Summary
Despite the strong evidence on circadian rhythm disruption in shift workers and consequent increased vulnerability for infection, longitudinal association between shift work and COVID‐19 infection is unexplored. In this study, data from UK Biobank participants who were tested for COVID‐19 infection (16 March to 7 September 2020) were used to explore the link between shift work and COVID‐19 infection. Using the baseline occupational information, participants were categorised as non‐shift workers, day shift workers, mixed shift workers and night shift workers. Multivariable regression models were used to assess the association between shift work and COVID‐19 infection. Among the 18,221 participants (9.4% positive cases), 11.2% were health workers, and 16.4% were involved in shift‐work‐based jobs. Ethnic minorities (18%) and people in night‐shift‐based jobs (18.1%) had a significantly higher prevalence of COVID‐19 infection than others. Adjusted logistics regression model suggest that, compared with their counterparts, people employed in a night‐shift‐based job were 1.85‐fold (95% CI: 1.42–2.41) more likely to have COVID‐19 infection. Sensitivity analysis focusing on people working in a non‐healthcare setting suggests that people in shift‐work‐based jobs had 1.81‐fold (95% CI: 1.04%–3.18%) higher odds of COVID‐19 infection than their counterparts. Shift workers, particularly night shift workers, irrespective of their occupational group, seem to be at high risk of COVID‐19 infection. If similar results are obtained from other studies, then it would mandate to revisit the criteria for defining high‐risk groups for COVID‐19 and implementing appropriate interventions to protect people in shift‐based jobs.
Keywords: ethnic minorities, occupation, UK Biobank
1. INTRODUCTION
Since the emergence of coronavirus disease‐19 (COVID‐19), there has been an unprecedented surge in research identifying the risk factors associated with COVID‐19 to facilitate better prevention and management of the virus (Almazeedi et al., 2020; Lassale et al., 2020). In the list of potential risk factors, advanced age, male sex, ethnic minority background, underlying medical conditions and weakened immune systems are identified as the key factors associated with increased vulnerability for COVID‐19 infection and severity (Chudasama et al., 2020; Patel et al., 2020).
In addition to sociodemographic predisposition, some occupational groups, for example essential workers, are found to be at greater risk of COVID‐19 than their counterparts (Koh, 2020a). The high rates of COVID‐19 in frontline health workers, due to their close contact with infected patients and colleagues, is well established (Bielicki et al., 2020; Mutambudzi et al., 2020). A more in‐depth analysis of the occupational group and COVID‐19 related death data from England and Wales reveals an unfortunate and tragic divide in occupation‐based COVID‐19 death rates (Windsor‐Shellard, 2020a). Compared with managerial and professional occupations, people from working‐class jobs, such as drivers, delivery workers, aged care and retail occupations, have a proportionately higher death rate (Windsor‐Shellard, 2020b). Unfortunately, most of the people in these occupational groups come from socio‐economically disadvantaged groups who need to step out to make ends meet and do not have the choice of working from home. Therefore, the uneven distribution of COVID‐19 infection risk across various occupational groups is further contributing to the socioeconomic divide in COVID‐19 infection and death rates (Lassale et al., 2020).
While the evidence on occupation type and increased vulnerability for COVID‐19 is growing (Bielicki et al., 2020; Sikkema et al., 2020), there is a dearth of evidence regarding whether job timing, i.e. shift work, is also playing a role in COVID‐19 infection (Bai et al., 2020). This is particularly concerning knowing that shift work interferes with the immune system and predisposes to infections, and that the majority of essential workers, particularly those employed in healthcare, work shift‐based roles (Almeida & Malheiro, 2016; Scheiermann et al., 2013).
The established role of circadian rhythm disruption in hormone regulation, immune system irregularities and consequent viral infection risk strongly suggest that night shift workers may face an increased risk of COVID‐19 infection (Zhuang et al., 2017). In a recent paper, Lim et al. proposed a plausible hypothesis linking clock genes, melatonin suppression and SARS‐CoV‐2 infection in night shift workers, underscoring the need for further research to assess the risk of COVID‐19 infection in shift workers (Lim et al., 2020).
Understanding that targeted prevention is the key to manage the growing burden of COVID‐19, it is important to confirm whether shift workers have increased physiological risk of COVID‐19, above and beyond their increased exposure to the virus due to the nature of their job. The UK Biobank, a community‐based cohort of 500,000 middle‐aged adults, with the rich sociodemographic, occupation, shift work and lifestyle data, has linked the Biobank records and National Health Service COVID‐19 test results in England, and thus offers an unparalleled opportunity to explore the link between shift work, particularly night shift work, and COVID‐19. The UK Biobank data were used to address: (1) whether shift work, after controlling for other established risk factors, is associated with increased vulnerability for COVID‐19 infection; and (2) whether shift work is contributing to the high rates of COVID‐19 in ethnic minorities.
2. METHODS
2.1. Study sample
The data from the UK Biobank were used for this study. The UK Biobank is a cohort study of middle‐aged adults where a wealth of data, for example, lifestyle, medical history, nutritional habits, physical measurements, and blood and urine samples, are collected to facilitate early prevention and management of chronic conditions and life‐threatening illnesses (Allen et al., 2014). UK Biobank cohort recruitment began in 2006 (n = 500,000), with follow‐up data collected between December 2009 and June 2013 (n = 20,346) and then again between April 2014 and November 2016 (n = 35,540). Participants provided informed consent for medical records data linkage. Recently, a swift dynamic linkage was implemented to allow the UK Biobank team to provide a regular feed of COVID‐19 (SARS‐CoV‐2) test results to facilitate rapid research into the COVID‐19 infection in the cohort (Armstrong et al., 2020).
