Abstract
Background
Coronavirus disease 2019 (COVID‐19) primarily causes lung infection, but recent studies have shown that cardiac involvement is associated with a worse prognosis.
Objectives
We conducted a systematic review and meta‐analysis to examine the prevalence of cardiac arrhythmias detected by the electrocardiogram and their relationships with adverse outcomes in patients with COVID‐19.
Methods
PubMed and Google were searched for studies that reported on cardiac arrhythmias and/or examined the relationship between arrhythmias and adverse outcomes.
Results
Thirty studies with 12,713 participants were included in the systematic review, and 28 studies (n = 12,499) in the meta‐analysis. The mean age was 61.3 ± 16.8 years; 39.3% were female. In 25 studies with 7578 patients, the overall prevalence of cardiac arrhythmias was 10.3% (95% confidence interval [CI]: 8.4%–12.3%). The most common arrhythmias documented during hospitalization were supraventricular arrhythmias (6.2%, 95% CI: 4.4%–8.1%) followed by ventricular arrhythmias (2.5%, 95% CI: 1.8%–3.1%). The incidence of cardiac arrhythmias was higher among critically ill patients (relative risk [RR]: 12.1, 95% CI: 8.5–17.3) and among non‐survivors (RR: 3.8, 95%, CI: 1.7–8.7). Eight studies reported changes in the QT interval. The prevalence of QTc > 500 ms was 12.3% (95% CI: 6.9%–17.8%). ST‐segment deviation was reported in eight studies, with a pooled estimate of 8.7% (95% CI: 7.3% to 10.0%).
Conclusion
Our meta‐analysis showed that QTc prolongation, ST‐segment deviation, and various other cardiac arrhythmias were observed in patients hospitalized with COVID‐19. The presence of cardiac arrhythmias was associated with a worse prognosis.
Keywords: atrial fibrillation, COVID‐19, ECG, ventricular tachyarrhythmias
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a potentially life‐threatening infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 SARS‐CoV‐2 utilizes the angiotensin‐converting enzyme 2 as the host receptor to gain entry into the cell. Recent studies have reported significant cardiac involvement in patients infected with COVID‐19, 3 , 4 and this presence has been associated with a worse prognosis. 5 , 6 The surface electrocardiogram (ECG) is one of the leading tools to assess potential cardiac involvement in hospitalized patients with COVID‐19. 4 Existing data shows that cardiac arrhythmias area common complication of a COVID‐19 infection, although their association with adverse outcomes remains to be fully defined. 7 The aim of our systematic review and meta‐analysis was to estimate the prevalence of ECG abnormalities and cardiac arrhythmias in hospitalized patients with COVID‐19 and to further evaluate the association of arrhythmias with patient outcomes.
2. METHODS
2.1. Study design and data sources
This systematic review and meta‐analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 8 The protocol was registered in the PROSPERO database of systematic reviews (registration number: CRD42020184448). PubMed was searched for studies that reported the prevalence of cardiac arrhythmias detected by the ECG and/or reported the relationship between cardiac arrhythmias and adverse outcomes in COVID‐19. The search was performed from database inception to August 30, 2020 without language restrictions. Search terms used were: “COVID” AND (“electrocardiogram” OR “ECG” OR “QT” OR “fibrillation” OR “arrhythm*” OR “LBBB” OR “RBBB” OR “bundle branch block” OR “QRS”). Gray literature and bibliographies of included studies were also searched to extend the search coverage. The exclusion criteria were: case reports, case series, reviews, preclinical studies, and preprints publications. Once duplicates were removed, two reviewers (YL and SGZ) independently screened titles and abstracts to ensure the capture of all relevant studies. Disagreements were resolved by discussion to achieve consensus.
2.2. Data extraction and quality assessment
Data were extracted into predetermined tables using a standardized protocol. The data extracted were: first author, country of study, number of included patients, study design, age of participants, gender, prevalence of risk factors, previous cardiovascular events, history of arrhythmias, pharmacological treatments for COVID‐19, type of ECG changes or arrhythmias, complications, and mortality during hospitalization for COVID‐19.
Two reviewers (GB and GT) independently completed a risk of bias assessment using the Newcastle–Ottawa scale (NOS). 9 The NOS point scoring scale uses a star system in which each study is judged based on three domains: selection of the study groups (four items); comparability of the groups (one item); and exposure (ascertainment of the outcome; three items). A study can be awarded a maximum of one star for each numbered item within the selection and exposure categories, and a maximum of two stars for comparability.
2.3. Patient and public involvement
We performed this meta‐analysis based on published data, and there were no patients or public involvement in this project.
2.4. Statistical analysis
For the outcomes of interest, mean differences with 95% confidence interval (CI) were extracted and subsequently pooled. 10 If the standard error (SE) or 95% CIs were not reported, calculations were made using the following formula:
SE = √ prevalence (1‐prevalence) / n, &
95% CI = p ± 1.96 X SE
where “n” is the sample size.
Heterogeneity across studies was determined using the variance between the studies (Tau‐square, τ²) and the I² statistic. The I² statistic, determined from the standard chi‐square test, describes the total variance explained by heterogeneity rather than chance. I 2 > 50% was considered to reflect significant statistical heterogeneity. If I 2 < 50%, a fixed effects model was used; otherwise, the random‐effects model using the inverse variance heterogeneity method was used. 11 , 12 , 13 To identify the source of the heterogeneity, sensitivity analysis using the leave‐one‐out method was used. To assess for possible publication bias, funnel plots, Begg's, and Egger's test were used. 14 Data analysis was performed using Review Manager (RevMan) (Version 5.3) and Stata (Version 13.0).
