensities of anticoagulation in critically ill patients with COVID‐19 unknown. Furthermore, the current COVID‐19 literature often lacks a comparator group to other critically ill patients with respiratory viral infections.
This retrospective cohort study compared major bleeding in patients with COVID‐19 to patients with respiratory failure from other viruses. In addition, we sought to compare the bleeding risk of critically ill patients with COVID‐19 by intensity of anticoagulation.
2. METHODS
2.1. Patient selection
A central electronic health record (EHR) data repository was used to identify consecutive adult patients ≥18 years of age admitted to an ICU in the University of Pennsylvania Health System for virus‐related respiratory failure and who received anticoagulation during their ICU stay. Two cohorts of patients with respiratory failure were identified: (1) COVID‐19 and (2) other respiratory viral illness (ORVI). The COVID‐19 cohort included patients with a COVID‐19 infection confirmed by a reverse transcription polymerase chain reaction test who were admitted or transferred to an ICU for respiratory failure between January 1, 2020, and May 30, 2020. The ORVI cohort included patients with respiratory failure from a viral infection other than COVID‐19 admitted to an ICU in a time‐matched period in 2017, 2018, and 2019 (01/01/2017–05/30/2017, 01/01/2018–05/30/2018, and 01/01/2019–05/30/2019). Patients with ORVI from 2020 were excluded given possibility of unrecognized co‐infection due to lack of testing. Respiratory failure was defined as oxygen requirement more than nasal cannula or face mask (including high‐flow nasal cannula, noninvasive ventilation, and mechanical ventilation), consistent with the World Health Organization clinical progression scale definition of “hospitalized severe disease.”19 Patients were excluded from the study if they were admitted to the ICU for a condition unrelated to viral infection or in the absence of respiratory failure. This study was determined to be exempt by the institutional review board at the University of Pennsylvania and a waiver of informed consent was granted for this retrospective study.
2.2. Outcomes
The primary endpoint of major bleeding was defined by the criteria of the International Society on Thrombosis and Haemostasis (ISTH) and included fatal bleeding, hemorrhage occurring at a critical area or organ, or bleeding causing a fall in hemoglobin of 2 g/dl or more, or leading to transfusion of 2 or more units of whole blood or red cells.20 To avoid misclassification of bleeding events from hemodilution or anemia of critical illness, we required the 2 g/dl hemoglobin drop or the 2‐unit packed red blood cells transfusion to occur within 24 h of a documented or suspected bleeding event identified on chart review.
2.3. Data collection
Major bleeding was recorded from day of admission to the ICU (time 0) until either hospital discharge, in‐hospital death, or data cutoff date (6/30/2020), whichever occurred first. All COVID‐19 patients who remained hospitalized at the time of end of study had at least 30 days follow‐up from the time of hospital admission. Study data were collected using REDCap (Research Electronic Data Capture) hosted at the University of Pennsylvania.21., 22. A group of data abstractors reviewed patients’ EHR to confirm study eligibility and collect data. All data abstractors received a guide with detailed instructions on data entry. The first 10% of each abstractors’ entries were reviewed in duplicate by one of the lead investigators (A. M. P. or R. H.). If errors in data entry were identified, further instructions were given to the reviewer and additional entries were reviewed in duplicate until consistent agreement. All major bleeding events recorded by data abstractors were verified by one of the lead investigators. Any discrepancies were resolved by discussion between R. H. and A. M. P.
2.4. Classification of anticoagulation intensity
Anticoagulation was classified as standard prophylactic‐intensity, intermediate‐intensity, or therapeutic‐intensity anticoagulation as follows:
-
1.
Standard prophylactic intensity: enoxaparin ≤40 mg once daily, enoxaparin 40 mg twice daily if body mass index (BMI) ≥ 40 kg/m2, subcutaneous heparin 5000 units or less 2 or 3 times a day, apixaban 2.5 mg twice daily (with intent of prophylaxis), rivaroxaban 10 mg daily, betrixaban 80 or 160 mg once daily, heparin infusion 200–500 units/h without partial thromboplastin time titration (used for continuous renal replacement therapy [CRRT]), fondaparinux 2.5 mg once daily.
-
2.
Intermediate intensity: enoxaparin 0.5 mg/kg twice daily, enoxaparin 40 mg twice daily for patients with BMI <40 kg/m2, or subcutaneous heparin 7500 units every 8 h.
-
3.
Therapeutic intensity: unfractionated heparin drip adjusted by partial thromboplastin time or anti‐XA level, argatroban infusion, bivalirudin infusion, enoxaparin 1 mg/kg twice a day or 1.5 mg/kg daily, fondaparinux ≥5 mg daily, warfarin, apixaban 5 or 10 mg twice daily, rivaroxaban 15 mg twice daily or 20 mg once daily, or dabigatran 150 mg twice daily.
We extracted from the EHR the highest intensity of anticoagulant received for each day from ICU admission to discharge/death or data cutoff. When classifying anticoagulation by day, we entered the maximum intensity of anticoagulation received on that day or the day prior. This was done because of expected lags in documentation, with daily progress notes summarizing events of the prior 24 h, including bleeding events occurring the day prior.
2.5. Statistical analysis
Patient characteristics, laboratory values, and intensity of anticoagulation on admission to the ICU were compared between the COVID‐19 and ORVI cohorts using standard descriptive statistics. The proportion of patients who experienced a bleeding event or in‐hospital mortality were also compared between the two cohorts using the chi‐square test or Fisher's exact test when indicated. Missing values were not imputed.
Fine and Gray's subdistribution hazard models and cumulative incidence functions were used to compare bleeding outcomes within 30 days of ICU admission between COVID‐19 and ORVI patients. Death and hospital discharge were considered competing events. Patients who remained in the hospital beyond 30 days without a bleeding event or remained in the hospital at data cutoff (6/30/2020) were censored.