The UK Biobank study has approval from the North West Multi‐centre Research Ethics Committee, the Patient Information Advisory Group, and the Community Health Index Advisory Group. Further details on the study design, sampling, data collection and ethics committee approval are detailed elsewhere (UK Biobank, 2007).
2.2. Study measures
2.2.1. Outcome
The primary outcome for this study was a positive test result for COVID‐19 infection. The test results, covering the period from 16 March to 7 September 2020 (UK Biobank, 2020b), were provided to the UK Biobank by Public Health England (UK Biobank, 2020a). The variables related to COVID‐19 infection included: test results based on a polymerase chain reaction (PCR) test, specimen date, specimen type, laboratory and origin (whether the participant was an inpatient or not). For this study, patients were considered positive if one or more of the tests performed was positive for COVID‐19. Although the baseline cohort offered information on about 500,000 participants, for the present work, the study sample was comprised of only those participants who were tested for COVID‐19 infection.
2.2.2. Exposure
The primary exposure was employment in a shift‐work‐based job at baseline. Shift workers were identified by asking “Does your work involve shift work?”, and responses were coded as “always”, “usually”, sometimes” and “never/rarely”. Participants who reported working in a shift‐work‐based job were further asked whether this involved night shifts, defined as “Night shifts are a work schedule that involves working through the normal sleeping hours, for instance, working through the hours from 12 a.m. to 6 a.m.” Response options were “always”, “usually”, “sometimes” and “never/rarely”. Based on those two questions, shift work status was derived. Participants were categorised as non‐shift workers (who did not work shifts at all), day shift workers (who “sometimes/usually/always” worked a shift but “never/rarely” worked a night shift), mixed shift workers (who “sometimes/usually/always” worked a shift but only “sometimes” worked a night shift) and night shift workers (who “sometimes/usually/always” worked a shift and “usually/always” worked a night shift). Participants who selected “prefer not to answer” and “do not know” responses were excluded from the analysis.
2.2.3. Covariates
Based on a priori evidence (Chudasama et al., 2020; Hamer et al., 2020; Niedzwiedz et al., 2020), three groups of covariates: (i) sociodemographic factors; (ii) health variables; and (iii) sleep, from the first wave of the UK Biobank (2006–2010), were considered in the regression models assessing the role of shift work in COVID‐19 infection.
Sociodemographic factors
The following were recorded: each participant's age; gender; ethnicity categorised as “white”, and “ethnic minorities” (Black, Asian and mixed); gross annual household income classified as “< £18,000”, “£18,000–30,999”, “£31,000–51,000”, “£52,000–100,000” and “> £100,000”; education level, coded as “College or University degree”, “A levels/AS levels or equivalent”, “O levels/GCSEs or equivalent”, or “others”. Participants reported their current or most recent job title, which was converted into a four‐digit Standard Occupational Classification‐2000 code (Office for National Statistics, 2016). Participants were classified into three broad groups: frontline healthcare workers (medical doctor, general practitioner, hospital consultant, nurse, paramedic, and ambulance paramedic); other healthcare workers; and other occupational groups.
The Townsend Deprivation Index was used as a measure of socioeconomic status (Townsend, 1979). This combines census data on housing, employment, social class and car availability based on the postal code of participants. The index was categorised into quintiles based on the baseline sample, from the least deprived (quintile 1) to the most deprived (quintile 5).
Health and obesity
Satisfaction with overall health was assessed by asking “In general how would you rate your health”. Response options were “excellent”, “good”, “fair” and “poor”. Body mass index was categorised as “underweight < 18.5 kg m−2”, “normal − between ≥ 18.5 kg m−2 and < 25 kg m−2, “overweight − between ≥ 25 kg m−2 and < 30 kg m−2”, and “obese ≥ 30 kg m−2” (World Health Organization, 1997).
2.2.4. Sleep variables
The following four sleep variables were recorded: (1) sleep duration; (2) daytime sleepiness; (3) sleeplessness; and (4) snoring. Sleep duration was recorded by asking “About how many hours sleep do you get in every 24 hr?” Daytime sleepiness was assessed based on the following questions: “How likely are you to doze off or fall asleep during the daytime when you don't mean to (e.g. when working, reading or driving)”, with responses “never/rarely”, “sometimes” and “often”. Sleeplessness was assessed by asking “Do you have trouble falling asleep at night or do you wake up in the middle of the night?”, with responses “never/rarely”, “sometimes” and “usually”. Snoring was assessed by asking “Does your partner or a close relative or friend complain about your snoring”, answering with “yes” or “no”.
2.3. Statistical analysis
To compare participants' baselines characteristics for COVID infection, t‐tests (for continuous variables) and Chi‐square tests (for categorical variables) were conducted. Multivariable logistic regression models were fitted to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between shift work and COVID‐19 infection. Collinearity among covariates was examined by computing the variance inflation factor. Sensitivity analyses were conducted to assess whether the association between shift work and COVID‐19 infection is driven by the high risk of COVID‐19 infection in health workers, a majority of whom are involved in shift work. A p‐value of .05 was adopted as a significance threshold for multivariable regression. All statistical analyses were undertaken using Stata IC 15.0 (Stata Statistical Software, College Station, TX, USA).
2.4. Missing data
Among the key covariates, age and gender did not have any missing values, while others reported varying degrees of missing data, for example, Townsend Deprivation Index (0.15%), sleeplessness (0.38%), ethnicity (0.64%), health (0.89%), sleep duration (1.2%), obesity (1.4%), snoring (8.3%), education (24.3%), income (17.3%). Maximum likelihood imputations, under the assumption of missing at random, were used to handle the missing data for covariates.
