Abstract
Neonatal ductus arteriosus aneurysm (DAA) is a rare abnormality that is typically diagnosed at fetal third trimester or early postnatal echocardiography. While echocardiography is usually adequate for diagnosis and clinical decision-making, cross-sectional imaging, including CT or cardiac MRI, may be necessary to clarify the diagnosis or delineate associated complications. Severe complications include thromboembolism, infection, compression of adjacent structures, airway erosion, and aneurysm rupture. This imaging essay reviews the pathophysiology and depicts the spectrum of cross-sectional imaging appearances of neonatal DAAs. Most neonatal DAAs will spontaneously regress and can be managed conservatively.
Keywords: CT, MRI, Cardiac, Aneurysms, Congenital
Supplemental material is available for this article.
©RSNA, 2021
Keywords: CT, MRI, Cardiac, Aneurysms, Congenital
Summary
The pathophysiology of neonatal ductus arteriosus aneurysms is reviewed and the spectrum of cross-sectional imaging appearances and complications for this relatively rare entity are depicted.
Key Points
■ Most neonatal ductus arteriosus aneurysms (DAAs) will spontaneously resolve without a clinical consequence, and thus can be followed with echocardiography.
■ CT or MRI is indicated when the diagnosis is in doubt or there is suspicion of an associated complication, such as thromboembolism, infection, compression or erosion of adjacent structures, or aneurysm rupture.
■ Accurate description of neonatal DAAs and potential associated complications is crucial to help guide clinical decision making between conservative versus surgical management.
Introduction
Neonatal ductus arteriosus aneurysm (DAA) is characterized by focal saccular or diffuse tubular dilatation of the ductus arteriosus and is typically diagnosed at fetal third trimester or early postnatal echocardiography. It is often an incidental finding with the reported incidence ranging from 1.5% to 8.8% (1–4). Most neonatal DAAs have a benign course with spontaneous regression by 6 to 12 weeks and without adverse outcome (3,5), with up to 100% resolving in one series (3). However, the true incidence and rate of complications of neonatal DAAs is unknown because there are no consensus diagnostic criteria, and most of the literature is in the form of aggregate review of case reports or retrospective data, which may miss DAAs that do not come to clinical attention. Potential serious complications of neonatal DAAs include thromboembolism, infection, compression of adjacent structures, airway erosion, and aneurysm rupture.
Diagnostically, the variable morphologic features of DAAs can occasionally result in perplexing imaging appearances. CT or MRI is indicated when needed to clarify echocardiographic findings or delineate complications of DAAs (6). A retrospective review, approved by the research ethics board at The Hospital of Sick Children with waiver of informed consent, found that over a 15-year period from January 2005 to January 2020, 80 cases of neonatal DAA were diagnosed with echocardiography. Six of these had further evaluation with CT or MRI. In this imaging essay, we review the pathophysiology of neonatal DAAs and depict the spectrum of cross-sectional imaging appearances and complications for this relatively rare entity.
Pathophysiology
Several theories have been proposed regarding the pathogenesis of neonatal DAAs, which is likely multifactorial. Most DAAs develop in the third trimester of fetal life or have a dilated fetal ductus arteriosus before progression to true aneurysm postnatally (1,2). The process of ductal dilatation and then aneurysm formation appears to initiate from the aortic end with propagation toward the pulmonary end (1). This happens as normal ductus arteriosus closure occurs in two steps, with the first step of the process more evident at the pulmonary end, whereby constriction of media smooth muscle induces ischemic hypoxia in the duct wall (7). Subsequently, intimal cushions protrude into the lumen and further thicken the ductal wall via connective and elastic tissue proliferation, which can be macroscopically apparent as luminal irregularity (6). These cushions eventually unite to obliterate the lumen (7). The second step of normal ductal closure involves further proliferation of connective tissue in the intima and media along with smooth muscle cell atrophy, which results in a noncontractile ligament of dense fibrous and elastic tissue. In some scenarios, the aortic end can remain open as an aortic ampulla after the pulmonary end of the duct has become occluded and ligamentous (7). Complete disruption of this process leads to a patent ductus arteriosus. Partial disruption of this process with only partial closure of the duct may predispose to DAA formation (7). Indeed, histologic examination of resected DAAs has shown reduced intimal cushions and/or abnormal elastin expression (2).
