Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic calls for rapid actions, now principally oriented to a world‐wide vaccination campaign. In this study we verified if, in individuals with a previous SARS‐CoV‐2 infection, a single dose of messenger RNA (mRNA) vaccine would be immunologically equivalent to a full vaccine schedule in naïve individuals. Health care workers (184) with a previous SARS‐CoV‐2 infection were sampled soon before the second dose of vaccine and between 7 and 10 days after the second dose, the last sampling time was applied to SARS‐CoV‐2 naïve individuals, too. Antibodies against SARS‐CoV‐2 were measured using Elecsys Anti‐SARS‐CoV‐2 S immunoassay. The study was powered for non‐inferiority. We used non parametric tests and Pearson correlation test to perform inferential analysis. After a single vaccine injection, the median titer of specific antibodies in individuals with previous coronavirus disease 2019 was 30.527 U/ml (interquartile range [IQR]: 19.992–39.288) and in subjects with previous SARS‐CoV‐2 asymptomatic infection was 19.367.5 U/ml (IQR: 14.688–31.353) (p = .032). Both results were far above the median titer in naïve individuals after a full vaccination schedule: 1974.5 U/ml (IQR: 895–3455) (p < .0001). Adverse events after vaccine injection were more frequent after the second dose of vaccine (mean: 0.95; 95% confidence interval [CI]: 0.75–1.14 vs. mean: 1.91; 95% CI: 1.63–2.19) (p < .0001) and in exposed compared to naïve (mean: 1.63; 95% CI: 1.28–1.98 vs. mean: 2.35; 95% CI: 1.87–2.82) (p = .015). In SARS‐CoV‐2 naturally infected individuals a single mRNA vaccine dose seems sufficient to reach immunity. Modifying current dosing schedules would speed‐up vaccination campaigns.
Keywords: COVID‐19, humoral immunity, SARS‐CoV‐2, spike m‐RNA vaccine, spike RBD
Highlights
1) Individuals COVID 19 positive shows a single dose antibody titers 10 fold higher than naive individuals with full vaccination schedule
2) In SARS‐CoV‐2 naturally infected individuals a single mRNA vaccine dose seems sufficient to reach immunity.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which was identified in China in December 2019, causes coronavirus disease 2019 (COVID‐19), a severe, acute respiratory syndrome with a complex, highly variable disease pathology. The severe and worldwide effect of the pandemic on human society calls for rapid actions, now principally oriented to a world‐wide vaccination campaign.
Two SARS‐CoV‐2 spike messenger RNA (mRNA) vaccines received emergency use authorization by the Food and Drug Administration in December 2020 (BNT162b2/Pfizer; mRNA‐1273/Moderna). 1 Both Phase 3 trials on these vaccines reported high efficacy in preventing symptomatic SARS‐CoV‐2 infections after two doses of the vaccine administered 3–4 weeks apart in subjects without previous SARS‐CoV‐2 infection. 2 , 3 However, little is known about subjects with previous exposure to SARS‐CoV‐2. Anecdotally, individuals with pre‐existing immunity experience more severe reactogenicity after the first doses compared to naïve individuals.
The present study was designed to verify if, in individuals with a previous SARS‐CoV‐2 infection, a single administration of BNT162b2/Pfizer vaccine would elicit an immunological response superimposable to a full vaccine schedule in naïve individuals, assuming that, for individuals with pre‐existing immunity to SARS‐CoV‐2, the first vaccine dose could immunologically resemble the booster dose in naïve individuals.
2. METHODS
The study was carried out on 184 health care workers. Individuals with a previous COVID‐19 symptomatic disease or a SARS‐CoV‐2 asymptomatic infection were sampled for measuring SARS‐CoV‐2 antibody responses soon before the second dose of vaccine and between 7 and 10 days after the second dose, the last sampling time was applied to SARS‐CoV‐2 naïve individuals, too.
Antibodies against SARS‐CoV‐2 in serum samples were measured using Elecsys Anti‐SARS‐CoV‐2 S immunoassay (Roche Diagnostics GmbH, Sandhofer Strasse 116, D‑68305 Mannheim) for the in vitro quantitative determination of antibodies (including immunoglobulin G) to the SARS‐CoV‐2 spike (S) protein receptor binding domain (RBD), 4 , 5 according to the manufacturer's instructions. Briefly, all serum samples were determined with ≥0.8 U/ml by the Elecsys Anti‐SARS‐CoV‐2 S test and the values above the measurement range (0.40 > 250 U/ml) were quantified using the recommended “Diluent Universal” on Cobas e602 analyzer. As reported by Roche Diagnostics, the specific U/ml units of the Roche Elecsys Anti‐SARS‐CoV‐2 S test can be considered equivalent to the binding antibody units (BAU)/ml of the first World Health Organization (WHO) International Standard for anti‐SARS‐CoV‐2 immunoglobulins. 6 , 7
2.1. Statistical analysis
The study had an 80% power to demonstrate no‐inferiority (alpha error 0.05) of a single dose in pre‐exposed subjects compared to two injections in naïve individuals, provided that the magnitude of immunological response, in terms of proportion of subjects responding to the vaccine was superimposable in the two groups and not inferior to 90%. Data are summarized as medians and interquartile range (IQR), means and 95% confidence intervals (CIs) or percentages. We used Mann–Whitney test, Wilcoxon rank test, and Pearson's correlation test to perform inferential analysis.