3. RESULTS
The study sample comprised of 18,221 participants (total 27,784 tests) with COVID‐19 test results (9.4% positive cases). The majority of samples tested for COVID‐19 were from inpatients (73.3%) and were from combined nose/throat swabs (28.4%). As shown in Table 1, the mean age of COVID‐19‐positive patients was 57.9 years (SD ± 8.22), and the patients were more likely to be male, from ethnic minorities, and most disadvantaged groups. While sleep duration, sleeplessness, snoring and overall health were not associated with COVID‐19 infection, overweight/obesity and daytime sleepiness were associated with higher prevalence of COVID‐19 infection than their counterparts. Additionally, people working in a healthcare service as well as those employed in shift‐work‐based roles were twice more likely to report COVID‐19 infection than their counterparts.
TABLE 1.
Sociodemographic, occupation, and self‐reported sleep and health information from the UK Biobank cohort, stratified by COVID‐19 test results from 18,221 participants
| Variable | COVID‐19 Test | |||
|---|---|---|---|---|
| Total n (%) | Negative n (%) | Positive n (%) | p‐valuea | |
| Age (mean ± SD) | 57.9 years (8.22) | 58.1 years (8.1) | 56.5 years (8.09) | < .001 |
| Gender | ||||
| Female | 9,440 (51.8) | 8,624 (91.4) | 816 (8.6) | < .001 |
| Male | 8,781 (48.2) | 7,884 (89.8) | 897 (10.2) | |
| Ethnicity | ||||
| White | 16,884 (93.3) | 15,403 (91.2) | 1,481 (8.8) | < .001 |
| Ethnic minoritiesb | 1,220 (6.7) | 1,000 (82) | 220 (18) | |
| Education | ||||
| College/university | 4,985 (36.2) | 4,580 (91.9) | 405 (8.1) | .001 |
| A levels/equivalent | 1,852 (13.4) | 1,699 (91.7) | 153 (8.3) | |
| O levels/CSE/equivalent | 4,584 (33.2) | 4,147 (90.5) | 437 (9.5) | |
| Others | 2,367 (17.2) | 2,112 (89.2) | 255 (10.2) | |
| Income (£/year) | ||||
| > 52,000 | 4,463 (29.6) | 4,010 (89.8) | 453 (10.2) | .025 |
| 31,000–51,999 | 3,842 (25.5) | 3,490 (90.8) | 352 (9.2) | |
| 18,000–30,999 | 3,488 (23.14) | 3,163 (90.7) | 325 (9.3) | |
| < 18,000 | 3,278 (21.7) | 3,012 (91.1) | 266 (8.1) | |
| Townsend deprivation score | ||||
| Quintile‐1 (least deprived) | 3,297 (18.1) | 3,061 (92.8) | 236 (7.2) | < .001 |
| Quintile‐2 | 3,380 (18.6) | 3,098 (91.7) | 282 (8.3) | |
| Quintile‐3 | 3,435 (18.9) | 3,132 (91.2) | 303 (8.8) | |
| Quintile‐4 | 3,630 (19.9) | 3,264 (90) | 366 (10.0) | |
| Quintile‐5 (most deprived) | 4,451 (24.4) | 3,926 (88.2) | 525 (11.8) | |
| Sleep duration (mean ± SD) | 7.15 hr (1.23) | 7.15 hr (1.23) | 7.11 hr (1.20) | .25 |
| Sleeplessness | ||||
| Rarely/never | 3,988 (22) | 3,591 (90) | 397 (10) | .190 |
| Sometimes | 8,445 (46.5) | 7,643 (90.5) | 802 (9.5) | |
| Often | 5,718 (31.5) | 5,210 (91.1) | 508 (9.9) | |
| Snoring | ||||
| No | 10,205 (61.1) | 9,260 (90.7) | 945 (9.3) | .578 |
| Yes | 6,504 (38.9) | 5,885 (90.5) | 619 (9.5) | |
| Daytime sleepiness | ||||
| Never | 12,907 (71.6) | 11,758 (91.1) | 1,149 (8.9) | .005 |
| Sometimes | 4,416 (24.5) | 3,956 (89.6) | 460 (10.4) | |
| Often | 709 (3.9) | 633 (89.3) | 76 (10.7) | |
| Overweight/obesity | ||||
| Normal | 4,984 (27.8) | 4,581 (91.9) | 403 (8.1) | < .001 |
| Overweight | 7,539 (42) | 6,822 (90.5) | 717 (9.5) | |
| Obese | 5,436 (30.2) | 4,869 (89.6) | 567 (10.4) | |
| Overall health | ||||
| Excellent/good | 11,705 (64.8) | 10,635 (90.9) | 1,070 (9.1) | .380 |
| Fair | 4,827 (26.7) | 4,353 (90.2) | 474 (9.8) | |
| Poor | 1,527 (8.5) | 1,381 (90.4) | 146 (9.6) | |
| Occupation | ||||
| All other occupational groups | 3,291 (87.2) | 3,094 (94) | 197 (6) | < .001 |
| Healthcare | 483 (12.8) | 429 (88.8) | 52 (11.2) | |
| Shift work | ||||
| No | 6,763 (76.4) | 6,191 (91.5) | 572 (8.5) | < .001 |
| Yes | 2,087 (23.6) | 1,745 (83.6) | 342 (16.4) | |
Data are presented as % (n) or mean ± SD.
aChi2 test.
bBlack, Asian and mixed ethnicity.
Within the shift‐work‐based jobs, people who reported “often” working night shift (17.6%) and those who worked mostly night shift were at the highest risk of COVID‐19 infection (18%; Table 2). Although shift work data used in the analysis were collected in the first wave, data from the successive waves (2009–2013 and 2014–2016) indicate that shift‐worker status was generally stable. About 7 in 10 people who worked in shift‐based roles at baseline, continued to be in the shift‐based roles at the subsequent follow‐ups.
TABLE 2.