3. RESULTS
3.1. Study selection
The search identified 488 records. After the removal of duplicates (n = 37), 451 records were screened based on titles and abstracts. Of these, 316 records were excluded because they were editorials, position papers from scientific societies, or reviews. Subsequently, full texts of 135 articles were reviewed. Of these, 102 articles were non related to the topic of interest; three articles were excluded because the ECG data were out of the scope of this review[15‐17]; Pavri et al. 15 were excluded because they evaluated the PR interval in hospitalized patients with COVID‐19; Shi et al. 16 were excluded because they described the frequencies of patients with abnormal ECG findings but did not further delineate the abnormalities observed; and Lei et al. 17 were excluded because they included all patients who underwent elective surgeries, but the diagnosis of COVID‐19 was made after the procedure. Finally, 30 studies were included in the qualitative review (Figure 1). Of these, 19 studies reported relevant ECG and/or arrhythmia findings. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 The other 11 studies only specified cardiac arrhythmias in general without further elaboration. 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 For the meta‐analysis, two studies (from the 30 included studies) were excluded as they only provided qualitative data on QT intervals. 30 , 32 The quality assessment of the included studies are summarized in Table S1 .
FIGURE 1.
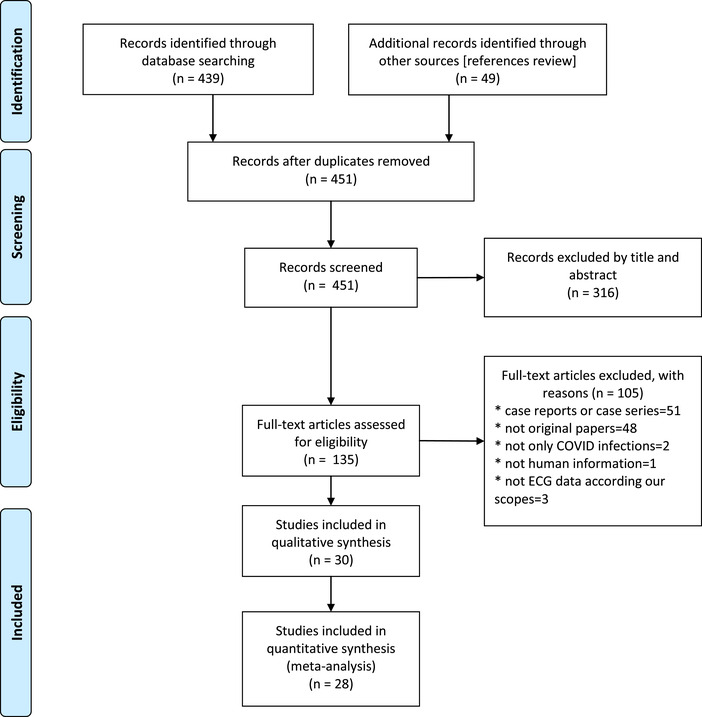
Flow diagram of included studies [Color figure can be viewed at wileyonlinelibrary.com]
Key messages
What is already known about this subject?
Coronavirus disease‐2019 (COVID‐19) is a novel viral infection with a wide spectrum of presentations, ranging from asymptomatic or mild symptomatic to severe forms. Cardiac involvement during COVID‐19 has been associated with significant morbidity and mortality. However, the frequency of cardiac arrhythmias or electrocardiographic abnormalities and their prognostic implications in patients with COVID‐19 remains uncertain.
What does this study add?
We found that new‐onset cardiac arrhythmias, ST‐segment deviation, and QTc prolongation were common findings in hospitalized patients with COVID‐19. These findings were seen in about one in 10 patients and occurred more frequently in critical care patients. Supraventricular arrhythmias were the most common, but any types of arrhythmias were associated with a worse prognosis.
How might this impact clinical practice?
Our findings suggest that the electrocardiogram can be a useful tool in the risk stratification of hospitalized patients with COVID‐19. Telemetry can also be considered for all critically ill patients to detect new‐onset cardiac arrhythmias.
3.2. Characteristics of the included studies and study participants
We included 30 studies with 12,713 participants. The sample size varied from 16 to 5700 patients (median: 128 patients, interquartile range: 90–380). The mean age was 61.3 ± 16.7 years, and 42.0% were female. All of the included studies were cohort studies, with the exception of Borba et al. 20 who reported the findings of a randomized controlled trial. Most studies originated from the United States (n = 13) and China (n = 11). All but one study 34 included adult patients, with most participants over 40 years of age. Samuel et al. 34 included pediatric and adolescent patients, with a mean age of 13 ± 6 years.
All studies reported medical comorbidities. The most common was hypertension (45%), followed by obesity (34%), and diabetes mellitus (24%). Obesity was most commonly reported among patients from the United States. 18 , 19 , 25 , 27 , 28 , 29 , 32 , 33 , 37 , 41 , 42 , 43 Overall, 11% of the patients had coronary artery disease. There were variations in the mortality rates amongst the studies (12% ± 2%, range: 0%–27.2%). There were variations in the disease severity of COVID‐19—ranging from stable to severe or critical patients requiring mechanical ventilation or cardiac support—and differences in the treatments offered (Tables 1 and 2).
TABLE 1.