Mechanical factors may also play a role in the development of DAAs. Fetuses that developed neonatal DAAs tend to have greater in utero curvature of the ductus compared with fetuses without neonatal DAAs (1). Greater in utero curvature of the ductus may lead to higher blood flow velocities at the aortic end causing vortex wall stress, thus contributing to DAA formation (1). In addition, after birth, delay in closure of the aortic end of the ductus can lead to a patent ductus stump on the aortic side. Continued exposure of ductal tissue to systemic arterial pressure may then cause myxoid degeneration of the media, weakening of the duct wall, and further contribute to DAA formation (8). The degree this latter process affects DAA formation is speculative because myxoid degeneration also appears to be part of the normal process of ductal closure (9,10).
Hormonal factors have also been implicated in DAA development. Maternal estrogen can activate smooth muscle in the ductus arteriosus and, conversely, is inhibited by progesterone which prevents ductal closure in utero (11,12). Withdrawal of progesterone and prostaglandins in late pregnancy contributes to ductal constriction after birth (9). Variation in these natural processes, including induction of labor with prostaglandin E2, may thus prolong ductal closure and potentially influence development of DAAs (13). In addition, there is a reported increased incidence of DAAs in infants who are large for gestational age because the mothers have poorly controlled gestational diabetes (3). It remains unclear what mechanism affecting DAA formation, if any, underlies these reported associations.
Finally, an association between neonatal DAAs and a wide variety of connective tissue disorders have been reported, such as Marfan, Ehlers-Danlos, Loeys-Dietz, and Larsen syndromes (2,6). In our series, 5% (four of 80) were confirmed to have a connective tissue disorder, three with Marfan syndrome, and one with Loeys-Dietz syndrome. The predisposition of neonates with connective tissue disorders to develop DAAs is presumably related to a weakened vessel wall as a result of the abnormal extracellular architecture seen in these patients. An important increased risk of spontaneous rupture of DAAs is seen in this population (2,6).
Diagnosis
Echocardiography is the first-line modality and is typically sufficient for the diagnosis and follow-up of DAAs. It is most commonly ordered to rule out congenital heart disease in the setting of a murmur, with secondary indications including evaluation for respiratory distress. However, there are no specific consensus diagnostic criteria for the definition of a neonatal DAA. Neonatal DAAs are often seen as a tortuous and dilated vascular structure protruding leftward of the aortic arch (Fig 1, Movie 1). Some authors consider the ductus arteriosus to be dilated if it is greater than 95% the size of the adjacent transverse arch or descending aorta (1). The absolute size of DAAs in reported series has ranged from 6.5 to 24 mm in maximal diameter, with some using greater than 10 mm as criteria for a large DAA (2,3).
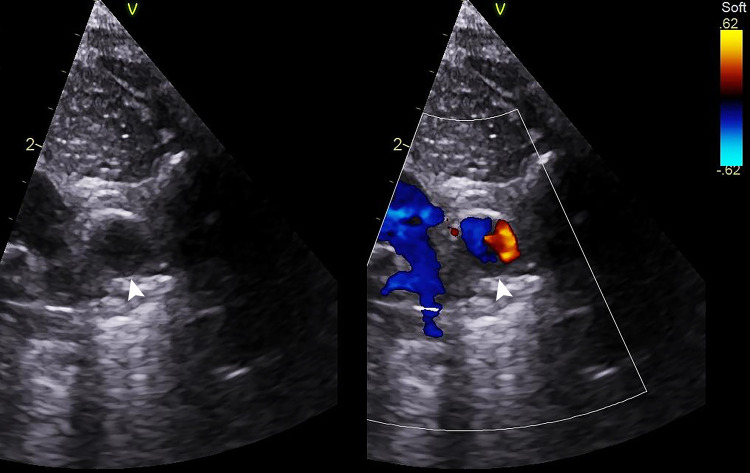
Figure 1:
A 3-day-old male neonate with an incidental ductus arteriosus aneurysm (DAA) showing classic appearance on echocardiogram. Two-dimensional and color Doppler ductal view echocardiograms show a dilated vascular structure to the left of the left pulmonary artery (LPA), measuring 8 × 7 mm, and consistent with a DAA (white arrowhead). Movie 1 shows a ductal aneurysm with unobstructed LPA and aortic arch to the right and left of the aneurysm, respectively. This patient underwent conservative management with serial echocardiography and eventual spontaneous regression of the DAA.