Association between shift work and COVID‐19 infection in the UK Biobank participants
| Shift work | COVID‐19 results | ||
|---|---|---|---|
| Negative n (%) | Positive n (%) | p‐value | |
| Shift‐work frequency | |||
| Rarely/never | 6,191 (91.5) | 572 (8.5) | < .001 |
| Sometimes | 713 (85.4) | 122 (14.6) | |
| Often | 1,032 (82.4) | 220 (17.6) | |
| Shift‐work type | |||
| None | 6,191 (91.5) | 572 (8.5) | < .001 |
| Mostly day shift | 815 (85.5) | 138 (14.5) | |
| Mixed day/night | 518 (82) | 114 (18) | |
| Mostly night | 909 (82) | 90 (18) | |
The regression model with only sociodemographic variables confirms that male sex, ethnic minority status and education were significant risk factors associated with higher odds of COVID‐19 infection (Model‐1; Table 3). The addition of overall health and obesity (Model‐2; Table 3) and sleep‐related variables (Model‐3; Table 3) did not affect the effect or statistical significance of gender, ethnicity or education on COVID‐19 infection. However, upon addition of the shift work variable (Model 4; Table 3), the role of gender difference became non‐significant. In the final model, after adjusting for age, gender, ethnic minority status, income, education, Townsend deprivation quintile, sleep variables, overall health and obesity, shift work emerged as strongly associated with higher odds of COVID‐19 infection. Compared with their counterparts, people employed in a night‐shift‐based job had significantly higher odds of having COVID‐19 infection (OR: 1.85; 95% CI: 1.42–2.41). In the fully adjusted model, in addition to shift work, ethnic minority status (OR: 1.42; 95% CI: 1.01–1.99) was also associated with higher odds of COVID‐19 infection. In the unadjusted model, both overweight and obesity were associated with COVID‐19 but, in the adjusted model, the effect of obesity became non‐significant.
TABLE 3.
Multivariable logistic regression results for shift work and COVID‐19 infection in the UK Biobank participants (n = results are presented as adjusted odds ratios [AOR] and 95% CIs)
| Risk factors | Model‐1 | Model‐2 | Model‐3 | Model‐4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| Age | 0.97 | 0.96–0.98 | 0.96 | 0.95–0.97 | 0.95 | 0.94–0.96 | 0.95 | 0.94–0.96 | 0.94 | 0.93–0.96 |
| Gender | ||||||||||
| Female (Ref) | ||||||||||
| Male | 1.20 | 1.05–1.38 | 1.22 | 1.03–1.45 | 1.18 | 1.01–1.40 | 1.17 | 1.00–1.38 | 1.05 | 0.83–1.34 |
| Ethnicity | ||||||||||
| White (Ref) | ||||||||||
| Ethnic minorities | 2.29 | 1.69–3.09 | 1.87 | 1.41–2.48 | 1.88 | 1.41–2.50 | 1.75 | 1.28–2.39 | 1.42 | 1.01–1.99 |
| Townsend Score | ||||||||||
| Quintile‐1 (Ref‐Least deprived) | ||||||||||
| Quintile‐2 | 1.18 | 0.95–1.47 | 1.11 | 0.87–1.42 | 1.08 | 0.85–1.37 | 1.06 | 0.84–1.35 | 1.03 | 0.77–1.38 |
| Quintile‐3 | 1.25 | 0.96–1.64 | 1.11 | 0.81–1.54 | 1.10 | 0.80–1.51 | 1.08 | 0.79–1.46 | 1.09 | 0.72–1.64 |
| Quintile‐4 | 1.45 | 1.18–1.80 | 1.22 | 0.95–1.56 | 1.20 | 0.94–1.51 | 1.17 | 0.93–1.47 | 1.20 | 0.88–1.62 |
| Quintile‐5 (Most deprived) | 1.73 | 1.29–2.33 | 1.26 | 0.89–1.78 | 1.23 | 0.88–1.72 | 1.18 | 0.85–1.64 | 1.14 | 0.77–1.68 |
| Annual household income (£) | ||||||||||
| < 18,000 (Ref) | ||||||||||
| 18,000–30,999 | 0.89 | 0.78–1.02 | 0.99 | 0.82–1.19 | 0.97 | 0.80–1.18 | 0.98 | 0.78–1.22 | 1.03 | 0.69–1.53 |
| 31,000–51,999 | 0.91 | 0.77–1.07 | 0.92 | 0.75–1.12 | 0.91 | 0.76–1.15 | 0.90 | 0.71–1.13 | 1.03 | 0.66–1.60 |
| > 52,000 | 0.78 | 0.64–0.95 | 0.78 | 0.58–1.04 | 0.77 | 0.59–1.10 | 0.78 | 0.57–1.08 | 0.90 | 0.54–1.50 |
| Education | ||||||||||
| College/Uni (Ref) | ||||||||||
| A levels/AS level or equivalent | 1.02 | 0.83–1.24 | 1.06 | 0.89–1.27 | 1.07 | 0.89–1.29 | 1.05 | 0.92–1.44 | 0.97 | 0.74–1.27 |
| O levels/GCSEs or equivalent | 1.19 | 0.97–1.45 | 1.18 | 0.96–1.43 | 1.17 | 0.95–1.42 | 1.20 | 0.99–1.45 | 1.11 | 0.89–1.44 |
| Others | 1.36 | 1.16–1.60 | 1.43 | 1.20–1.70 | 1.43 | 1.22–1.68 | 1.49 | 1.26–1.77 | 1.27 | 1.03–1.58 |
| Overweight/obesity | ||||||||||
| Normal (Ref) | ||||||||||
| Overweight | 1.19 | 1.05–1.36 | 1.24 | 1.10–1.39 | 1.22 | 1.12–1.33 | 1.19 | 1.01–1.41 | ||
| Obese | 1.32 | 1.16–1.50 | 1.28 | 1.10–1.49 | 1.30 | 1.11–1.52 | 1.19 | 0.97–1.46 | ||
| Overall health | ||||||||||
| Excellent/good (Ref) | ||||||||||
| Fair | 1.08 | 0.95–1.23 | 0.99 | 0.84–1.18 | 0.97 | 0.82–1.14 | 1.01 | 0.83–1.21 | ||
| Poor | 1.05 | 0.87–1.26 | 0.87 | 0.67–1.12 | 0.84 | 0.60–1.17 | 1.16 | 0.65–2.07 | ||