Demographic and baseline characteristics of included studies with electrocardiographic data
| Author | Sample size | Country | Age | Female sex | Risk factors/Comorbidities | Prior CV events and Arrhythmias | Treatments | Severity / evolutions | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Angeli F 18 | 50 | Italy | 64 ± 15 | 38% | BMI 26.8 ± 4,4 kg/m 2 ; 50% HTN; 12% DM; 2% COPD; 10% smoker | 10% CAD; 6% CHF; |
82% HCQ 56% Macrolides 54% antiviral |
4% ICU | None |
| Bhatla A 19 | 700 | United States | 50 ± 18 | 55% | BMI 31 ± 9 kg/m 2 ; 50% HTN; 26% DM; 9% COPD; 11% CKD; 9% smoker |
11% CAD; 13% CHF; 6% AF |
25% HCQ 8% RDV |
11% ICU; 8% remained hospitalized | 4% |
| Borba MGS 20 | 81 | Brazil | 51.1 ± 13.9 | 24.7% | 45.5% HTN; 25.5% DM; 7.4% CKD; 8.3% smoker | 3.6% CAD; 3.6% CHF; 1.9% Atrioventricular block |
100% Chloroquine 100% Macrolides 86.4% antiviral |
45.7% ICU | 27.2% |
| Chen Q 21 | 54 | China |
56.1 ± 13.5 61.7 ± 9.6 ƛ |
33.3% | 29.6% HTN; 46.3% DM; 0% COPD | 11.1% CAD; 0% CHF; 1.9% CeVD; 1.9% AF | NA | 27.8% critical patients 1 | NA |
| Chorin E 22 | 251 | United States & Italy | 64 ± 13 | 25% | 54% HTN; 27% DM; 7% COPD; 11% CKD | 12% CAD; 3% CHF |
100% HCQ 100% Macrolides |
NA | 20% |
| Colon CM 23 | 115 | United States | 56 ± 17 | 46% | 70% HTN; 39% DM; 13% COPD; 14% CKD; 42% smoker € | 16% CAD; 4.4% AF |
6.1% HCQ 43.5% Macrolides 7% RDV |
60% ICU | 4.4% |
| Deng Q 24 | 112 | China |
65 (49–70.8) |
49.1% | 32.1% HTN; 17% DM; 3.6% COPD | 13.4% CAD; 3.6% AF | NA | 23.2% ICU; 54.5% remained hospitalized | 12.5% |
| Goyal P 25 | 393 | United States |
62,2 (48.6–73.7) |
39.4% | 35.8% obesity; 50.1% HTN; 25.2% DM; 5.1% COPD; 5.1% smoker | 13,7% CAD |
64% HCQ 4.3% RDV |
33.1% mechanical ventilation | 10.2% |
| Guo T 26 | 187 | China | 58.5 ± 14.7 | 51.3% | 32.6% HTN; 15% DM; 3.2% CKD | 11.2% CAD; 4.3% CHF |
98% antibiotics 89% antiviral |
24.1% mechanical ventilation | 23% |
| Jain S 27 | 459 | United States | 68.2 ± 15.2 | 37.9% | 16.5% morbid obesity; 59.2% HTN; 48.5% DM; 31.1% CKD | 18.4% CAD; 15.5% CHF; 13.6% CeVD; 19.4% AF/AFL |
90.4% HCQ 2.9% Macrolides 20.5% antiviral *5.7% RDV* |
58.3% ICU during hospitalization | NA |
| McCullough SA 28 | 756 | United States | 63.3 ± 16 | 36.8% | 37.3% obesity; 56.5% HTN; 29.4% DM; 18.8% pulmonary disease; 9.5% renal disease | 14.4% CAD; 7.3% CHF; 7.3% CeVD | None | NA | 11.9% |
| Mercuro NJ 29 | 90 | United States | 60.1 ± 16.7 | 48.9% | BMI 31.5 ± 6.6 kg/m 2 ; 53.3% HTN; 28.9% DM; 20% COPD | 11.1% CAD; 10% CHF; 13.3% AF |
100% HCQ 58.9% Macrolides |
33.3% ICU; 50% remained hospitalized | 4.4% |
|
Öztürk F 30 |
91 £ | Turkey | 49.2 ± 16.7 | 43.1% | 11.8% HTN; 11.7% DM; other risk factors excluded | Prior CV disease and arrhythmias excluded | NA | NA | 5.9% |
| Ramireddy A 31 | 98 | United States | 62.3 ± 17 | 39% | 60% HTN; 22% DM; 26% COPD; 14% CKD | 20% CHF; prior arrhythmias excluded |
72.5% HCQ 89.8% Macrolides |
49% ICU | NA |
|
Rath D 32 |
123 | Germany | 68 ± 15 | 37.4% | 19.5% obesity; 69.9% HTN; 24.4% DM; 11.4% CKD; 0.8% smoker € |
22.8% CAD 22.8% AF |
NA | 45.5% ICU | 13% |
| Sala S 33 | 132 | Italy | 65 ± 14 | NA | 14% obesity; 45% HTN; 20% DM; 6% COPD |
7% CAD 12% AF; 0,9% PSVT |
100% HCQ 100% Macrolides |
All clinically stable patients | NA |
| Samuel S 34 | 36 | United States | 12.6 ± 6 | 44,4% | 5.5% obesity; 17% malignancy; 8% asthma; 11% sickle cell; | 55.5% on home medications; 8% cerebral palsy /seizures |
69.4% HCQ 25% Macrolides 5.6% RDV |
58% ICU | 2.8% |
| Si D 35 | 1159 | China | 61.5 (32–69) vs 64 (24–70) Ω | 45,3% | 55.9% HTN; 21.8% DM; 5.3% CKD; 6.5% COPD | 17.7% CAD; 3.5% CeVD | NA 2 | NA | 16% |
| Yenercag M 36 | 75 | Turkey | 55.5 ± 17.1 | 48% | BMI 24.1 ± 3.5 kg/m 2 ; 52% HTN; 36% DM; 37% smoker | CAD, CHF, AF, CeVD and CKD were excluded | NA | NA | NA |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; BMI, body mass index; CAD, coronary artery disease; CeVD, cerebrovascular disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular.; CVD, cardiovascular disease; DM, diabetes mellitus; HCQ, hydroxycloroquine; HTN, hypertension; ICU, intensive care units; IQR, interquartile range; NA, not available; PSVT, paroxysmal supraventricular tachycardia; RDV, Remdesivir; vs, versus.