In the COVID‐19 cohort, we assessed the effect of the intensity of anticoagulation received on ICU admission on bleeding risk. We used an inverse probability treatment weighting (IPTW) model to account for potential differences in the severity of illness and/or bleeding risk in patients who received different intensities of anticoagulation. The following laboratory values were considered for inclusion in the model based on prior studies showing an association with bleeding: international normalized ratio, platelet count, creatinine clearance (CrCl), and total bilirubin.23., 24. We also assessed age ≥65 years, use of antiplatelet agent during hospitalization, as well as indicators of severity of illness (use of extracorporeal membrane oxygenator [ECMO], CRRT, and mechanical ventilation).24., 25. For each potential variable, we performed univariate analyses predicting time to first bleeding event in Fine and Gray subdistribution hazard models with discharge and death as competing risks. Those variables with p < .05 or with a parameter estimate >2 were included in a logistic regression to develop a propensity score. Patients missing any of the covariates selected for the IPTW model were excluded. Anticoagulation intensity on ICU admission was classified by the maximum intensity of anticoagulation received within 2 days of ICU admission. Patients who did not receive anticoagulation within 2 days of ICU admission were also excluded from the IPTW model.15 Anticoagulation intensity was entered as a predictor of bleeding using IPTW in a Fine and Gray subdistribution hazard model. We derived cumulative incidence function estimates of bleeding events by anticoagulation intensity.
We expected frequent changes of anticoagulation intensity may occur in patients with COVID‐19 because there were changes in institutional guidelines and published work describing increased risk of thrombosis during the study period. Thus, a second competing risk analysis was performed as a sensitivity analysis with anticoagulation intensity as a time‐varying covariate. The intensity of anticoagulation was allowed to vary by day and classified as the maximum intensity received on that hospital day or the previous hospital day as described in the section “Classification of anticoagulation intensity.” Variables found to be associated with bleeding in our analysis of the whole cohort were entered into the model with anticoagulation intensity as a time‐varying covariate. All analyses were performed using Stata v.14.0 or SAS v.9.4.
3. RESULTS
3.1. Patient characteristics
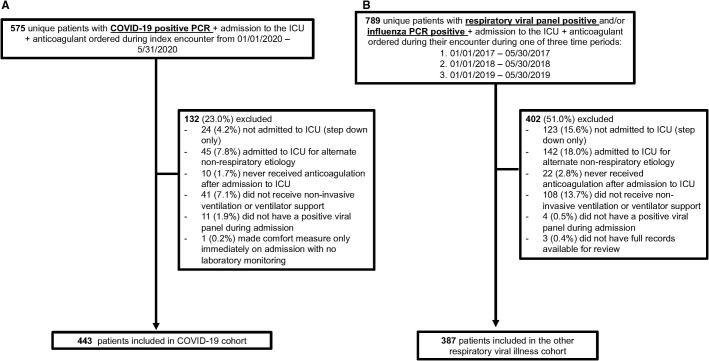
In the COVID‐19 cohort, 575 unique patients were identified during the period of interest. A total of 443 (77.0%) patients were eligible for inclusion in this study (Figure 1A ). For the ORVI cohort, 789 unique patients were identified based on ICU admission for acute respiratory failure with positive influenza and/or other respiratory viral polymerase chain reaction during the period of interest. Of these, 387 (49.0%) patients were eligible for inclusion in this study (Figure 1B).
FIGURE 1.
Cohort selection flow diagram. (A) Cohort selection diagram for the COVID‐19 cohort. (B) Cohort selection diagram for the ORVI cohort. Respiratory viral panel includes influenza A, influenza B, parainfluenza 1–4, respiratory syncytial virus A/B, adenovirus, human metapneumovirus, coronavirus (non–SARS‐COV‐2), rhinovirus/enterovirus, Chlamydia pneumonia, Mycoplasma pneumonia. Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; ORVI, other respiratory virus infection; PCR, polymerase chain reaction; SARS‐COV‐2, severe acute respiratory syndrome coronavirus 2
The baseline demographic and clinical characteristics of the two cohorts are summarized in Table 1 . There were differences between the two cohorts with respect to race and ethnicity as recorded in the EHR, with fewer White individuals (34.3% vs. 46.5%, p < .001) and more patients of Hispanic/Latino ethnicity (10.6% vs. 2.1%, p < 0.001) in the COVID‐19 cohort. Mean (standard deviation) BMI was higher in the COVID‐19 cohort compared with the ORVI cohort (31.3 [9.6] kg/m2 vs. 29.2 [9.6] kg/m2, p = 0.002). More ORVI patients were prescribed anticoagulation as outpatients before the index admission (22.5% vs. 14.4%, p = 0.003).
TABLE 1.
Baseline demographics and clinical features prior to admission for COVID‐19 versus other respiratory viral illness cohorts
| COVID‐19 N = 443 |
ORVI N = 387 |
p | |
|---|---|---|---|
| Age (y), median (IQR) | 66 (55−75) | 64 (55−75) | .28 |
| Female, N (%) | 192 (43.3) | 189 (48.8) | .11 |
| Race, N (%) | |||
| White | 148 (34.3) | 173 (46.5) | <.001 |
| Black | 234 (54.3) | 173 (46.5) | |
| Asian or Pacific islander | 32 (7.4) | 11 (3.0) | |
| Other | 17 (3.9) | 15 (4.0) | |
| Unknown/missing | 12 (.) | 15 (.) | |
| Hispanic/Latino ethnicity, N (%) | 46 (10.6) | 8 (2.1) | <.001 |
| Body mass index, mean (SD) | 31.3 (9.6) | 29.2 (9.6) | .002 |
| Coronary artery diseasea, N (%) | 56 (12.6) | 55 (14.3) | .49 |
| Hypertensiona, N (%) | 295 (72.0) | 221 (60.5) | <.001 |
| Dyslipidemiaa, N (%) | 176 (44.1) | 108 (29.8) | <.001 |
| Diabetesa, N (%) | 166 (37.5) | 102 (26.5) | <.001 |
| Heart failurea, N (%) | 57 (12.9) | 76 (19.7) | .007 |
| Presence of left ventricular assist devicea, N (%) | 1 (0.2) | 1 (0.3) | .92 |
| Strokea, N (%) | 46 (10.4) | 31 (8.1) | .28 |
| Chronic lung diseasea, N (%) | 100 (22.6) | 178 (46.2) | <.001 |
| Chronic liver diseasea, N (%) | 9 (2.0) | 6 (1.6) | .80 |
| Chronic kidney diseasea, N (%) | 42 (9.5) | 30 (7.8) | .46 |
| End stage renal disease on dialysisa, N (%) | 14 (3.2) | 14 (3.6) | .71 |
| History of prior or current malignancya, N (%) | 71 (16.0) | 113 (29.4) | <.001 |
| Organ transplant recipienta, N (%) | 3 (0.7) | 30 (7.8) | <.001 |
| Smoking (any), N (%) | 160 (36.1) | 235 (60.7) | <.001 |
| Active, N (%) | 19 (4.2) | 56 (14.4) | |
| Former, N (%) | 141 (31.8) | 179 (46.2) | |
| Missing, N (%) | 73 (16.5) | 24 (6.2) | |
| Charlson Comorbidity Indexb, mean (SD) | 1.2 (1.85) | 1.9 (2.18) | <.001 |
| Use of anticoagulant prior to hospitalization, N (%) | 64 (14.4) | 87 (22.5) | .003 |
| Indication for anticoagulant use before hospitalizationc, N (%) | |||
| None | 379 (85.6) | 300 (77.5) | .003 |
| Prior venous thromboembolism | 28 (6.3) | 34 (8.8) | .18 |
| Atrial fibrillation or flutter | 29 (6.5) | 45 (11.6) | .01 |
| Mechanical or bioprosthetic valve | 0 | 1 (0.3) | .47 |
| Prior stroke | 3 (0.7) | 5 (1.3) | .48 |
| Other indication | 9 (2.0) | 10 (2.6) | .60 |
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range; ORVI, other respiratory virus infection; SD, standard deviation.