Movie 1.
Echocardiogram shows a classic DAA appearance on echocardiogram, with unobstructed aortic arch and left pulmonary artery. There is a small amount of eccentric thrombus within the DAA.
Contrast-enhanced cardiac CT can clearly depict the anatomy of DAAs (Fig 2, Movie 2). Cardiac CT angiography is the appropriate next-step modality when echocardiography is equivocal, the diagnosis is in doubt, or complications are suspected. For example, in our series of 80 neonatal DAAs, one was mistaken at echocardiography for an anomalous pulmonary vein (Fig 3, Movie 3) and another as a pulmonary sling (Fig 4, Movie 4). At our institution, a nongated examination is typically preferred because we find that electrocardiographic gating or triggering is not necessary for diagnosis given that most DAAs are relatively remote from the regions most affected by cardiac motion, and vascular pulsation artifacts are negligible. However, choice of gating may vary by institutional practice and type of scanner available, especially if one has access to newer generation equipment. As an alternative to cardiac CT angiography, contrast-enhanced cardiac MR angiography can also be performed if other imaging is indicated during the same examination (ie, combined with MRI brain and/or spine examinations for workup of connective tissue disorders). Otherwise, CT is typically preferred because MRI has poorer spatial resolution and often requires general anesthesia in the neonatal age range. While feed and sleep techniques could be attempted, in our experience, contrast-enhanced cardiac MR angiography with feed and sleep techniques is suboptimal in the neonatal range. Occasionally neonatal DAAs may be seen as an incidental finding when CT is performed for other indications (Fig 5). In such scenarios, DAAs could prove a diagnostic conundrum to radiologists unfamiliar with this entity.
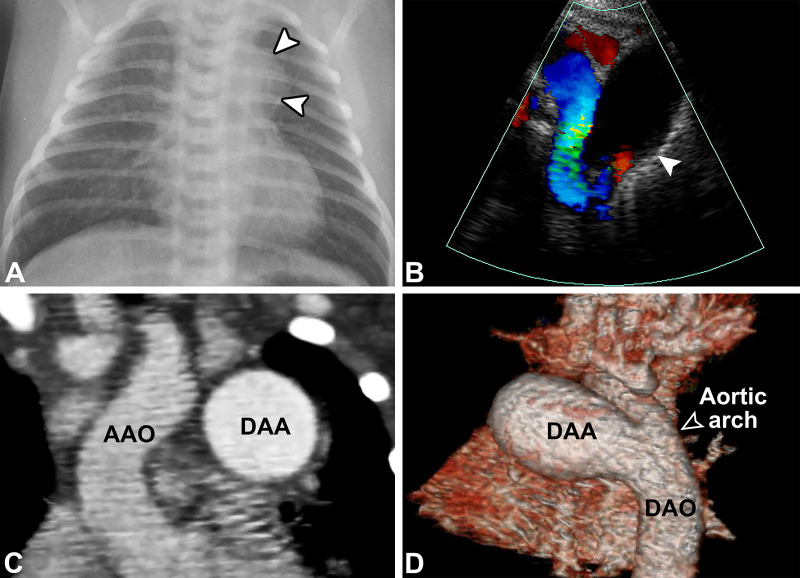
Figure 2:
A 13-day-old male neonate with a large ductus arteriosus aneurysm (DAA). (A) Posteroanterior chest radiograph shows a rounded opacity along the left superior mediastinum (white arrowheads), corresponding to a DAA. (B) Color Doppler ductal view echocardiogram (Movie 2) shows a 17- × 13-mm DAA (white arrowhead) with laminar flow of the open aortic end. Nongated contrast-enhanced cardiac CT angiograms with (C) coronal and (D) lateral-oblique three-dimensional volume-rendered reconstructions show a DAA greater than twice the size of the aorta with a widely patent aortic end (open arrowhead). The pulmonary end was closed. Surgical resection and ligation of the DAA were performed due to risk of thrombus formation with embolization and/or aneurysm rupture. AAO = ascending aorta, DAO = descending aorta.