Any one of the following: respiratory failure and an artificial airway required for invasive mechanical ventilation; shock; combining failure of other organs which requires ICU monitoring and treatment.
Reported only for a subset of patients.
Severe and critical patients, respective.
*Could be slightly overestimated due to some treatments were reported in combination.
Current or former smoker.
51 COVID‐19 patients and 40 controls.
Discharged alive and died in hospital, respective.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
TABLE 2.
Demographic and baseline characteristics of included studies without electrocardiographic data
| Author | Sample size | Country | Age | Female sex | Risk factors / Comorbidities | Prior CV events | Treatments | Severity / evolutions | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Aggarwal S 37 | 16 | United States |
67 (38–95) |
25% | 50% obesity; 57% HTN; 31% DM; 38% CKD; 13% COPD; 0% smoker | 19% CAD; 25% CHF; 13% CeVD |
69% HCQ 43% Macrolides |
50% ICU | 19% |
| Cao J 38 | 102 | China |
54 (37–67) |
48% | BMI IQR 21.8–26; 27.5% HTN; 10.8% DM; 3.9% CKD; 9.8% respiratory disease | 4.9% CVD disease; 5.9% CeVD |
99% antibiotics 98% antiviral |
17.6% ICU | 16.7% |
| Du Y 39 | 85 | China | 65.8 ± 14.2 | 27.1% | 37.6% HTN; 22.4% DM; 3.5% CKD; 2.4% COPD | 11.8% CVD; 8.2% CeVD |
90.6% antibiotics 91.8% antiviral |
Include all fatal cases in 2 hospitals | 100% |
| Hou W 40 | 101 | China | 50.9 ± 20.1 | 56.4% | 20.8% HTN; 5.9% DM; 4% COPD | 10.9% CAD; 3% CeVD | 34.7% antiviral | 10% ICU | 5% |
| Mani VR 41 | 184 | United States | 64.7 ± 14.9 | 39.7% | 38.6% obesity; 65.8% HTN; 43.5% DM; 17.4% CKD; 9.2% COPD | 20.1% CAD |
70.7% HCQ 61.4% Macrolides |
16.3% mechanical ventilation | 17.4% |
| Richardson S 42 | 5700 | United States | 63 (52–75) | 39.7% | 41.7% obesity; 56.6% HTN; 33.8% DM; 5% CKD; 5.4% COPD; 15.6% smoker€ | 11.1% CAD; 6.9% CHF | NA | 14.2% ICU; 53.8% remained hospitalized | 21% |
| Rosenberg ES 43 | 1438 | United States | 63.4 (IQR NA) | 40.3% | 30.5% obesity; 56.8% HTN; 35.1% DM; 13% CKD; 18% respiratory disease | 12% CAD; 6.7% CHF |
69.9% HCQ 65.8% Macrolides |
22.8% ICU | 20.3% |
| Wang D 44 | 138 | China | 56 (42–68) | 45.7% | 31.2% HTN; 10.1% DM; 2.9% CKD; 2.9% COPD | 14.5% CVD; 5.1% CeVD |
18.1% Macrolides 89.9% antiviral |
26.1% ICU; 61.6% remained hospitalized | 4.3% |
| Wang L 45 | 339 | China | 69 (65–76) | 51% | 40.8% HTN; 16% DM; 3.8% CKD; 6.2% COPD | 15.7% CVD; 6.2% CeVD | NA | NA | 19.2% |
| Zeng JH 46 | 416 | China |
45 (33–57) 64 (60–68)¥ |
52.4% | 14.4% HTN; 5.5% DM; 0.5% CKD; 1.2% COPD |
3.1% CAD; 1% prior arrhythmias |
NA | 8.4% ICU | 0.7% |
| Zhang G 47 | 221 | China |
55 (39–66.5) |
51.1% | 24.4% HTN; 10% DM; 2.7% CKD; 2.7% COPD | 10% CVD; 6.8% CeVD | 88.7% antiviral | 19.9% ICU | 5.4% |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CeVD, cerebrovascular disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular.; CVD, cardiovascular disease; DM, diabetes mellitus; HCQ, hydroxychloroquine; HTN, hypertension; ICU, intensive care units; IQR, interquartile range; NA, not available; RDV, Remdesivir.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In our meta‐analysis of 28 studies, 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 a total of 12,499 patients (mean age: 61.3 ± 16.8 years; 39.3% female) were included. Prior history of cardiac arrhythmias was only reported in 10 studies, 19 , 20 , 21 , 23 , 24 , 27 , 29 , 32 , 33 , 46 which showed a prevalence of 8.7% ± 2.5% (Tables 1 and 2). Atrial fibrillation was the most observed arrhythmia in eight of the mentioned studies.
The overall prevalence of cardiac arrhythmias during COVID‐19 hospitalization was 10.3% (95% CI: 8.4%–12.3%) amongst 25 studies (n = 7578), though there was substantial heterogeneity between the studies (I 2 = 100%) (Figure 1A). Sensitivity analysis, excluding data from pediatric and adolescent patients, did not significantly alter the overall frequency of arrhythmias (10%, 95% CI: 8.1%–12%) (Figure S1).