| Sleep duration | 0.98 | 0.94–1.01 | 0.98 | 0.92–1.03 | 0.98 | 0.93–1.04 | ||||
| Sleeplessness | ||||||||||
| Rarely/never (ref) | ||||||||||
| Sometimes | 0.95 | 0.89–1.01 | 0.96 | 0.86–1.06 | 0.97 | 0.86–1.09 | ||||
| Often | 0.88 | 0.81–0.96 | 0.86 | 0.74–1.01 | 0.92 | 0.78–1.08 | ||||
| Snoring | ||||||||||
| No (ref) | ||||||||||
| Yes | 1.03 | 0.90–1.18 | 0.94 | 0.80–1.09 | 0.95 | 0.79–1.15 | ||||
| Daytime sleepiness | ||||||||||
| Never (ref) | ||||||||||
| Sometimes | 1.19 | 1.06–1.32 | 1.19 | 1.02–1.39 | 1.30 | 1.01–1.63 | ||||
| Often | 1.23 | 0.86–1.75 | 1.07 | 0.64–1.78 | 1.22 | 0.72–2.14 | ||||
| Shift‐work type | ||||||||||
| None (ref) | ||||||||||
| Mostly day shift | 1.83 | 1.57–2.13 | 1.54 | 1.30–1.81 | ||||||
| Mixed day/night | 2.38 | 1.90–2.98 | 1.79 | 1.38–2.33 | ||||||
| Mostly night | 2.38 | 1.86–3.04 | 1.85 | 1.42–2.41 | ||||||
Model‐1: concurrent consideration of all sociodemographic variables.
Model‐2: Model 1 + overweight/obesity, overall health.
Model‐3: Model 2 + sleep duration, sleep problems, daytime sleepiness.
Model‐4: Model 3 + shiftwork.
Significant associations are highlighted in bold.
AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
3.1. Sensitivity analysis
Although health workers were found to have a significantly higher risk of COVID‐19 infection than other occupational groups (11.2% versus 6%), and a significantly higher proportion of the healthcare workforce was involved in shift‐based jobs than in other occupational groups (24% versus 11.6%), this did not confound the association between shift work and COVID‐19 infection. Shift workers, irrespective of whether they were working in a healthcare setting (14.4%) or other occupational groups (13%), reported significantly higher rates of COVID‐19 infection than their counterparts (Figure 1). Sensitivity analysis, focusing on people working in a non‐healthcare setting, suggests that people in shift‐work‐based jobs had 1.81‐fold (95% CI: 1.04%–3.18%) higher odds of COVID‐19 infection than their counterparts.
FIGURE 1.
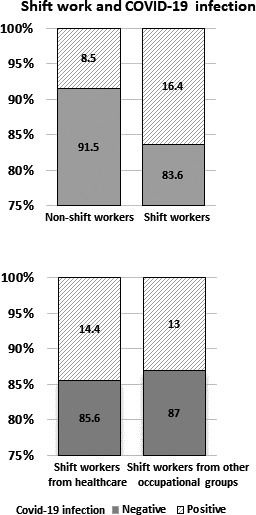
Association between shift work and COVID‐19 infection based on the UK Biobank data
3.2. Covid‐19 infection risk in shift‐working ethnic minorities
People from ethnic minorities, who were more involved in shift‐work‐type jobs than their counterparts (32.2% versus 16.3%), were also at higher risk of COVID‐19 infection (18% versus 8.8%). Regression analysis indicated that, compared with White British people in non‐shift‐based jobs, ethnic minorities in shift‐based jobs had 2.43‐fold (95% CI: 1.70–3.47) higher odds of having COVID‐19 infection.
4. DISCUSSION
This paper reports on data from over 18,000 participants of the UK Biobank, who have had at least one test for COVID‐19. Our findings suggest that shift workers, particularly night shift workers, are at a greater risk of COVID‐19 infection. While the extant literature primarily focuses on the increased risk of COVID‐19 in essential workers, specifically front line health workers (Nguyen et al., 2020), other occupational groups who work shift‐based roles also have a comparable risk for COVID‐19 infection. Evidence from other research suggests that people working in the retail and hospitality industries, transport and security workers, have significantly higher rates of COVID‐19 infection due to their frequent interaction with people with suspected or confirmed COVID‐19 (Koh, 2020b). In addition to ensuring the availability and adequacy of personal protective equipment to healthcare workers, the focus on infection control strategies, safe work practices and staff training on infection prevention is essential to prevent occupational exposure and transmission of COVID‐19 infection. For people employed in shift‐based roles, it is important to offer appropriate education on sleep health and hygiene, to ensure safety and health in and outside of the workplace. Acknowledging the pervasive impact of COVID‐19 on population sleep, the Society of Behavioural Sleep Medicine convened a task force to disseminate key information in addressing sleep concerns and help individuals in protecting their, as well as others’, sleep during this pandemic (Crew et al., 2020). In addition to maintaining appropriate sleep hygiene, the task recommends that people working in long shifts who are not getting enough sleep at night should take naps of 15–30 min, which might help in physical and mental wellbeing (Crew et al., 2020).