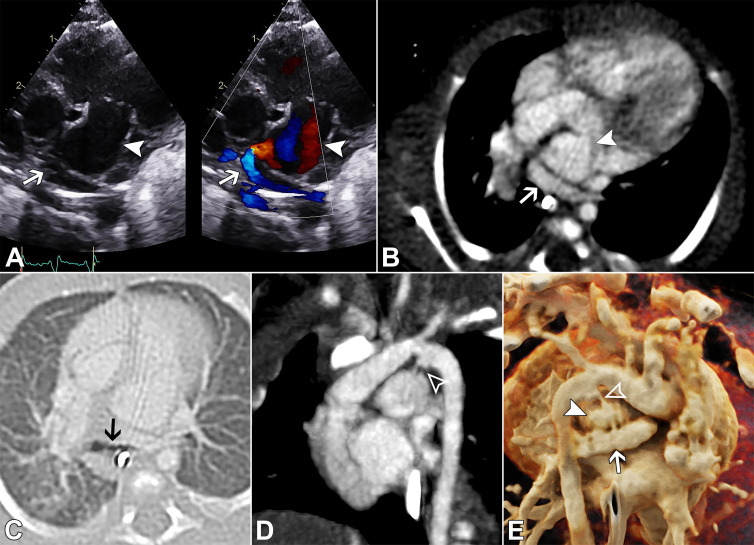
Figure 3:
A 5-day-old male neonate with hypoxic ischemic encephalopathy and unclear dilated vascular structure on echocardiogram requiring CT. (A) Gray-scale and color Doppler ductal view echocardiograms (Movie 3) show a tortuous and tubular ductus arteriosus aneurysm (DAA; white arrowhead) with upward directional left-to-right flow at the aortic end (white arrow), mistaken for an anomalous pulmonary vein in the setting of innominate vein and superior vena cava dilatation. Nongated contrast-enhanced cardiac CT angiograms with (B) axial-oblique and (C) sagittal-oblique maximum intensity projection reconstructions obtained the following week show the vascular structure to represent a part of the tortuous DAA, now with development of circumferential hypoattenuation representing partially occlusive thrombus (white arrowheads), creating irregular contour of the patent lumen. The systemic and pulmonary venous connections were otherwise normal. Follow-up echocardiogram showed complete occlusion of the ductal aneurysm, and no intervention was required. The thrombus in this case likely a consequence of the natural evolution of neonatal DAA during regression. Ao = aorta, DAO = descending aorta, MPA = main pulmonary artery, LPA = left pulmonary artery, RPA = right pulmonary artery.
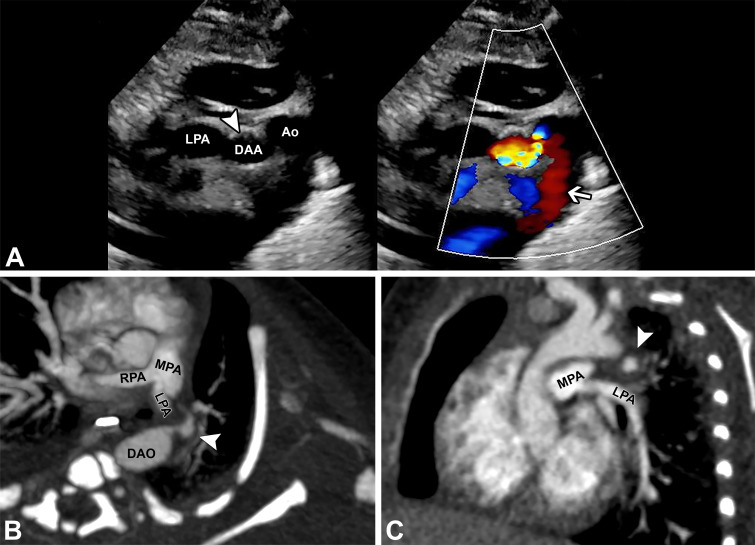
Figure 4:
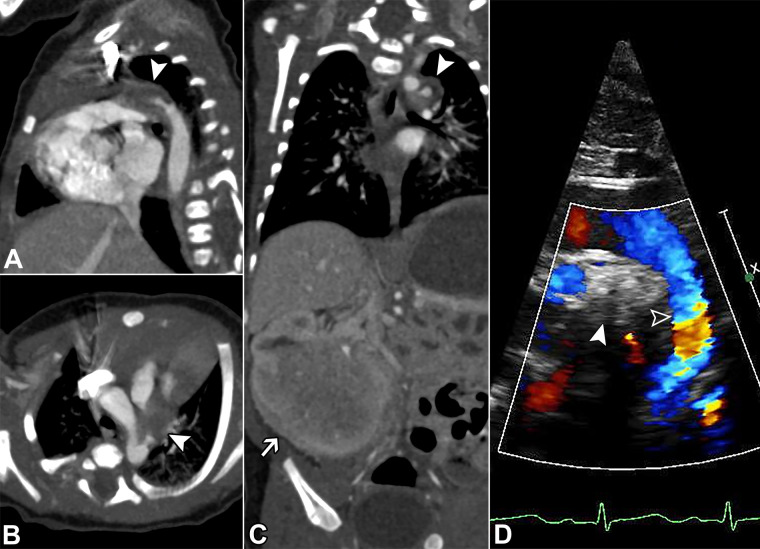
A 21-day-old ex-premature (32 weeks +2 days) female neonate presenting with apneas and desaturations and found to have a ductus arteriosus aneurysm (DAA) displacing and compressing the pulmonary artery origins and left mainstem bronchus, which also caused false appearance of a left pulmonary artery (LPA) sling at echocardiography. (A) Two-dimensional and color Doppler ductal view echocardiograms (Movie 4) show a DAA (white arrowhead) causing displacement of the LPA, which was mistaken for a pulmonary artery sling (white arrow). Nongated contrast-enhanced cardiac CT angiography with (B, C) axial-oblique, (D) sagittal-oblique, and (E) three-dimensional cinematic-rendering reconstructions show a large DAA (white arrowheads), displacing the LPA downward and rightward (white arrows). There is compression of the LPA origin and left mainstem bronchus (black arrow). The aortic end of the DAA is thread-like (open arrowheads). Subsequent follow-up imaging revealed progressive LPA and airway stenosis, and the patient underwent surgical DAA resection and ligation.