Presence of medical condition noted in encounter before index hospitalization admission date. In patients without prior encounters, initial admission note was reviewed for presence of the medical condition.
Calculated from International Classification of Diseases‐10 codes from any encounters before admission.
Patients could have more than one indication for anticoagulation.
Table 2 describes the clinical features during the index hospitalization and laboratory values on admission to the ICU for the two cohorts. The median duration of the ICU admission in patients in the COVID‐19 cohort was 11 (interquartile range 5–20) days versus 4.0 (interquartile range 2.0–10.0) days in the ORVI cohort. A total of 71.8% of COVID‐19 patients required mechanical ventilation compared with 55.0% of ORVI patients (p < .001). In‐hospital mortality was higher in the COVID‐19 group compared with the ORVI cohort (37.5% vs. 21.4%, p = .003). A total of 4.3% (19/443) of patients in the COVID‐19 cohort remained hospitalized at time of data cutoff and were classified as alive in this analysis. Inflammatory and coagulation markers (C‐reactive protein, fibrinogen, and D‐dimer) were elevated in COVID‐19 patients; however, these markers were not frequently drawn in the ORVI cohort (>50% missing) and thus comparisons were not performed.
TABLE 2.
Clinical features and laboratory values during index hospitalization for the COVID‐19 and ORVI cohorts
| COVID‐19 N = 443 |
ORVI N = 387 | p | |
|---|---|---|---|
| Clinical features during index admission | |||
| Type of viral infection, N (%) | |||
| COVID‐19 | 443 (100.0) | 0 | NRa |
| Adenovirus | 1 (0.8) | 18 (4.7) | |
| Metapneumovirus | 0 | 48 (12.4) | |
| Influenza A/B | 0 | 136 (35.2) | |
| Coronavirus (not COVID‐19) | 0 | 42 (10.9) | |
| Mycoplasma pneumonia | 0 | 1 (0.3) | |
| Parainfluenza | 0 | 34 (8.8) | |
| Rhinovirus or enterovirus | 0 | 70 (18.1) | |
| Respiratory syncytial virus A or B | 0 | 39 (10.1) | |
| Total duration of hospitalizationb (d), median (IQR) | 18 (10.0–31.0) | 10.0 (6.0–21.0) | <.001 |
| Days in ICUc, median (IQR) | 11 (5.0 −20.0) | 4.0 (2.0–10.0) | <.001 |
| Mechanical ventilator use, N (%) | 318 (71.8) | 213 (55.0) | <.001 |
| CRRT and/or dialysis, N (%) | 89 (20.1) | 67 (17.3) | .31 |
| ECMO use, N (%) | 22 (5.0) | 32 (8.3) | .05 |
| Patient status at discharge, N (%) | |||
| Alive | 258 (58.2) | 304 (78.6) | <.001 |
| Deceased | 166 (37.5) | 83 (21.4) | |
| Remains hospitalized at end of study period | 19 (4.3) | 0 (0) | |
| Medications received during hospitalization | |||
| Any antiplatelet agent, N (%) | 174 (39.3) | 158 (40.8) | .65 |
| Aspirin, N (%) | 170 (38.4) | 151 (39.0) | |
| Clopidogrel, N (%) | 27 (6.1) | 23 (5.9) | |
| Ticagrelor, N (%) | 3 (0.7) | 4 (1.0) | |
| Prasugrel, N (%) | 0 | 0 | |
| Nonsteroidal anti‐inflammatory drugs, N (%) | 53 (12.0) | 51 (13.2) | .60 |
| Lipid‐lowering medications, N (%) | 212 (47.9) | 170 (43.9) | .26 |
| At least one pressor received during hospitalization, N (%) | 288 (65.0) | 172 (44.5) | <.001 |
| ACE inhibitor, ARB, or angiotensin receptor antagonist/neprilysin inhibitor, N (%) | 73 (16.5) | 90 (23.3) | .01 |
| Hydroxychloroquine, N (%) | 244 (55.1) | 4 (1.0) | <.001 |
| Oseltamivir, N (%) | 2 (0.5) | 185 (47.8) | <.001 |
| Remdesivir, N (%) | 89 (20.1) | 0 | <.001 |
| Tocilizumab, N (%) | 16 (3.6) | 0 | <.001 |
| Azithromycin, N (%) | 118 (26.6) | 234 (60.5) | <.001 |
| Lopinavir‐ritonavir, N (%) | 1 (0.2) | 0 | 1 |
| Zinc, N (%) | 35 (7.9) | 5 (1.3) | <.001 |
| Steroids, N (%) | 320 (72.2) | 267 (69.0) | .31 |
| Convalescent plasma, N (%) | 19 (4.3) | 0 | NRa |
| Laboratory values on ICU admissiond | |||
| Hemoglobin (g/dl), mean ± SD Missing, N (%) |
12.1 ± 2.2 0 (0) |
11.1 ± 2.2 0 (0) |
<.001 |
| White blood count (k/µl), mean ± SD Missing, N (%) |
9.5 ± 5.1 1 (0.22) |
11.0 ± 6.9 1 (0.26) |
.13e |
| Platelets (×109/L), mean ± SD Missing, N (%) |
226 ±99 0 (0) |
202 ± 100 3 (0.77) |
<.001 |
| Creatinine (mg/dl), mean ± SD Missing, N (%) |
2.01 ± 3.04 0 (0) |
1.76 ± 1.98 0 (0) |
.82e |
| Total bilirubin (mg/dl), mean ± SD Missing, N (%) |
0.7 ± 0.4 6 (1.4) |
0.9 ± 2.0 54 (14.0) |
.21e |
| INR, mean ± SD Missing, N (%) |
1.35 ± 0.5 54 (12.1) |
1.32 ± 0.6 58 (0) |
.08e |
| Fibrinogen (mg/dl), mean ± SD Missing, N (%) |
578 ± 197 302 (68.2) |
534 ± 243 338 (87.3) |
NRa |
| Noncardiac CRP (mg/dl), mean ± SD Missing, N (%) |
30.9 ± 50.6 146 (33.0) |
79.7 ± 96.4 368 (95.1) |
NRa |
| D‐dimer [µg/ml (FEU)], mean ± SD Missing, N (%) |
3.06 ± 3.38 130 (29.3) |
3.46 ± 3.46 | NRa |
| 349 (90.2) | |||
| Troponin (ng/ml), mean ± SD Missing, N (%) |
0.13 ± 0.78 140 (31.6) |
0.17 ± 0.64 132 (34.1) |
.002e |
| NT‐pro BNP (pg/ml), mean ± SD Missing, N (%) |
4738 ± 9182 252 (56.9) |
7214 ± 10,668 184 (47.4) |
.001e |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CRP, C‐reactive protein; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile ratio; NR, not reported; NT‐pro BNP, N‐terminal pro B type natriuretic peptide; ORVI, other respiratory virus infection; PTT, partial thromboplastin time; SD, standard deviation.