We should consider at least two possible mechanisms that explain the observed risks of shift work. First, Lim and colleagues have postulated that disruption of circadian rhythms resulting from night‐shift working could predispose someone to be more at risk of infection with COVID‐19 (Lim et al., 2020). This could be a function of reduced melatonin levels, and abnormal immune cell and cytokine levels. The role of sleep deprivation on the immune response is also highlighted in the work of Prather et al., who found that short sleep is associated with increased susceptibility to colds (Prather et al., 2015). The work by Prather et al. suggests that circadian rhythms exert substantial regulatory effects on the immune system, hence complete or partial sleep deprivation triggers functional changes relevant to host resistance, manifesting in the form of poor immunological response (Besedovsky et al., 2012). Whilst this is plausible given the evidence of links between sleep disruption, circadian rhythm and a diverse range of physiological systems, we should also consider behavioural mechanisms that might explain the link. In addition to the physiological effects of sleep disruption, we also know that poor sleep quality has a diverse range of adverse effects on cognitive functioning, particularly executive function and self‐regulation (Waters & Bucks, 2011). Prevention of infection by COVID‐19 requires persistent enactment of a diverse range of behaviours to reduce the risk of exposure to the virus. This requires consistent self‐regulation of our behaviour, a capability that is diminished by the chronically poor quality sleep that is a function of regular night shift work (Rouch et al., 2005).
Though weakened executive functioning and self‐regulation seem to be plausible behavioural mechanisms by which shift work could increase someone's risk of catching COVID‐19, one might also argue that such higher‐order mental skills operate through simpler facets of cognitive abilities such as vigilance, attentional capacity, etc. (Lim & Dinges, 2010). There is other evidence highlighting that sleepiness is a key factor for the attention deficits in patients with sleep apnea (Verstraeten et al., 2004). Therefore, while weakened executive functioning and self‐regulation might contribute to the increased risk of COVID‐19 in shift workers, there seem to be other factors driving the role of shift work in COVID‐19 infection.
In addition to physiological and behavioural mechanisms, a potential explanation may be that night shift work is a marker for lower socioeconomic status, which is associated with increased vulnerability for COVID‐19 infection. If one looks at the hospital at night, the medical care is limited to emergency care only; senior staff are much thinner on the ground at night; and the majority of the staff are those who are working at lower levels. Therefore, the effect of night shift work on COVID‐19 infection could be driven by socioeconomic disparities in the health workforce. While this explanation seems plausible, considering that in our analysis the association between night shift work and COVID‐19 infection risk was robust to adjustment for socioeconomic status, weakens the argument that shift work and COVID‐19 infection risk is primarily driven by socioeconomic disadvantages.
Though daytime sleepiness had some role in COVID‐19 infection, sleep duration did not seem to be associated with increased risk of COVID‐19 infection. However, it is worth mentioning that sleep duration (assessed by self‐reported hours of sleep) does not capture the inter‐individual variability in sleep needs between individuals. The inter‐individual variations in the accumulation of sleep need, as well as the dissipation of sleep need, are influenced by factors that significantly vary between individuals, for example, circadian, behavioural and environmental factors (Klerman & Dijk, 2005). In future research, in addition to enquiring about sleep duration, information on perceived sleep needs will help in contrasting the differences between perceived sleep needs and actual hours of sleep. The mismatch between sleep needs and sleep hours would be a better indicator of sleep deprivation and help in the robust assessment of the association between sleep loss and infection vulnerability.
This is the first study highlighting the link between shift work and COVID‐19, and takes into account a range of sociodemographic covariates associated with increased vulnerability for COVID‐19 infection. In addition to being a well‐characterised prospective cohort, the UK Biobank provides objective data for COVID‐19 status. Nonetheless, there are a few caveats that should be mentioned. First, the UK Biobank cohort is not representative of the general UK population and, being an observational study, the results are prone to bias. However, we used a sound analytical approach and adjusted for a range of established risk factors in our analyses. Second, we recognise that the data on shift work were not obtained at the same time as exposure to COVID‐19. These data were obtained from the UK Biobank some years before the pandemic started. However, we do know that being in shift work is a relatively stable variable across the different waves of the UK Biobank, with 67% of individuals remaining in the same category across 10 years of the study. Alternatively, the data may represent the long‐term effects of shift work, rather than short‐term effects of working on shifts. The effect could possibly be argued to be a function of exposure. Although the small sample size for sensitivity analysis is a limitation, nonetheless the effect of shift work was seen in both health workers and non‐health workers. While shift workers may be more common amongst service industries, increasing the risk of exposure to people who may have had COVID‐19, this would not account for the effect of shift work for health workers. Arguably, night shift health workers would be working when there is reduced activity in the system and possibly less risk of exposure to COVID‐19. Third, one can critique the analysis here for not being a clear systematic process for COVID‐19 testing in the UK. However, we have examined relationships only in those who had a test, which reflects an ecologically valid examination of the data. Obviously, this analysis needs to be replicated and tested in other data sets from other countries, but given it is based on testing an a priori hypothesis provided by another group of researchers, it would be sensible to consider these results as identifying a valid marker of risk.
Though this is the first study to establish a link between shift work and COVID‐19, evidence from other studies across the globe is needed to validate our results. If similar results are obtained from other studies, then it would mandate to revisit the criteria for defining high‐risk groups, and developing and implementing appropriate policies and protocols to protect people in shift‐work‐based jobs from not just COVID‐19 but all other poor health outcomes consequent to disrupted circadian rhythms.