Specific types of arrhythmias were reported by 15 studies (Table 3). The most common was supraventricular tachyarrhythmias (n = 3395 from nine studies, 6.2%, 95% CI: 4.4%–8.1%; I 2 = 100%) (Figure 1B), 18 , 19 , 21 , 23 , 25 , 28 , 33 , 34 , 35 followed by ventricular tachyarrhythmias (n = 3485 from 11 studies, 2.5%, 95% CI: 1.8%–3.1%; I 2 = 100%) (Figure 1C). 19 , 20 , 21 , 22 , 25 , 26 , 27 , 29 , 34 , 35 , 36 Bradyarrhythmias were the least common (n = 1560 from four studies, 1.8%, 95% CI: 1.0%–2.5%; I 2 = 100%) (Figure S2). 18 , 19 , 21 , 28
TABLE 3.
Arrhythmias observed in the included studies
| Study | Supraventricular | Ventricular | Bradyarrhythmias | Combined |
|---|---|---|---|---|
| AngeliF 18 | 3 AF | – | 1 tachy/brady | – |
| BhatlaA 19 | 25 incident AF | 9 cardiac arrests, 10 NSVTs | 9 bradyarrhythmias | – |
| BorbaMGS 20 | – | 2 VT | – | – |
| Chen Q 21 | 1 AF 1 | 3 VT | 2 complete AV block | – |
| ChorinE 22 | – | 1 TdP | – | – |
| Colon CM 23 | 12 AF, 6 AFL, 1AT | – | – | – |
| Goyal P 25 | 28* | 1 VT | – | – |
| Guo T 26 | – | 11 VT/VF | – | – |
| Jain S 27 | – | 2 VT | – | – |
| McCullough SA 28 | 42 AF or AFL | – | 1 complete AV block | – |
| Mercuro NJ 29 | – | 1 TdP | – | – |
| Sala S 33 | 8 AF, 3 AT & 1 PSVT | – | – | – |
| Samuel S 34 | 1 AT | 5 VT | – | – |
| Si D 35 | 22 AT/AF | 3 VT/VF | – | 3 AT/AF + VT/VF |
| Yenercag M 36 | – | 2 VT | – | – |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia.; AV block, atrioventricular block; NSVT, non sustained ventricular tachycardia; tachy/brady, tachycardia‐bradycardia syndrome; TdP, torsade de pointes; VF, ventricular fibrillation; VT, ventricular tachycardia.
*Declared as “Atrial arrhythmia”.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.3. ST‐segment changes and QT interval prolongation
Changes in the QT interval during COVID‐19 hospitalization were reported by 16 studies (Table 4). 18 , 20 , 22 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 41 , 42 , 43 The most frequent criteria to define substantial QTc prolongation was QTc duration > 500 ms when the QRS duration was < 120 ms and QTc > 550 ms when the QRS duration was ≥120 ms. Due to disparities in the reported data, only six studies (n = 4812) were included in our meta‐analysis for QT prolongation. 18 , 20 , 22 , 29 , 31 , 42 The overall prevalence of QTc > 500 ms was 12.3% (95% CI: 6.9%–17.8%; I 2 = 100%) (Figure 2A).
TABLE 4.
QTc information in the included studies
| Author | Baseline QTc (ms) | SD (+/‐) | Comments |
|---|---|---|---|
| AngeliF 18 | 428 | 26 ms | 1 patient with QTc > 500 ms |
| BorbaMGS 20 | 424.7 | 27.4 | 15.1% QTc > 500 ms |
| ChorinE 22 | 439 | 29 | 23% QTc > 500 ms |
| Jain S 27 | 22.4% patients with QT prolongation £ . No episodes of torsades de pointes. 2 episodes of VT. | ||
| Mani VR 41 | Isolated QT prolongation in 3.8% of the patients and sinus tachycardia with QT prolongation in 1.9% of the patients | ||
| McCullough SA 28 | 449 | 144 ms | No differences in QTc between survivors and non‐survivors of COVID‐19. |
| MercuroNJ 29 | 455 | 430–474 | 20% QTc > 500 ms |
| ÖztürkF 30 | 410.4 | 24.5 ms | 3 patients died, all with QTc < 430 ms |
| RamireddyA 31 | 448 | 29 | Prolonged QTc in 12% of the patients post‐treatment (QTc > 500 ms if QRS < 120 ms, and QTc > 550 ms if QRS ≥120 ms OR QTc > 60 ms from baseline) |
| Rath D 32 | 445 | 33 ms | No differences in QTc between survivors and non‐survivors of COVID‐19 |
| Richardson S 42 | 6.1% of patients with QTc > 500 ms over 4250 patients (from automated ECG reading) | ||
| Rosenberg ES 43 | 11% of QTc prolongation with HCQ+AZN; 14.4% QTc prolongation with HCQ alone; 7.1% QTc prolongation with AZN alone & 5.9% QTc prolongation with neither drug (p = .006) | ||
| Sala S 33 | No patients with QTc interval > 450 ms, despite drugs administered for COVID‐19 treatment | ||
| Samuel S 34 | 412 | 19 ms | Use of HCQ with or without AZN was associated with QTc prolongation (411 ± 19 ms vs. 426 ± 15 ms, p < .0001). QTc was not different in patients with and without arrhythmias (425 ± 15 ms vs. 425 ± 15 ms, p = 1.0) |
| Si D 35 | QTc interval was measured in 35 patients and was prolonged by an average of 45 ms in those treated with QT‐prolonging medications (455 ms [423–480] vs. 410 ms [364–430], p = .01). Fatal VT/VF occurred in6 patients, but only 2 had ECGs recorded before death (1 with QTc prolongation and the other with normal QTc) | ||
| Yenercag M 36 | 411.1 | 23.9 ms | Patients using QT‐prolonging medications were excluded |
Abbreviations: AZN, Azithromycin; HCQ, Hydroxychloroquine; ms, milliseconds; QTc, corrected QT interval; SD, standard deviation; VF, ventricular fibrillation.; VT, ventricular tachycardia.