Comparison between cohorts was not reported if less than 20% of either cohort had laboratory value performed.
Time of hospitalization until discharge from the health system. Time includes discharge to inpatient hospice facility if part of the hospital system.
Multiple ICU transfers could occur during index admissions (e.g., patient transferred to the ICU then to the general medical floor and then ICU). Days in ICU reflect all the days spent in the ICU during the index admission and exclude time spent in the general medical floor.
Laboratory values on day of ICU admission or within 48 h if not drawn on day of ICU admission.
Data log transformed and results of t test of log‐transformed data listed.
3.2. Anticoagulation following ICU admission
Table 3 summarizes the intensity of anticoagulation therapy received on admission to the ICU (day 0) and any subsequent increase in anticoagulation intensity from time of ICU admission to hospital discharge or in‐hospital death. In the COVID‐19 cohort, more patients had at least one increase in intensity of anticoagulation following admission to the ICU compared with the ORVI cohort (59.8% vs. 25.8%, p < 0.001). The most common reason for increasing intensity of anticoagulation in the COVID‐19 cohort was “deterioration in clinical status” (48.2%) in comparison to “atrial fibrillation/flutter” (26.0%) in the ORVI cohort.
TABLE 3.
Anticoagulation on admission to intensive care unit by cohort and increases in anticoagulation intensity following intensive care unit admission by cohort
| COVID‐19 N = 443 |
ORVI N = 387 |
p | |
|---|---|---|---|
| Anticoagulation intensity on admission to ICU, N (%) | |||
| Standard prophylactic | 216 (48.8) | 252 (65.1) | <.001 |
| Intermediate | 84 (19.0) | 8 (2.1) | |
| Treatment | 127 (28.7) | 104 (26.9) | |
| Home warfarin held because of therapeutic/supratherapeutic INR | 4 (1.0) | 4 (1.0) | |
| Nonea | 12 (2.7) | 19 (4.9) | |
| Anticoagulation intensity increase following ICU admission, N (%) | |||
| Yes | 189 (59.8) | 73 (25.8) | <.001 |
| No | 127 (40.1) | 210 (74.2) | |
| Ineligible (already on therapeutic intensity) | 127 (28.7) | 104 (26.9) | |
| Reason for increase in anticoagulation intensity, N (%)b | |||
| Suspected thrombosis | 15 (7.9) | 5 (5.5) | <.001 |
| Confirmed thrombosis | 27 (14.3) | 12 (16.4) | |
| Deterioration in clinical status | 91 (48.2) | 4 (5.5) | |
| Atrial fibrillation or flutter | 14 (7.4) | 19 (26.0) | |
| Initiation of ECMO | 1 (0.53) | 8 (11.0) | |
| Concern for heparin‐induced thrombocytopenia | 1 (0.53) | 1 (1.4) | |
| Elevated D‐dimer/concern for hypercoagulability | 12 (6.4) | 0 (0) | |
| Institutional guidelines | 3 (1.6) | 0 (0) | |
| Contraindication to anticoagulation resolvedc | 6 (3.2) | 7 (9.6) | |
| Other/unknown | 19 (10.6) | 17 (23.2) | |
Abbreviations: COVID‐19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenator; ICU, intensive care unit; INR, international normalized ratio; ORVI, other respiratory virus infection.
Patients were not on anticoagulation on day of admission to the ICU. All patients eventually received anticoagulation at a later date.
If patient had increase in anticoagulation intensity, the reason for anticoagulation intensity increase was reflected in clinical progress notes.
Number of patients who had anticoagulation initially held because of contraindication such as bleeding, severe thrombocytopenia, or elevated INR and then resumed anticoagulation when contraindication resolved.
3.3. Outcomes
3.3.1. Description of major bleeding events
Table 4 describes the major bleeding events that occurred in the COVID‐19 and ORVI cohorts. A major bleeding event occurred in 14.7% (65/443) of patients in the COVID‐19 cohort. A total of 94 bleeding events occurred in these 65 patients. Nonfatal critical organ bleed occurred in 2.0% (9/443) of COVID‐19 patients and fatal bleed occurred in 0.9% (4/443) . Anticoagulation was administered in 93.8% of COVID‐19 patients on the day of or day before the first major bleeding event, with 75.4% receiving therapeutic‐intensity anticoagulation. All six patients with COVID‐19 who experienced an intracranial hemorrhage received therapeutic intensity anticoagulation on the day of bleeding. Notably, five of the six patients required ECMO support during their hospitalization.
TABLE 4.