Figure 5:
An 11-day-old male neonate with a congenital renal mass and incidental finding of a partially thrombosed ductus arteriosus aneurysm (DAA) during workup with CT, which could be mistaken for mediastinal disease. Nongated contrast-enhanced CT of the chest, abdomen, and pelvis with (A) sagittal-oblique, (B) axial-oblique, and (C) coronal reconstructions show a DAA with internal hypoattenuation representing occlusive thrombus (white arrowheads). A small patent portion at the aortic end persists. In addition, there is a large right renal mass that was proven to be a congenital mesoblastic nephroma (white arrow). (D) Follow-up color Doppler ductal view echocardiogram shows progressive echogenic thrombus occluding the ductal aneurysm (white arrowhead), with no residual shunt and unobstructed laminar flow of the aortic arch and isthmus (open arrowhead). No intervention was required.
Movie 2.
Echocardiogram shows a large DAA with open aortic end.
Movie 3.
Echocardiogram shows a tortuous and tubular DAA with upward directional left-to-right flow in the aortic end, mistaken for an anomalous pulmonary vein.
Movie 4.
Echocardiogram shows a DAA causing displacement of the left pulmonary artery, which was mistaken for a pulmonary artery sling.
With CT or MRI, DAAs may have variable appearances but typically will be seen as a vascular structure connecting to the aortic isthmus and projecting inferiorly or leftward of the aortic arch. The pulmonary end may or may not be patent. The wall-to-wall size is typically greater than the arch itself, although the lumen may be smaller or irregular if there is mural thrombus. It is important to report the precise DAA location, length, diameter, luminal patency, presence and extent of calcification and/or thrombus, relationship to adjacent anatomic structures, and any associated complications. A proposed diagnostic checklist is included in the Table. Multiplanar and three-dimensional reconstructions may be particularly helpful in characterizing the anatomy and providing accurate measurements (14). Such reconstructions may also assist with planning for potential interventional endovascular therapy (6,15).