QTc interval > 470 ms for QRS duration ≤120 ms or QTc interval > 500 ms for QRS duration > 120 ms.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
FIGURE 2.
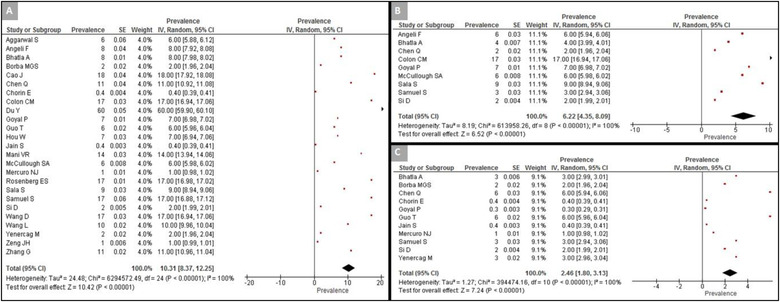
Forest plot of the prevalence of arrhythmias in hospitalized patients with COVID‐19. (A) Prevalence of arrhythmias in analyzed studies; (B) prevalence of supraventricular arrhythmias; (C) prevalence of ventricular arrhythmias [Color figure can be viewed at wileyonlinelibrary.com]
The presence or absence of ST‐segment deviation was reported in eight studies (n = 1598) with a pooled estimate of 8.7% (95% CI: 7.35%–10.0%; I 2 = 100%) (Figure 2B). 18 , 21 , 23 , 24 , 27 , 34 , 37
3.4. Relationship between cardiac arrhythmias and adverse outcomes
Eight studies reported the relationship between new‐onset cardiac arrhythmias and adverse outcomes in patients with COVID‐19 (n = 2112, 56.1 ± 17.8 years, 46.4% female). 19 , 21 , 23 , 25 , 36 , 44 , 46 , 47 Regarding the definition of disease severity, Bhatla et al., 19 Colon et al., 23 Yenercag et al., 36 Wang et al., 44 and Zeng et al. 46 classified disease severity based on ICU admission, whereas Goyal et al. 25 classified it based on the need for mechanical ventilation. Chen et al. 21 and Zhang et al. 47 classified disease severity based on ICU admission and/or other clinical parameters, including oxygen saturation ≤93%, respiratory rate ≥30 times per minute, or severe respiratory distress (Figure 3).
FIGURE 3.
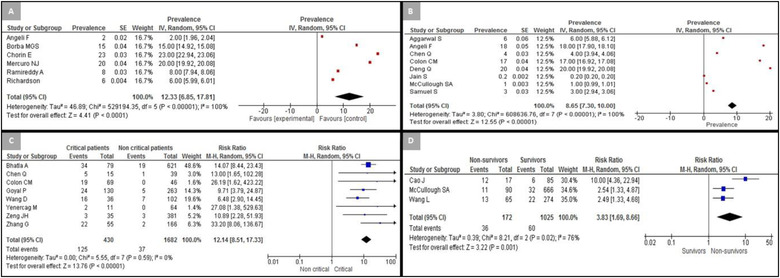
Forest plot for QT prolongation, ST prevalence, and arrhythmias among hospitalized patients with COVID‐19. (A) Prevalence of corrected QT > 500 ms; (B) prevalence of ST‐segment deviation; (C) prevalence of arrhythmias in critical and non‐critical patients; (D) prevalence of arrhythmias in survivors and non‐survivors of COVID‐19 [Color figure can be viewed at wileyonlinelibrary.com]
Critically ill patients showed a higher risk of developing cardiac arrhythmias compared to those who were not critically ill (risk ratio [RR]: 12.1, 95% CI: 8.5–17.3; I 2 = 0%) (Figure 2C), both for ventricular arrhythmias (RR: 10.5, 95% CI: 3.9–27.9; I 2 = 0%) (Figure S3) and supraventricular arrhythmias (RR: 10.1, 95% CI: 5.7–17.2; I 2 = 0%) (Figure S4).
The relationship between cardiac arrhythmias and inpatient mortality was only reported by three studies (n = 1197, 62 ± 16 years old, 45.0% female). 28 , 36 , 45 Non‐survivors were more likely to develop cardiac arrhythmias compared to survivors during their inpatient stay (RR: 3.8, 95% CI: 1.7–8.7; I 2 = 76%) (Figure 2D). Of these three studies, only one reported the specific type of arrhythmias observed, which was supraventricular arrhythmias observed in all patients. 28 In this study, non‐survivors were also found to be at a higher risk of developing supraventricular arrhythmias (RR: 2.3, 95% CI: 1.2–4.5) (Figure S5).
In Figure 4, we have summarized the main findings our study relating to cardiac arrhythmias and ECG abnormalities among hospitalized COVID‐19.
FIGURE 4.
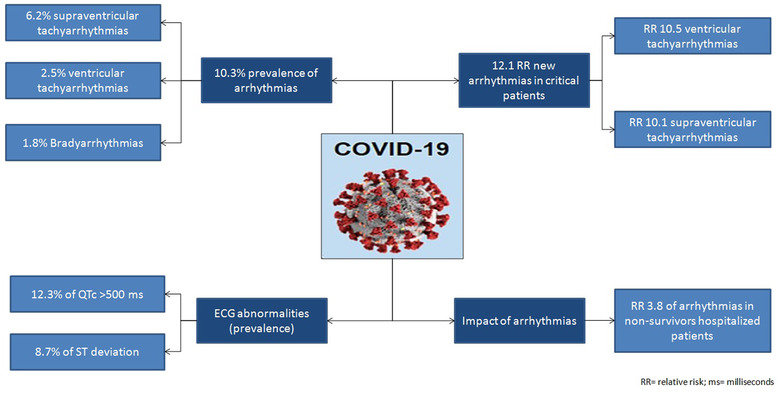
Cardiac arrhythmias and electrocardiographic abnormalities in hospitalized patients with COVID‐19 [Color figure can be viewed at wileyonlinelibrary.com]
Regarding potential publication bias between analyzed studies, a visual inspection of funnel plots suggested an asymmetric distribution of the occurrence of cardiac arrhythmias in hospitalized COVID‐19 patients (Figure S6). The Begg's test suggested no significant publication bias (z = 1.38; p = .17). The Egger test demonstrated significant asymmetry (t‐value −3.0; p = .006) (Figure S7). Sensitivity analysis, excluding one study at a time (leave‐one‐out method), did not reduce the heterogeneity of the results.