ISTH major bleeding events description in the COVID‐19 and ORVI cohorts
| COVID−19 N = 443 |
ORVI N = 387 |
p | |
|---|---|---|---|
| Any ISTH major bleeding event, N (%)a | |||
| Yes | 65 (14.7) | 44 (11.4) | .16 |
| No | 378 (85.3) | 343 (86.3) | |
| ISTH criteria met (highest severity)b, N (%) | |||
| Decrease in hemoglobin ≥2 g/dl and/or 2‐unit pRBC transfusionc | 52 (11.7) | 32 (8.3) | _ |
| Nonfatal critical organ bleed | 9 (2.0) | 8 (2.1) | |
| Fatal bleed | 4 (0.9) | 4 (1.0) | |
| Maximum intensity of anticoagulation received on day of or day before the first bleeding event, N (%) | |||
| None | 4 (6.2) | 5 (11.4) | _ |
| Standard prophylactic | 8 (12.3) | 6 (13.6) | |
| Intermediate | 4 (6.2) | 0 (0) | |
| Therapeutic | 49 (75.4) | 33 (75.0) | |
| Type of major bleeding event(s)d, N (%) | |||
| Gastrointestinal bleed | 25 (5.6) | 17 (4.4) | _ |
| Hemoptysis | 2 (0.5) | 3 (0.8) | |
| Hematuria | 3 (0.7) | 2 (0.5) | |
| Epistaxis | 1 (0.2) | 1 (0.4) | |
| Surgical or procedural site bleeding | 11 (2.5) | 7 (1.8) | |
| Intracranial bleed | 6 (1.4) | 6 (1.6) | |
| Retroperitoneal bleed | 6 (1.4) | 5 (1.3) | |
| Pericardial bleed | 0 (0) | 1 (0.3) | |
| Intramuscular bleed without compartment syndrome | 8 (1.8) | 5 (1.3) | |
| Vaginal bleed | 2 (0.5) | 1 (0.3) | |
| Other | 9 (2.0) | 6 (1.6) | |
Abbreviations: COVID‐19, coronavirus disease 2019; ISTH, International Society on Thrombosis and Haemostasis; ORVI, other respiratory virus infection; pRBC, packed red blood cells.
Number reflects one event per patient. In the COVID cohort, 94 total events occurred in 65 patients. In the ORVI cohort, 65 total events occurred in 44 patients.
The clinically most severe event that occurred during the hospitalization is recorded in the following order of severity: fatal bleed >critical organ bleed >packed red blood cell transfusion and/or 2 g/dl hemoglobin drop.
Required 2 g/dl hemoglobin decrease and/or 2‐unit pRBC transfusion to occur within 24 h.
Numbers reflect the presence of bleeding at specific site during the study period (includes first event and recurrent bleeding events if they were at different sites). Patients could have more than one type of major bleeding event. Multiple events of the same type occurring in a single patient are not recorded.
In the ORVI cohort, 11.4% (44/387) of patients had a major bleeding event. A total of 65 events occurred in these 44 patients. Nonfatal critical organ bleed occurred in 2.1% (8/387) of patients and 1.0% (4/387) of patients experienced a fatal bleed . Anticoagulation was administered in 88.6% of ORVI patients on the day of or day before the first major bleeding event, with 75% receiving therapeutic‐intensity anticoagulation. Three of the six ORVI patients who experienced an intracranial hemorrhage were receiving therapeutic anticoagulation on the day of bleed. All three of these patients required ECMO during their hospital stay.
3.3.2. Comparison of bleeding outcomes between COVID‐19 and ORVI cohorts
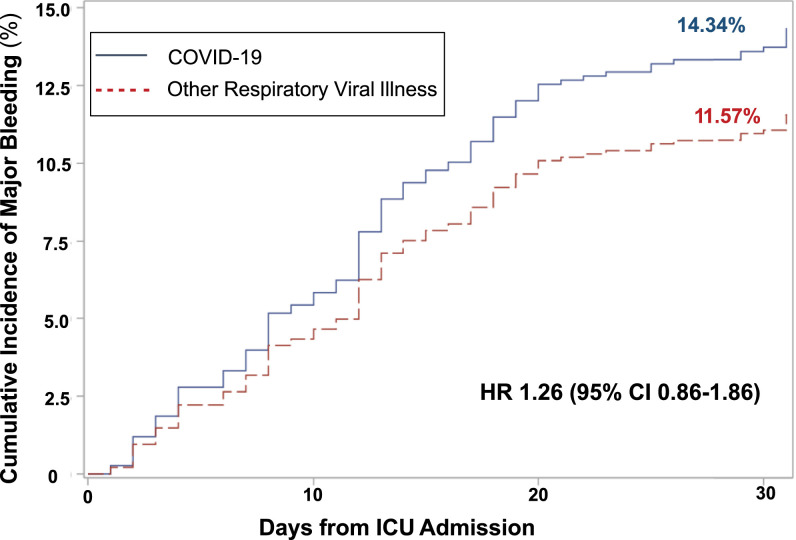
The estimated cumulative incidence of major bleeding at 7, 14, and 30 days was 5.2%, 9.8%, and 14.3% in the COVID‐19 cohort versus 4.1%, 7.8%, and 11.6% in the ORVI cohort (Figure 2 ). The hazard ratio of major bleeding for the COVID‐19 cohort relative to the ORVI cohort was 1.26 (95% confidence interval [CI]: 0.86–1.86).
FIGURE 2.
Cumulative incidence of major bleeding events in the entire population (COVID‐19 and ORVI cohorts). The primary analysis used Fine and Gray's subdistribution hazard models to determine the association of COVID‐19 compared with ORVI with bleeding outcomes within 30 days of ICU admission. Death and hospital discharge were considered competing events. Patients who remained in the hospital beyond 30 days without a bleeding event or remained in the hospital at data cutoff (6/30/2020) were censored. Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; ORVI, other respiratory virus infection
3.3.3. Comparison of bleeding outcomes by anticoagulation intensity in the COVID‐19 cohort
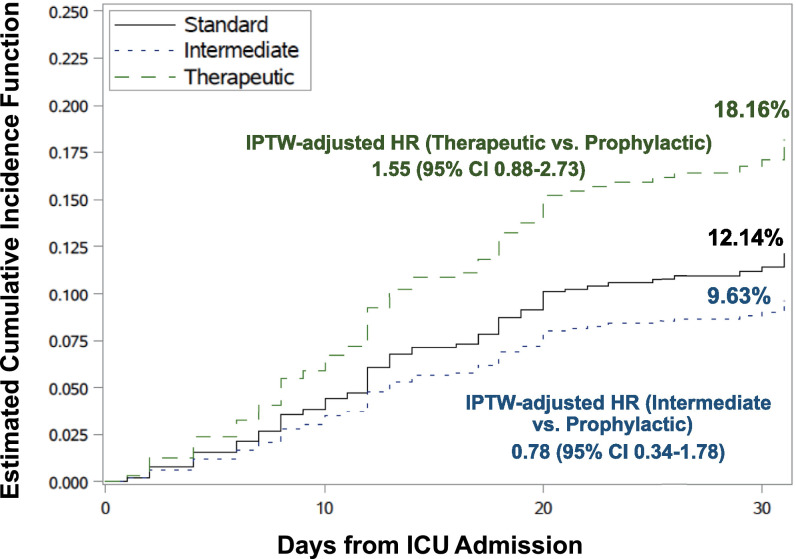
Variables found to be associated with time to first bleeding event in the COVID‐19 cohort in univariate analysis included ECMO, mechanical ventilation, dialysis, or CRRT, and CrCl <30 ml/min on admission to the ICU and were included in the final balanced model for the IPTW method. One patient did not have baseline CrCl available and was excluded from the analysis. In this IPTW model, where anticoagulation intensity was defined as the maximum intensity of anticoagulation administered within 2 days of ICU admission, 9/443 (2.03%) of patients did not receive any anticoagulation within 48 h of ICU admission and were excluded from the analysis. Figure 3 shows the stabilized weight‐adjusted cumulative incidence curves for the effect of anticoagulation intensity on major bleeding in the COVID‐19 cohort. Among 433 patients with COVID‐19, the IPTW‐adjusted estimated cumulative incidence of major bleeding at 30 days was 12.14%, 9.64%, and 18.16% with standard prophylactic‐intensity, intermediate‐intensity, and therapeutic‐intensity anticoagulation, respectively. Compared with standard prophylactic intensity, intermediate‐intensity anticoagulation was not associated with an increased risk of major bleeding in the IPTW model (adjusted hazard ratio [aHR]: 0.78; 95% CI: 0.34–1.78). Therapeutic‐intensity anticoagulation on ICU admission was not associated with increased risk of major bleeding compared to standard thromboprophylaxis (aHR: 1.55; 95% CI: 0.88–2.73).