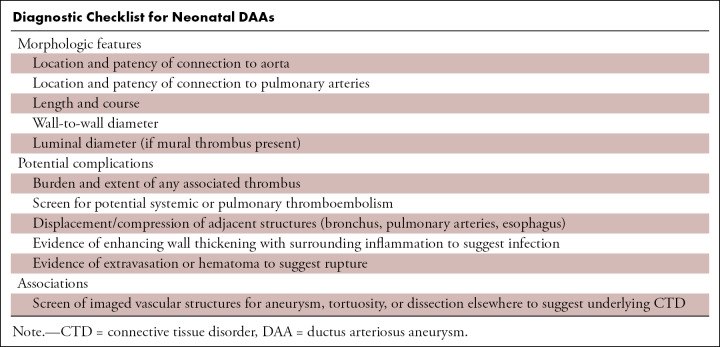
Diagnostic Checklist for Neonatal DAAs
Complications
The complication rate of neonatal DAAs has historically been reported to be as high as 31% (4), with thromboembolism and rupture reported as most common, accounting for 12% and 9%, respectively, in one series (2). These rates are likely overestimated due to presentation and reporting bias. The true incidence of complications in neonatal DAAs is unknown because many affected infants have a benign course with eventual regression and resolution (4). Thus, these patients may not come to clinical attention and so may not be captured in retrospective series and are underrepresented in cumulative reviews of case reports. Indeed, a prospective study by Jan et al, albeit using a liberal definition of DAA, reported a high (8.8%) incidence of isolated DAAs in term newborns, all of which were asymptomatic and regressed by 35 days of life without adverse events (3). In our series, only 5% (four of 80) of patients required surgery for the DAA, 1% (one of 80) underwent elective surgery for concomitant tetralogy of Fallot, and the remaining 94% (75 of 80) spontaneously regressed without complication. Nonetheless, CT or MRI are indicated when complications of neonatal DAAs arise or are suspected, such as thromboembolism, infection (arteritis and/or endocarditis), compression of surrounding structures, or rupture.
Thromboembolism
Compared with echocardiography, contrast-enhanced CT or MRI are more suitable for differentiating slow flow from thrombosis and delineating the full extent of thrombus burden, including potential extension to the pulmonary arteries, which can assist with potential surgical planning (Fig 6, Movie 5). Care needs to be taken to not mistake circumferential thrombus as normal mediastinal tissue because the DAA wall may not always be obviously apparent, and the attenuation of thrombus and normal mediastinal tissue can be similar. It should be noted that thrombus formation may also represent the normal pathway for DAA resolution, as shown in a small case series published by Koneti et al with three out of four neonates showing evolution of DAAs through various stages of thrombus formation and organization, with eventual spontaneous regression of the DAA over a period of 6 weeks to 3 months (5). Indeed, Jan et al showed that up to 30% of neonatal DAAs that spontaneously regressed developed thrombus during the process (3).
Figure 6:
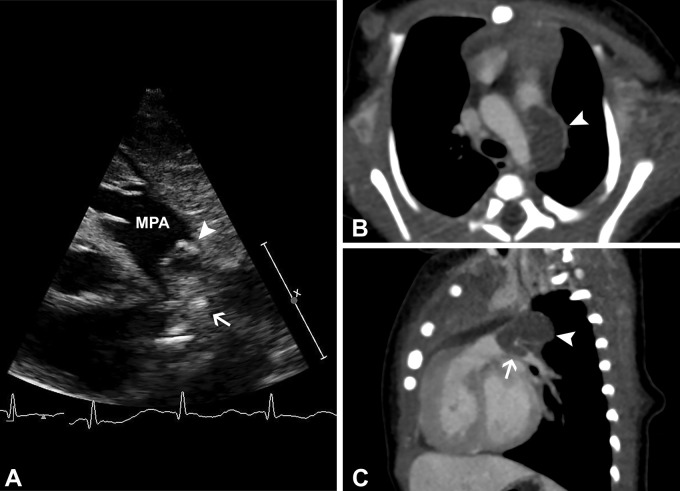
A 2-day-old male neonate with thrombosed ductus arteriosus aneurysm (DAA) and extension of thrombus into the left pulmonary artery. (A) Gray-scale and color Doppler (shown in Movie 5) ductal view echocardiogram shows a heterogeneous mass in the expected region of the ductus arteriosus (white arrowhead), which protrudes into the left pulmonary artery (white arrow). Nongated contrast-enhanced cardiac CT angiography with (B) axial-oblique and (C) sagittal-oblique reconstructions confirm occlusive thrombus of a DAA (white arrowhead) that extends into the left pulmonary artery (white arrow). The DAA was surgically ligated with complete removal of left pulmonary artery thrombus. MPA = main pulmonary artery.