5. CONCLUSION
The findings of this paper provide preliminary but strong evidence for the role of shift work in COVID‐19 infection. Thus, when we are considering risk factors for COVID‐19 and/or vulnerability for the condition, which may prompt testing, shift work might be one of the additional factors to be considered for timely intervention and management of at‐risk patients. This study is based on the most recent data from the UK Biobank and over 35% of the study participants were tested more than once; nonetheless, considering that the pandemic is still ongoing, many of the individuals who were negative at the time of the data analysis might not still be negative. The change in COVID infection status might affect the strength of the association between shift work and COVID‐19 infection. Therefore, as more data become available, an addendum to the current work will be useful in the current estimation of the impact of shift work on COVID‐19 infection risk.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All authors were involved in designing the study protocol, developing the analytical approach, interpreting the data and critical review of the paper. YF and AAM conducted the analysis; YF, RB and TS drafted the manuscript; and all authors helped in revising and improving the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This research was conducted using the UK Biobank data (UK Biobank application number 19705). The authors acknowledge and thank the UK Biobank participants and investigators for making this study possible.
Fatima Y, Bucks RS, Mamun AA, et al. Shift work is associated with increased risk of COVID‐19: Findings from the UK Biobank cohort. J Sleep Res. 2021;30:e13326. 10.1111/jsr.13326
DATA AVAILABILITY STATEMENT
The UK Biobank data are available upon request subject to data access request approval.
REFERENCES
- Allen, N. E. , Sudlow, C. , Peakman, T. , & Collins, R. (2014). UK biobank data: Come and get it. Science Translational Medicine, 6(224), 224ed4. 10.1126/scitranslmed.3008601 [DOI] [PubMed] [Google Scholar]
- Almazeedi, S. , Al‐Youha, S. , Jamal, M. H. , Al‐Haddad, M. , Al‐Muhaini, A. , Al‐Ghimlas, F. , & Al‐Sabah, S. (2020). Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID‐19 in Kuwait. EClinicalMedicine, 24, 100448. 10.1016/j.eclinm.2020.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, C. M. O. , & Malheiro, A. (2016). Sleep, immunity and shift workers: A review. Sleep Science, 9(3), 164–168. 10.1016/j.slsci.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. , Rudkin, J. K. , Allen, N. , Crook, D. W. , Wilson, D. J. , Wyllie, D. H. , & O’Connell, A. M. (2020). Dynamic linkage of COVID‐19 test results between Public Health England’s Second Generation Surveillance System and UK Biobank. Microbial Genomics, 6(7), mgen000397. 10.1099/mgen.0.000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Wang, X. , Huang, Q. , Wang, H. , Gurarie, D. , Ndeffo‐Mbah, M. , Fan, F. , Fu, P. , Horn, M. A. , Xu, S. , Mondal, A. , Jiang, X. , & Zhao, H. (2020). SARS‐CoV‐2 infection in health care workers: A retrospective analysis and a model study. In: medRxiv. [DOI] [PMC free article] [PubMed]
- Besedovsky, L. , Lange, T. , & Born, J. (2012). Sleep and immune function. Pflugers Archiv: European Journal of Physiology, 463(1), 121–137. 10.1007/s00424-011-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielicki, J. A. , Duval, X. , Gobat, N. , Goossens, H. , Koopmans, M. , Tacconelli, E. , & van der Werf, S. (2020). Monitoring approaches for health‐care workers during the COVID‐19 pandemic. The Lancet Infectious Diseases, 20(10), e261–e267. 10.1016/S1473-3099(20)30458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama, Y. V. , Gillies, C. L. , Appiah, K. , Zaccardi, F. , Razieh, C. , Davies, M. J. , Yates, T. , & Khunti, K. (2020). Multimorbidity and SARS‐CoV‐2 infection in UK Biobank. Diabetes & Metabolic Syndrome, 14(5), 775–776. 10.1016/j.dsx.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew, E. C. , Baron, K. G. , Grandner, M. A. , Ievers‐Landis, C. E. , McCrae, C. S. , Nadorff, M. R. , Nowakowski, S. , Ochsner Margolies, S. , & Hansen, K. (2020). The Society of Behavioral Sleep Medicine (SBSM) COVID‐19 Task Force: Objectives and summary recommendations for managing sleep during a pandemic. Behavioral Sleep Medicine, 18(4), 570–572. 10.1080/15402002.2020.1776288 [DOI] [PubMed] [Google Scholar]
- Hamer, M. , Kivimäki, M. , Gale, C. R. , & Batty, G. D. (2020). Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: A community‐based cohort study of 387,109 adults in UK. Brain, Behavior, and Immunity, 87, 184–187. 10.1016/j.bbi.2020.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman, E. B. , & Dijk, D.‐J. (2005). Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep, 28(10), 1253–1259. 10.1093/sleep/28.10.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, D. (2020a). Occupational risks for COVID‐19 infection. Occupational Medicine, 70(1), 3–5. 10.1093/occmed/kqaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassale, C. , Gaye, B. , Hamer, M. , Gale, C. R. , & Batty, G. D. (2020). Ethnic disparities in hospitalisation for COVID‐19 in England: The role of socioeconomic factors, mental health, and inflammatory and pro‐inflammatory factors in a community‐based cohort study. Brain, Behavior, and Immunity, 88, 44–49. 10.1016/j.bbi.2020.05.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J. , & Dinges, D. F. (2010). A meta‐analysis of the impact of short‐term sleep deprivation on cognitive variables. Psychological Bulletin, 136(3), 375–389. 10.1037/a0018883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, R. K. , Wambier, C. G. , & Goren, A. (2020). Are night shift workers at an increased risk for COVID‐19? Medical Hypotheses, 144, 110147. 10.1016/j.mehy.2020.110147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutambudzi, M. , Niedzwiedz, C. L. , Macdonald, E. B. , Leyland, A. H. , Mair, F. S. , Anderson, J. J. , Celis‐Morales, C. A. , Cleland, J. G. , Forbes, J. , Gill, J. M. R. , Hastie, C. E. , Ho, F. K. , Jani, B. D. , Mackay, D. F. , Nicholl, B. I. , O'Donnell, C. A. , Sattar, N. , Welsh, P. , Pell, J. P. , … Demou, E. (2020). Occupation and risk of COVID‐19: Prospective cohort study of 120,621 UK Biobank participants. In: medRxiv. [DOI] [PMC free article] [PubMed]