FIGURE 3.
Inverse probability treatment weighting (IPTW). Adjusted cumulative incidence of major bleeding by anticoagulation intensity in the COVID‐19 cohort. The IPTW adjusted cumulative incidences of bleeding by various intensities of anticoagulation defined as the maximum intensity of anticoagulation received within 2 days of ICU admission in COVID‐19 patients are shown. A total of 10 patients were excluded from the IPTW analysis (nine did not receive anticoagulation within 2 days of ICU admission and 1 had missing baseline creatinine clearance); thus, 433 patients were included in the IPTW analysis. Death and hospital discharge were considered competing events. Patients who remained in the hospital beyond 30 days without a bleeding event or remained in the hospital at data cutoff (6/30/2020) were censored. Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit
In the COVID‐19 cohort, we noted frequent changes in anticoagulation intensity from the time of ICU admission compared with day of exit from the analysis. A total of 29.3% of patients initially on standard prophylactic intensity and 23.9% of patients initially on intermediate intensity escalated to therapeutic intensity anticoagulation. De‐escalations in therapy were also frequent, with 23.6% of patients on therapeutic intensity changing to intermediate or standard prophylactic‐intensity anticoagulation at the end of the observation period. Thus, we also assessed anticoagulation as a time‐varying covariate in the model by classifying the intensity of maximum intensity received on that day or the day before account for these frequent changes in doses. After adjustment for ECMO, mechanical ventilation, CRRT, and dialysis, therapeutic‐intensity anticoagulation was associated with an increased risk of bleeding compared with standard thromboprophylaxis (aHR: 2.59; 95% CI: 1.20–5.57) (Table 5 ). Intermediate‐intensity anticoagulation was not associated with increased risk of bleeding compared with standard‐intensity thromboprophylaxis (aHR: 0.66; 95% CI: 0.20–2.23).
TABLE 5.
Fine and Gray competing risk model for major bleeding by anticoagulation intensity in COVID‐19 patients
| Unadjusted Subhazard Ratios (95% CI) a | p | Adjusted Subhazard Ratios (95% CI) a | p | |
|---|---|---|---|---|
| Anticoagulation Intensity (varying by day)b | ||||
| Standard prophylactic | Reference | ‐ | Reference | ‐ |
| Intermediate | 0.75 (0.22–2.52) | .642 | 0.66 (0.20–2.23) | .50 |
| Therapeutic | 3.02 (1.43–6.38) | .004 | 2.59 (1.20–5.57) | .02 |
| None | 2.36 (0.63–8.86) | .203 | 1.92 (0.49–7.51) | .35 |
| ECMOc | 4.56 (2.33–8.94) | <0.001 | 2.84 (1.42–5.69) | .003 |
| Mechanical ventilationc | 6.04 (2.18–16.70) | <0.001 | 1.91 (0.64–5.75) | .25 |
| CRRT/Dialysis c | 0.52 (0.08–3.29) | 0.488 | 2.74 (1.58–4.72) | <.001 |
| CRRT/Dialysis*timec, d | 2.40 (1.16–4.97) | 0.018 | ||
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenator.
Fine and Gray subdistribution hazard ratios with competing risk of discharge or death was used for this analysis, with anticoagulation as time‐varying covariate.
Anticoagulation was classified by the maximum dose of anticoagulation received on the same day or day prior. Anticoagulation was entered as a time‐varying covariate in the model.
Use of listed organ support device at any time during hospitalization.
Effect of CRRT/dialysis varied with time in the univariate Fine and Gray competing risk model (violated proportional hazards). Covariate reported with the interaction with time.
4. DISCUSSION
Many studies report an increased thrombotic risk in COVID‐19, but few provide a detailed evaluation of bleeding events.11 A large multiplatform randomized controlled trial paused enrollment for ICU patients with COVID‐19 because of futility of therapeutic anticoagulation (NCT04505774).26 Preliminary results showed therapeutic anticoagulation did not improve hospital survival or days free of organ support compared with usual care pharmacological thromboprophylaxis without difference in major bleeding compared with standard thromboprophylaxis.27 We performed a methodologically rigorous retrospective study to determine the incidence of major bleeding in critically ill patients with COVID‐19. First, we found that the bleeding risk in COVID‐19 was similar to that of other respiratory viral illnesses. Next, we focused on the COVID‐19 cohort to determine whether the intensity of anticoagulation received was associated with an increased risk of major bleeding. When examining only the anticoagulant received on ICU admission in an IPTW model, we did not find an effect of the intensity of anticoagulation on bleeding risk.15 However, when we accounted for frequent changes in the anticoagulation intensity after admission to the ICU, we found an increased risk of bleeding among patients on therapeutic versus standard prophylactic‐intensity anticoagulation.