Movie 5.
Echocardiogram shows a thrombosed DAA with extension of thrombus into the left pulmonary artery.
However, on the other end of the spectrum, thrombus formation can also be rarely associated with poor outcome with reported cases of DAA leading to systemic thromboembolism with acute cerebral infarction (16), and even death (17). Unfortunately, there are no evidence-based criteria for discriminating between thrombus that could be part of the normal process of involution versus thrombus that is at risk for serious thromboembolic complication. Because most DAA thrombi are likely benign and serious thromboembolic complications are relatively rare, intervention on neonatal DAAs for thrombus alone is controversial. Ultimately, decisions on treatment will continue to depend on a holistic consideration of thrombus burden, extension to adjacent vessels, comorbidities, institutional practice, and case-by-case multidisciplinary discussion.
Infection
Infection of a ductus arteriosus or DAA is a rare and potentially fatal clinical entity. Bacteremia or septic embolization causing infectious arteritis of a patent ductus arteriosus can weaken the wall and subsequently lead to the development of a mycotic DAA (18–20). Historically, infective endarteritis of a patent ductus arteriosus, predominantly by Staphylococcus aureus, was the most common cause of death in the setting of a patent duct, accounting for 45% of reported deaths (21). In the modern era of antibiotics and improved hygiene, both the incidence of and mortality from infective ductus arteritis are now very low and nearly nonexistent (22). Nonetheless, serious complications of ductus arteriosus endarteritis include septic embolism, mycotic aneurysm, and death (23,24). Diagnosis of infected DAA is often made based on clinical suspicion and laboratory tests. Echocardiographic images may show echogenic pus within a DAA. CT or MRI helps delineate the precise anatomy and may potentially show wall thickening and inflammation. Treatment is with antibiotics and surgical or transcatheter closure of the DAA.
Compression of Adjacent Structures
Neonatal DAAs can cause compression of adjacent structures and lead to potential erosion into the bronchus, esophagus, or other surrounding vascular structures (4). The exact size of the DAA is less important than the morphologic feature and relationship with nearby structures in this regard. The adjacent pulmonary arteries can be particularly compromised in the setting of DAAs due to external compression by the DAA. Compression of the phrenic nerve, recurrent laryngeal nerve, or adjacent large airways have all also been described, which can lead to stridor and/or respiratory distress (25–27). Erosion into the bronchus or esophagus has been reported in 2% of neonatal DAAs (4), although again, these numbers are likely overestimated due to presentation and reporting bias as we had none in our series. CT or MRI can easily depict the precise anatomic relationships in these scenarios for appropriate preoperative surgical planning.
Aneurysm Rupture
DAA rupture is a more commonly reported complication in older children and adults compared with infants (4). A prior review of DAA case reports found rupture to occur in 9% of infants younger than 2 months, 44% of children between ages 2 months and 15 years, and in 28% of adults with a DAA (4). We emphasize that these are likely vastly overestimated incidences. Nonetheless, rupture of DAAs can result in catastrophic outcomes and spontaneous death (17). There are no established criteria to determine which DAAs are at increased risk of rupture. Although larger DAAs may potentially be at greater risk of rupture (28,29), rupture can occur in aneurysms as small as 8 mm in diameter, and thus the decision for surgical resection should not be based on size alone (2,30). However, the presence of any underlying connective tissue disorder does increase the risk of rupture, and a potential DAA rupture should always be a consideration in these patients (31,32) (Fig 7 and Movie 6).