- Nguyen, L. H. , Drew, D. A. , Graham, M. S. , Joshi, A. D. , Guo, C.‐G. , Ma, W. , Mehta, R. S. , Warner, E. T. , Sikavi, D. R. , Lo, C.‐H. , Kwon, S. , Song, M. , Mucci, L. A. , Stampfer, M. J. , Willett, W. C. , Eliassen, A. H. , Hart, J. E. , Chavarro, J. E. , Rich‐Edwards, J. W. , … Zhang, F. (2020). Risk of COVID‐19 among front‐line health‐care workers and the general community: A prospective cohort study. The Lancet Public Health, 5(9), e475–e483. 10.1016/S2468-2667(20)30164-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiedz, C. L. , O’Donnell, C. A. , Jani, B. D. , Demou, E. , Ho, F. K. , Celis‐Morales, C. , Nicholl, B. I. , Mair, F. S. , Welsh, P. , Sattar, N. , Pell, J. P. , & Katikireddi, S. V. (2020). Ethnic and socioeconomic differences in SARS‐CoV‐2 infection: Prospective cohort study using UK Biobank. BMC Medicine, 18(1), 160. 10.1186/s12916-020-01640-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics . (2016). Standard occupational classification (SOC 2000) and NS‐SEC on the labour force survey. Retrieved from https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/methodologies/standardoccupationalclassificationsoc2000andnsseconthelabourforcesurvey#soc‐2000 [Google Scholar]
- Patel, A. P. , Paranjpe, M. D. , Kathiresan, N. P. , Rivas, M. A. , & Khera, A. V. (2020). Race, socioeconomic deprivation, and hospitalization for COVID‐19 in English participants of a national biobank. International Journal for Equity in Health, 19(1), 114. 10.1186/s12939-020-01227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, A. A. , Janicki‐Deverts, D. , Hall, M. H. , & Cohen, S. (2015). Behaviorally assessed sleep and susceptibility to the common cold. Sleep, 38(9), 1353–1359. 10.5665/sleep.4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch, I. , Wild, P. , Ansiau, D. , & Marquié, J.‐C. (2005). Shiftwork experience, age and cognitive performance. Ergonomics, 48(10), 1282–1293. 10.1080/00140130500241670 [DOI] [PubMed] [Google Scholar]
- Scheiermann, C. , Kunisaki, Y. , & Frenette, P. S. (2013). Circadian control of the immune system. Nature Reviews Immunology, 13(3), 190–198. 10.1038/nri3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema, R. S. , Pas, S. D. , Nieuwenhuijse, D. F. , O'Toole, Á. , Verweij, J. , van der Linden, A. , Chestakova, I. , Schapendonk, C. , Pronk, M. , Lexmond, P. , Bestebroer, T. , Overmars, R. J. , van Nieuwkoop, S. , van den Bijllaardt, W. , Bentvelsen, R. G. , van Rijen, M. M. L. , Buiting, A. G. M. , van Oudheusden, A. J. G. , Diederen, B. M. , … Koopmans, M. P. G. (2020). COVID‐19 in health‐care workers in three hospitals in the south of the Netherlands: A cross‐sectional study. The Lancet Infectious Diseases, 20(11), 1273–1280. 10.1016/S1473-3099(20)30527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, P. (1979). Poverty in the United Kingdom. Allen Lane and Penguin Books’. [Google Scholar]
- UK Biobank . (2007). UK Biobank: Protocol for a large‐scale prospective epidemiological resource. Retrieved from http://www.ukbiobank.ac.uk/wp‐content/uploads/2011/11/UK‐Biobank‐Protocol.pdf [Google Scholar]
- UK Biobank . (2020a). COVID‐19 test results data. Retrieved from http://biobank.ndph.ox.ac.uk/ukb/exinfo.cgi?src=COVID19_tests [Google Scholar]
- UK Biobank . (2020b). Current and future availability of COVID‐19 related health outcome data. Retrieved from http://biobank.ndph.ox.ac.uk/showcase/exinfo.cgi?src=COVID19_availability [Google Scholar]
- Verstraeten, E. , Cluydts, R. , Pevernagie, D. , & Hoffmann, G. (2004). Executive function in sleep apnea: Controlling for attentional capacity in assessing executive attention. Sleep, 27(4), 685–693. [PubMed] [Google Scholar]
- Waters, F. , & Bucks, R. S. (2011). Neuropsychological effects of sleep loss: Implication for neuropsychologists. Journal of the International Neuropsychological Society, 17(4), 571–586. 10.1017/s1355617711000610 [DOI] [PubMed] [Google Scholar]
- Windsor‐Shellard, B. (2020a). Coronavirus (COVID‐19) related deaths by occupation, England and Wales. Retrieved from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/datasets/coronaviruscovid19relateddeathsbyoccupationenglandandwales [Google Scholar]
- Windsor‐Shellard, B. (2020b). Coronavirus (COVID‐19) related deaths by occupation, England and Wales: deaths registered between 9 March and 25 May 2020. Retrieved from https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/coronaviruscovid19relateddeathsbyoccupationenglandandwales/latest [Google Scholar]
- World Health Organization . (1997). Obesity: Preventing and managing the global epidemic. Retrieved from Switzerland. [PubMed] [Google Scholar]
- Zhuang, X. , Rambhatla, S. B. , Lai, A. G. , & McKeating, J. A. (2017). Interplay between circadian clock and viral infection. Journal of Molecular Medicine, 95(12), 1283–1289. 10.1007/s00109-017-1592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The UK Biobank data are available upon request subject to data access request approval.