4. DISCUSSION
The main findings of this systematic review and meta‐analysis are: (i) cardiac arrhythmias are a common complication among hospitalized COVID‐19 patients, and (ii) cardiac arrhythmias can be considered a marker of worsening prognosis.
To the best of our knowledge, this is the largest meta‐analysis reporting on the prevalence of ECG findings in hospitalized COVID‐19 patients. We found that the frequency of cardiac arrhythmias showed a wide range across the studies, which could be attributable to the differences in the patients' comorbidities and variations seen across disease severity and treatments offered. In our study, premature beats were excluded in order to avoid an overestimation of the clinically significant arrhythmias. Among specific arrhythmias reported across the studies, supraventricular arrhythmias were the most frequent, followed by ventricular arrhythmias. In contrast, bradyarrhythmias were the least observed arrhythmias. Furthermore, the risk of cardiac arrhythmias was higher among non‐survivors and critically ill patients hospitalized with COVID‐19.
ST‐segment changes were the most frequently reported ECG finding. This is a significant ECG abnormality caused by different pathologies such as pericarditis, Takotsubo cardiomyopathy, and acute coronary syndrome. Additionally, QTc prolongation is a significant concern during COVID‐19 infection and could be largely attributable to drugs that cause delayed repolarization. 3 However, we were unable to fully evaluate the prognostic implications of ST‐segment changes and QT prolongation due to the significant disparities in the reported data across the studies.
Cardiac involvement in COVID‐19 has a wide spectrum, and contemporary high sensitivity troponin tests might be elevated in critically ill patients even without apparent myocardial involvement. 48 , 49 Nevertheless, previous studies have linked cardiac involvement with a worse prognosis in COVID‐19. 50 ECG abnormalities are intrinsically related to cardiac pathology, and our findings are in agreement with these observations.
4.1. Limitations
Our study has important limitations. Firstly, about half of the included articles did not report the types of arrhythmias observed. We were, therefore, unable to accurately estimate the incidence and prevalence of electrocardiographic abnormalities in hospitalized patients with COVID‐19. Secondly, we could not investigate the relationship between specific types of arrhythmias and the severity of COVID‐19 infection. This information was not consistently reported across the included studies, likely because most studies did not examine cardiac arrhythmias as a specific risk factor for adverse outcomes. Finally, we found high heterogeneity for most comparisons except for the occurrence of arrhythmias in critically ill patients. Nevertheless, such heterogeneity could have a clinical origin due to the differences in the study cohorts amongst the included studies.
5. CONCLUSION
Our systematic review and meta‐analysis showed that QTc prolongation, ST‐segment deviation, and other forms of cardiac arrhythmias were observed in patients hospitalized with COVID‐19. The presence of cardiac arrhythmias was associated with a worse prognosis. Future studies are needed to explore the possible role of arrhythmias in relation to patient outcomes.
CONFLICT OF INTEREST
Nothing to declare.
ETHICAL APPROVAL
Our meta‐analysis was based on published data and did not require approval from an Ethical committee.
AUTHOR CONTRIBUTIONS
Conception and design of the project:
Gary Tse, Sebastian Garcia‐Zamora, George Bazoukis, Adrian Baranchuk.
Data collection:
Sebastian Garcia‐Zamora, Sharen Lee, Sohaib Haseeb, George Bazoukis, Gary Tse, Jesus Alvarez‐Garcia, Enes Elvin Gul, Göksel Çinier, Bryce Alexander, Marcelo Martins Pinto‐Filho.
Data analysis and interpretation:
Sebastian Garcia‐Zamora, Sharen Lee, Gary Tse, George Bazoukis, Jesus Alvarez‐Garcia, Göksel Çinier, Tong Liu, Adrian Baranchuk.
Drafting the article:
Sebastian Garcia‐Zamora, Sharen Lee, Sohaib Haseeb, George Bazoukis, Gary Tse, Jesus Alvarez‐Garcia, Enes Elvin Gul, Göksel Çinier, Bryce Alexander, Marcelo Martins Pinto‐Filho.
Critical revision of the article:
Gary Tse, George Bazoukis, Tong Liu, Adrian Baranchuk.
Final approval of the version to be published:
Sebastian Garcia‐Zamora, Sharen Lee, Sohaib Haseeb, George Bazoukis, Gary Tse, Jesus Alvarez‐Garcia, Enes Elvin Gul, Göksel Çinier, Bryce Alexander, Marcelo Martins Pinto‐Filho, Tong Liu, Adrian Baranchuk
Responsibles for the overall content as guarantors:
Sebastian Garcia‐Zamora, George Bazoukis, Gary Tse, Adrian Baranchuk.
Supporting information
Supporting information
ACKNOWLEDGEMENTS
The present systematic review and meta‐analysis has not received any grants or financial support.
Garcia‐Zamora S, Lee S, Haseeb S. Arrhythmias and Electrocardiographic findings in Coronavirus disease 2019: a systematic review and meta‐analysis. Pacing Clin Electrophysiol. 2021;44:1062–1074. 10.1111/pace.14247
Registration: PROSPERO ID: CRD42020184448
DATA AVAILABILITY STATEMENT
The data that support the findings of this systematic review and meta‐analysis are available in the studies listed in references.