2.2. Ethics
The Provincial Ethical Committee approved the study and all participants gave their informed consent.
2.3. Funding
The study has no external funding.
3. RESULTS
Out of 184 health care workers, joining the National vaccination campaign, 53 were previously diagnosed with COVID‐19; 21 had been previously tested positive (nasal swab or serological test) for SARS‐CoV‐2 without any symptom of COVID‐19 and 110 were naïve individuals. Overall, 125 (67.9%) were of feminine gender (34 in the first group, 16 in the second and 75 in the third) and the median age was 50 years (IQR: 39–56). Median age was years 49 (95% CI: 43–55) for subjects with previous COVID‐19; 50 years (95% CI: 42.5–55) for subjects with previous SARS‐CoV‐2 infection and 51 years (95% CI: 39.7–56) for subjects naïve to SARS‐CoV‐2 infection. All were of Caucasian origin, were in good health at the moment of sampling and all received two doses of BNT162b2/Pfizer vaccine.
After a single vaccine injection, the median titer of specific antibodies in individuals with previous COVID‐19 was 30,527 U/ml (IQR: 19,992–39,288) and in subjects with previous SARS‐CoV‐2 asymptomatic infection was 19,367.5 U/ml (IQR: 14,688–31,353) (p = .032) (Figure 1, panel A). Both results were far above the median titer in naïve individuals after a full vaccination schedule: 1974.5 U/ml (IQR: 895–3455) (p < .0001) (Figure 1, panel A). With the second dose, in previously exposed individuals, median titers raised to 43,073 U/ml (IQR: 31,605–61,903) (p < .0001) (Figure 1, panel B). As all but two individuals with previous SARS‐CoV‐2 infection and most naïve individuals showed antibody titers higher than 1000 BAU/ml (the value assigned to WHO International Standard), we used throughout the study the definition U/ml, considering that the correlation between the specific U/ml units of the Roche Elecsys Anti‐SARS‐CoV‐2 S test and the BAU/mI, was confirmed up to 1000 BAU/ml.
Figure 1.
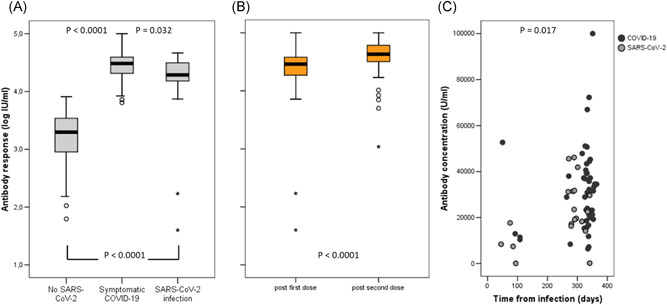
Panel A: Specific antibody titers after a full vaccination schedule (two injections) in SARS‐CoV‐2 naïve individuals and a single vaccine injection in subjects with previous exposure to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) either symptomatic or not. Panel B: Specific antibody titers in subjects with previous exposure to SARS‐CoV‐2 after a single or two doses of vaccine. Panel C: Specific antibody titers in SARS‐CoV‐2 pre‐exposed subjects according to time of infection. Values are differentiated between subjects with previous asymptomatic infection and subjects with previous COVID‐19. COVID‐19, coronavirus disease 2019
Titers were slightly higher in subjects who acquired the infection during the first wave, observed in March/April 2020, 8–11 months before testing, compared to those with more recent infection (2–3 months) (p = .017) (Figure 1, panel C), but titers were not influenced by age (p = .083). Adverse events after vaccine injection were more frequent after the second dose of vaccine (mean: 0.95; 95% CI: 0.75–1.14 vs. mean: 1.91; 95% CI: 1.63–2.19) (p < .0001).
Adverse events were also less frequent in naïve compared to exposed individuals both after the first (mean: 0.77; 95% CI: 0.55–1.00 vs. mean 1.23, 95% CI from 0.89 to 1.50) (p = .002); or the second (mean: 1.63; 95% CI: 1.28–1.98 vs. mean: 2.35; 95% CI: 1.87–2.82) (p = .015) vaccine dose (Figure 2).
Figure 2.