Prior studies have reported an incidence of major bleeding in COVID‐19 patients of approximately 3% to 8% of all patients.11., 14., 15., 28. The preliminary results of the clinical trial on therapeutic anticoagulation in critically ill COVID‐19 patients reported a rate of major bleeding of 3.5% in the therapeutic‐intensity anticoagulation arm, though the final results are still pending.27 Compared with these studies, we found a higher proportion of COVID‐19 patients experienced major bleeding events (14.7%). This is likely because of differences in our study population and design. First, we only studied critically ill patients who likely have a higher bleeding risk than that of ward patients. Second, we also included patients who required ECMO. Third, roughly one‐half of the patients in our study received an intensity of anticoagulation higher than standard prophylaxis on admission to the ICU. This is in contrast to some studies, such as Al‐Samkari et al., where 86% of patients received standard prophylaxis.14 Last, we performed chart review to evaluate for bleeding events using the ISTH definition, which differed from some of the other studies that used administrative data or other bleeding definitions (e.g., World Health Organization definition of major bleeding). Our study findings were more consistent with a single center French study of critically ill COVID patients, where the incidence of hemorrhagic events was 21% patients.29 Overall, it is difficult to compare bleeding outcomes between studies because of these differences.
A few studies showed the risk of thrombosis is higher in COVID‐19 compared with other viral infections.30 However, to the best of our knowledge, no study compared major bleeding outcomes between COVID‐19 and patients with other viral respiratory illnesses. A nationwide cohort using administrative data reported a higher incidence of hemorrhagic stroke in patients with COVID‐19 compared to those with influenza infection.31 In our study, a similar proportion of patients in each cohort experienced an intracranial hemorrhage (1.4% in the COVID‐19 cohort and 1.6% in the ORVI cohort).
In our study, when anticoagulation was assessed as a time‐varying covariate, we found an increased bleeding risk with therapeutic anticoagulation in the COVID‐19 cohort. This is similar to findings in a non‐critically ill cohort of patients with COVID‐19, in which higher doses of anticoagulation had an incident rate for major or clinically‐relevant non‐major bleeding of 24 per 100 person‐months compared with 6.9 per 100 person‐months in patients receiving standard prophylactic‐intensity anticoagulation.32 Another study in critically ill patients with COVID‐19 found that two‐thirds of major bleeding events occurred while patients were receiving therapeutic‐intensity anticoagulation.33 Together with our study, these findings caution against the use of higher than standard prophylactic‐intensity anticoagulation while we await results of randomized trials to confirm or deny their benefit. Although we did not find an increased risk of bleeding in intermediate intensity compared with standard prophylaxis, our findings are limited by the relatively small number of patients receiving intermediate‐intensity anticoagulation. Randomized clinical trials of intermediate‐ versus therapeutic‐intensity anticoagulation in COVID‐19 are under way (NCT 04367831).
This study has several limitations. We divided anticoagulation intensity into three discrete categories. Although our classification scheme is similar to that used by the American Society of Hematology guideline on COVID‐19 and anticoagulation,18 we acknowledge that such classification is arbitrary and that, in reality, anticoagulant intensity is a continuous rather than a categorical variable. Importantly, in this retrospective study, the anticoagulation intensity was determined by treating clinicians. There are likely differences in baseline bleeding risk between patients who were treated with therapeutic‐intensity anticoagulation compared with those treated with standard thromboprophylaxis or intermediate‐intensity anticoagulation. We attempted to account for this potential bias with use of an IPTW model, which adjusted for known risk factors for bleeding. However, it is possible that there are other factors that affect the bleeding risk that are not accounted for by this method.
Moreover, anticoagulation intensity was frequently changed during a patient's hospital course, particularly in the COVID‐19 cohort. A frequent indication for escalation of anticoagulation intensity was deterioration in clinical status, which may have served as a confounding variable in our analysis because clinical deterioration may, itself, be a risk factor for major bleeding. With the classification not remaining constant over time, it was challenging to study the effect of the intensity of anticoagulation on bleeding. The majority of previous studies on COVID‐19 have not accounted for changes in anticoagulant intensity during hospital admission.14., 15., 33. We attempted to account for these changes by assessing anticoagulation intensity as a time‐varying covariate. Major bleeding is difficult to adjudicate retrospectively as event adjudication depends on the quality of EHR documentation. Decreases in hemoglobin in critical illness can be due to causes other than bleeding such as hemolysis and hemodilution.34 To minimize misclassification of bleeding events, we required documentation of bleeding in the progress notes or imaging reports and were rigorous in our adjudication of events.
In addition, we did not exclude patients who had a chronic indication for anticoagulation. It is possible that patients who had tolerated anticoagulation previously may be less likely to experience bleeding events; however, this is unknown in the setting of COVID‐19 and associated coagulopathy. Also, there are many hemostatic derangements in critical illness (such as thrombocytopenia, renal failure, and hepatic dysfunction) that may alter the tolerance of anticoagulation among patients on chronic anticoagulation; therefore, we believed it was important to include these patients in our study. In the entire study population (ORVI and COVID‐19 cohorts), 151 of 830 (18.2%) patients were on chronic anticoagulation; of those 151 patients, 115 (76.2%) received therapeutic dose anticoagulation on ICU admission. Major bleeding occurred in 109/830 (13.1%) patients in the entire study population (COVID‐19 and ORVI cohorts) compared with 23/151 (15.2%) among those who were on chronic anticoagulation at baseline. Thus, the rate of major bleeding was not lower in patients on chronic anticoagulation. Although patients with an indication for chronic anticoagulation were generally excluded from the thromboprophylaxis randomized controlled trials, we believe the inclusion of these patients is important as heightened bleeding risk may suggest consideration of temporarily withholding therapeutic anticoagulation for indications with low daily thrombotic risk (e.g., some patients with atrial fibrillation).
In this study, we did not exclude patients with an episode of major bleeding before ICU admission. Although patients could theoretically have had ongoing bleeding before admission, we believe this would be a rare event as most patients with ongoing or very recent major bleeding would have anticoagulation held and would therefore be excluded from our cohort for not receiving anticoagulation. We did not report prior episodes of major bleeding as a risk factor for bleeding in this study. With retrospective data from a single health system, we could not reliably report if a patient had a previous episode of major bleeding as they may have presented to a hospital outside of our health system. Finally, we anticipated frequent escalations in anticoagulation because many studies during our observation period reported increased thrombotic risk in critically ill patients with COVID‐19.12 Thus, we extracted from the EHR recorded reasons for escalations in anticoagulation intensity. We did not collect data on why anticoagulation intensity was de‐escalated. Possible reasons for de‐escalation include bleeding or concerns about bleeding risk, need for invasive procedures, diagnostic imaging revealing no thrombosis, or clinical improvement. Further prospective studies are needed to better describe these practice patterns.