REFERENCES
- 1. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X, Guan B, Su T, Wei L, et al. Impact of cardiovascular disease and cardiac injury on in‐hospital mortality in patients with COVID‐19: a systematic review and meta‐analysis. Heart. 2020;106:1142‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haseeb S, Gul EE, Çinier G, et al. Value of electrocardiography in coronavirus disease 2019 (COVID‐19). J Electrocardiol. 2020;62:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID‐19. Circulation. 2020;141:1930‐1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of Coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831‐840. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Roever L, Tse G, Liu T. 2019‐Novel Coronavirus‐related acute cardiac injury cannot be ignored. Curr Atheroscler Rep. 2020;22:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jain V, Yuan J‐M. Predictive symptoms and comorbidities for severe COVID‐19 and intensive care unit admission: a systematic review and meta‐analysis. Int J Public Health. 2020;65:533‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells A, Shea B, O'Connell D, et al. The Newcastle‐ Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspn.d
- 10. Wang X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. www.cochrane‐handbook.orgn.d. Updated March 2011. [Google Scholar]
- 15. Pavri BB, Kloo J, Farzad D, Riley JM. Behavior of the PR interval with increasing heart rate in patients with COVID‐19. Heart Rhythm. 2020;17:1434‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine. 2020;21:100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Angeli F, Spanevello A, De Ponti R, et al. Electrocardiographic features of patients with COVID‐19 pneumonia. Eur J Intern Med. 2020;78:101‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhatla A, Mayer MM, Adusumalli S, et al. COVID‐19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. [DOI] [PubMed] [Google Scholar]
- 21. Chen Q, Xu L, Dai Y, et al. Cardiovascular manifestations in severe and critical patients with COVID‐19. Clin Cardiol. 2020;43:796‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID‐19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colon CM, Barrios JG, Chiles JW, et al. Atrial Arrhythmias in COVID‐19 Patients. JACC Clin Electrophysiol. 2020;6:1189‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID‐19: evidence from front‐line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal P, Choi JJ, Pinheiro LC. Clinical Characteristics of COVID‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jain S, Workman V, Ganeshan R, et al. Enhanced electrocardiographic monitoring of patients with Coronavirus Disease. Heart Rhythm. 2019;17:1417‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCullough SA, Goyal P, Krishnan U, Choi JJ, Safford MM, Okin PM. Electrocardiographic findings in coronavirus disease‐19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26:626‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1036‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Öztürk F, Karaduman M, Çoldur R, İncecik Ş, Güneş Y, Tuncer M. Interpretation of arrhythmogenic effects of COVID‐19 disease through ECG. Aging Male. 2020:1‐4. 10.1080/13685538.2020.1769058. [DOI] [PubMed] [Google Scholar]
- 31. Ramireddy A, Chugh H, Reinier K, et al. Experience with hydroxychloroquine and azithromycin in the Coronavirus Disease 2019 Pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9:e017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rath D, Petersen‐Uribe Á, Avdiu A, et al. Impaired cardiac function is associated with mortality in patients with acute COVID‐19 infection. Clin Res Cardiol. 2020:1‐9. 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sala S, Peretto G, De Luca G. Low prevalence of arrhythmias in clinically stable COVID‐19 patients. Pacing Clin Electrophysiol. 2020;43(8):891‐893. 10.1111/pace.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samuel S, Friedman RA, Sharma C, et al. Incidence of arrhythmias and electrocardiographic abnormalities in symptomatic pediatric patients with PCR‐positive SARS‐CoV‐2 infection, including drug‐induced changes in the corrected QT interval. Heart Rhythm. 2020;17:1960‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Si D, Du B, Ni L, et al. Death, discharge and arrhythmias among patients with COVID‐19 and cardiac injury. CMAJ. 2020;192:E791‐E798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yenerçağ M, Arslan U, Doğduş M, et al. Evaluation of electrocardiographic ventricular repolarization variables in patients with newly diagnosed COVID‐19. J Electrocardiol. 2020;62:5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aggarwal S, Garcia‐Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID‐19): early report from the United States. Diagnosis (Berl). 2020;7:91‐96. [DOI] [PubMed] [Google Scholar]
- 38. Cao J, Tu W‐J, Cheng W, et al. Clinical features and short‐term outcomes of 102 patients with Coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 from Wuhan. a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou W, Zhang W, Jin R, Liang L, Xu B, Hu Z. Risk factors for disease progression in hospitalized patients with COVID‐19: a retrospective cohort study. Infect Dis (Lond). 2020;52:498‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mani VR, Kalabin A, Valdivieso SC, Murray‐Ramcharan M, Donaldson B. At the epicenter of the American Coronavirus outbreak ‐ New York inner city hospital COVID‐19 experience and current data: a retrospective analysis. J Med Internet Res. 2020;22:e20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city Area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York state. JAMA. 2020;323:2493‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80:639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng JH, Wu W‐B, Qu JX, et al. Cardiac manifestations of COVID‐19 in Shenzhen, China. Infection. 2020;48(6):861‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Momtazmanesh S, Shobeiri P, Hanaei S, Mahmoud‐Elsayed H, Dalvi B. Malakan Rad E. Cardiovascular disease in COVID‐19: a systematic review and meta‐analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt Heart J. 2020;72:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60:2427‐2463. [DOI] [PubMed] [Google Scholar]
- 50. Li JW, Han TW, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta‐analysis. Prog Cardiovasc Dis. 2020;63:518‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data that support the findings of this systematic review and meta‐analysis are available in the studies listed in references.