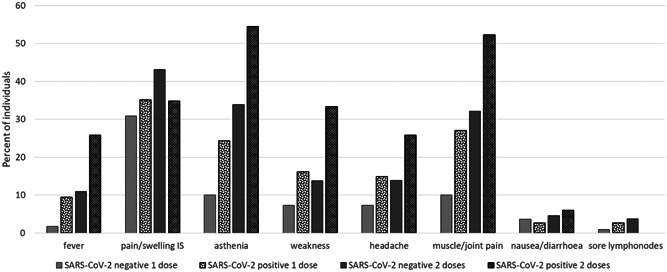
Most frequent "AE" observed after vaccine injection
4. DISCUSSION
A world‐wide vaccination campaign is an expensive, time and labor consuming effort. Eventually, considering a single vaccine dose in subjects previously exposed to SARS‐CoV‐2 would offer advantages in terms of costs, timing and possibility to reach a greater number of subjects in a shorter period of time.
Our findings suggest that a single dose of mRNA vaccine elicits a very strong immune response in seropositive individuals with post‐dose antibody titers 10‐fold higher to those observed in naïve individuals who received a full vaccination schedule. Interestingly, this type of response is present whether or not individuals developed a symptomatic COVID‐19 disease. These observations are in line with the hypothesis that the first vaccine dose serves as a boost in naturally infected individuals. 8 Further, the antibody response was higher in subjects infected during the first wave of the pandemic, compared to those who acquired the infection months later. We do not have a definitive answer to this, it might be because there were more cases of severe infection in the first wave, nevertheless our data indicate a long lasting immune memory that would allow to modify the vaccination schedule in SARS‐CoV‐2 infected individuals irrespective of the timing of infection.
Our study has some limitations. Being conducted in health care workers, the age of enrolled individuals ranged from 24 to 66 years and we cannot exclude that younger or older subjects could react differently. Furthermore, all our subjects were Caucasian and we therefore cannot extend our observation to different ethnicities.
Finally, with the test we used, we measured spike (S) protein RBD antibodies not neutralizing antibodies that are considered to better correlate to protection. However, the used test has been compared to a test that uses purified ACE2 and RBD proteins to evaluate the rate of inhibition of the interaction RBD/ACE2 by neutralizing antibodies. It has been shown that 15 IU/ml was a minimal cut‐off to define concordance with the presence of neutralizing antibodies. Out of 494 samples reactive to RBD/ACE2 test, 439 were also positive to the test used in this study for a final positive predictive value of 99.1% (95% CI: 97.7%–99.6%). 9 , 10
In our experience, all patients but two with a pre‐exposure to SARS‐CoV‐2 showed antibody titers well above the 1000 IU/ml after a single vaccine dose. That appears reassuring in light of a differentiated vaccination schedule.
In SARS‐CoV‐2 naturally infected individuals a single mRNA vaccine dose seems sufficient to reach immunity. Modifying currently implemented dosing schedules would speed‐up vaccination campaigns and would limit occurrence of adverse events in this sub‐group of individuals.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Annapaola Callegaro is the person who takes responsibility for the integrity of the work as a whole, from inception to published article; study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Daniela Borleri, Claudio Farina, Gavino Napolitano, and Marco Rizzi contributed to critical revision of the manuscript for important intellectual content. Daniela Valenti contributed to acquisition of data; critical revision of the manuscript for important intellectual content. Franco Maggiolo contriburted to study concept and design; acquisition of data; analysis and interpretation of data; statistical analysis; drafting of the manuscript.
Callegaro A, Borleri D, Farina C, et al. Antibody response to SARS‐CoV‐2 vaccination is extremely vivacious in subjects with previous SARS‐CoV‐2 infection. J Med Virol. 2021;93:4612‐4615. 10.1002/jmv.26982
REFERENCES
- 1. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586(7830):516‐527. 10.1038/s41586-020-2798-3 [DOI] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salazar E, Kuchipudi SV, Christensen PA, et al. Relationship between anti‐spike protein antibody titers and SARS‐CoV‐2 in vitro virus neutralization in convalescent plasma. bioRxiv. 2020. 10.1101/2020.06.08.138990 [DOI] [Google Scholar]
- 5. Premkumar L, Segovia‐Chumbez B, Jadi R, et al. The receptor‐binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Sci Immunol. 2020;5(48.eabc8413). 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Establishment of the WHO International Standard and Reference Panel for anti‐SARS‐CoV‐2 antibody. 2020. WHO/BS/2020.2403. https://www.who.int/publications/m/item/WHO-BS-2020.24032
- 7. NIBSC . First WHO international standard anti‐SARS‐CoV‐2 immunoglobulin (human). https://www.nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=20/136 [DOI] [PMC free article] [PubMed]
- 8. Abu Jabal K, Ben‐Amram H, Beiruti K, et al. Impact of age, ethnicity, sex, and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID‐19 vaccine: real‐world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6). 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer B, Torriani G, Yerly S, et al. Validation of a commerciably available SARS‐CoV‐2 serological immunoassay. Clin Microbiol Infect. 2020;26(10):1386‐1394. 10.1016/j.cmi.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elecsys® Anti‐SARS‐CoV‐2 . https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html