Therapeutic anticoagulation was associated with an increased risk of major bleeding compared to standard‐intensity thromboprophylaxis in critically‐ill patients with COVID‐19. The optimal strategy for thromboprophylaxis in COVID‐19 critical illness remains unclear. This real‐world study in addition to the early reports of futility from randomized controlled trials question the safety and efficacy of therapeutic anticoagulation in ICU patients with COVID‐19.
CONFLICT OF INTEREST
A. Cuker has served as a consultant for Synergy and his institution has received research support on his behalf from Alexion, Bayer, Novartis, Novo Nordisk, Pfizer, Sanofi, and Spark. A. Pishko has received research funding from an educational grant from Sanofi Genzyme and Novo Nordisk. J. Giri has received grant funding to the institution from Boston Scientific and St Jude Medical, and served on advisory boards for Astra Zeneca and Inari Medical. C. Domenico had consulting fees from Pfizer. The remaining authors have no conflicts of interests to disclose.
AUTHOR CONTRIBUTIONS
Allyson M. Pishko and Rim Halaby contributed to the concept and design of the study, data collection and analysis, and drafted the manuscript. Adam Cuker, Jay Giri, Todd E. H. Hecht, and Steven Pugliese contributed to the concept and design of the study and critically revised the manuscript. Ann Tierney contributed to the data analysis and critical revision of manuscript. Christopher Domenico and Jennifer Yui contributed to the concept and design of the study, data collection, and critical revision of manuscript. Tara Cooper, Pardis Niami, Elizabeth Traxler, Andrew Matthews, Ella Ishaaya, and Nathalie Van der Rijst contributed to data collection and critical revision of the manuscript. Jacob Gutsche and Srinath Adusumalli contributed to the critical revision of manuscript. All authors approved the final manuscript.
ACKNOWLEDGMENTS
We acknowledge Emily Schriver, Penn Data Analytics Center (DAC), and the Institute for Biomedical Informatics Clinical Research Informatics Core (CIC) for their assistance with data extraction. This project was supported by the Penn Cardiovascular Disease Fellowship Innovation Fund. A. Pishko is supported by a 2019 HTRS Mentored Research Award supported by Sanofi Genzyme.
Hemostasis and Thrombosis Research SocietyMentored Research Award supported by Sanofi Genzym
Penn Cardiovascular Disease Fellowship Innovation Fund
Footnotes
Manuscript handled by: Walter Ageno
Final decision: Walter Ageno, 23 March 2021
REFERENCES
- 1.Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. Jama Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. New Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connors J.M., Levy J.H. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schünemann H.J., Cushman M., Burnett A.E., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group PI for the CCCTG and the Australian and NZICSCT. Cook D., Meade M., et al. Dalteparin versus unfractionated heparin in critically Ill patients. N Engl J Med. 2011;364:1305–1314. doi: 10.1056/NEJMoa1014475. [DOI] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M.j.h.a., van der Meer N.j.m., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intens Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez D., García‐Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta‐analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosovsky R.P., Sanfilippo K.M., Wang T.F., et al. Anticoagulation practice patterns in COVID‐19: a global survey. Res Pract Thrombosis Haemostasis. 2020;4:969–983. doi: 10.1002/rth2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks A.L., Auerbach A.D., Schnipper J.L., et al. COVID‐19 coagulopathy and thrombosis: analysis of hospital protocols in response to the rapidly evolving pandemic. Thrombosis Research. 2020;196:355–358. doi: 10.1016/j.thromres.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al‐Samkari H., Leaf R.S.K., Dzik W.H., et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS‐CoV2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID‐19: a single health system study. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolys. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuker A., Tseng E.K., Nieuwlaat R., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID‐19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall J.C., Murthy S., Diaz J., et al. A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Soceity on Thrombosis and Haemostatis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients: definitions of major bleeding in clinical studies. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Minor B.L., et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauzier F., Arnold D.M., Rabbat C., et al. Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intens Care Med. 2013;39:2135–2143. doi: 10.1007/s00134-013-3044-3. [DOI] [PubMed] [Google Scholar]
- 24.Decousus H., Tapson V.F., Bergmann J.‐.F., et al. Investigators for the I. factors at admission associated with bleeding risk in medical patients. Chest. 2011;139:69–79. doi: 10.1378/chest.09-3081. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M., Hajage D., Lebreton G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NIH. ACTIV Trial of blood thinners pauses enrollment of critically ill COVID‐19 patients. National Institute of Health website. December 22, 2020. Accessed on January 23, 2021. https://www.nih.gov/news‐events/news‐releases/nih‐activ‐trial‐blood‐thinners‐pa
- 27.Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation in critically ill patients with Covid‐19‐preliminary report. medRxiv. 2021 http://medrxiv.org/content/early/2021/03/12/2021.03.10.21252749.abstract [Google Scholar]
- 28.Daughety M.M., Morgan A., Frost E., et al. COVID‐19 associated coagulopathy: thrombosis, hemorrhage and mortality rates with an escalated‐dose thromboprophylaxis strategy. Thromb Res. 2020;196:483–485. doi: 10.1016/j.thromres.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID‐19 patients: a French monocenter retrospective study. Crit Care. 2020;24:275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smilowitz N.R., Subashchandran V., Yuriditsky E., et al. Thrombosis in hospitalized patients with viral respiratory infections versus COVID‐19. Am Heart J. 2020;231:93–95. doi: 10.1016/j.ahj.2020.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piroth L., Cottenet J., Mariet A.‐.S., et al. Comparison of the characteristics, morbidity, and mortality of COVID‐19 and seasonal influenza: a nationwide, population‐based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pesavento R., Ceccato D., Pasquetto G., et al. The hazard of (sub)therapeutic doses of anticoagulants in non‐critically ill patients with Covid‐19: the Padua province experience. J Thromb Haemost. 2020;18:2629–2635. doi: 10.1111/jth.15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al‐Samkari H., Gupta S., Leaf R.K., et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID‐19. Ann Intern Med. 2021 doi: 10.7326/M20-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikkert W.J., Tijssen J.G.P., Piek J.J., Henriques J.P.S. Challenges in the adjudication of major bleeding events in acute coronary syndrome: a plea for a standardized approach and guidance to adjudication. Eur Heart J. 2016;37:1104–1112. doi: 10.1093/eurheartj/ehv584. [DOI] [PubMed] [Google Scholar]