Figure 7:
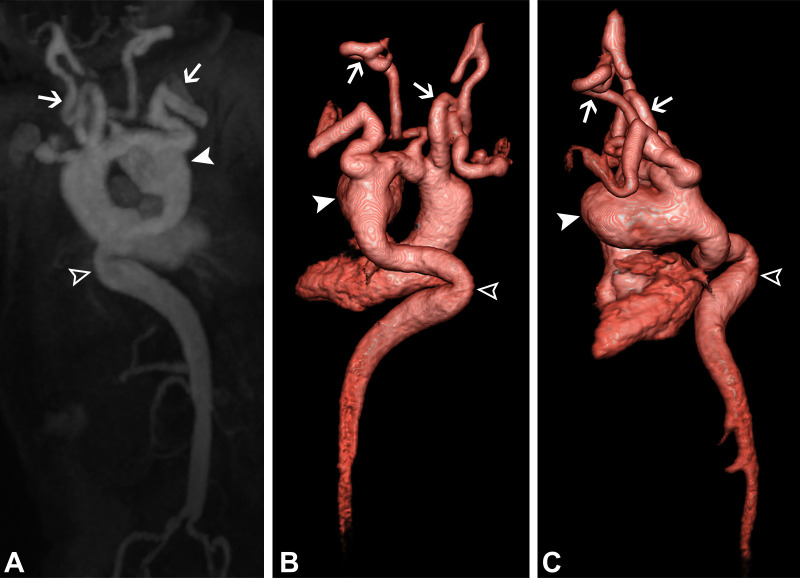
A 30-day-old male infant with Loeys-Dietz syndrome (TGFBR2 mutation) and contained rupture of a ductus arteriosus aneurysm (DAA). (A) Contrast-enhanced MR angiography with coronal-oblique maximum intensity projection and (B, C) three-dimensional volume-rendered (Movie 6) reconstructions show a large DAA (white arrowheads). There is also a dilated, tortuous descending aorta (open arrowheads) with significant bilateral subclavian and vertebral artery tortuosity (white arrows), consistent with Loeys-Dietz syndrome. Contained rupture of the DAA was found during surgical resection. An end-to-end aortic anastomosis was performed.
Movie 6.
MRI 3D volume-rendered reconstruction shows a large DAA and dilated tortuous descending aorta, bilateral subclavian and vertebral arteries consistent with Loeys-Dietz syndrome.
The only reliable imaging sign of DAA rupture is visualization of frank extravasation or hematoma in the mediastinum (33). Otherwise, the diagnosis is typically only confirmed during surgery or with postmortem examination (34). Therefore, there is the possibility of finding unexpected DAA rupture during surgery, which may require an adaptive intraoperative surgical approach including the potential need for cardiopulmonary bypass.
Management
The level of evidence regarding the management of neonatal DAAs remains low because most data are from case reports or single institution retrospective series, and many DAAs with a benign course may not be captured. Mitchell et al recommended treatment for DAAs with a diameter greater than 3 cm (35); however, there is no good evidence for size criteria to discriminate between DAAs suitable for conservative expectant management versus those requiring surgical treatment to prevent potential complications. In general, the presence of a large DAA should not necessarily itself be an indication for surgery (2). For infants younger than 2 months, close clinical observation along with serial echocardiography is often sufficient to identify high-risk cases that may require surgical intervention, with the expectation that most cases will spontaneously regress by age 6 to 12 weeks (3–5). Surgery may be indicated for neonatal DAAs that fail to regress after 2 months of observation, in the setting of any of the previously described complications, or in the presence of an underlying connective tissue disorder. For children older than 2 months, some authors have advocated for surgical treatment for all spontaneous or postoperative DAAs in this cohort as these may have a high complication rate and are unlikely to regress on their own (4). Interventional endovascular transcatheter closure using stent grafts or occluding devices are potential emerging therapeutic options for patients with a high risk of surgical resection (6,14).
Conclusion
Neonatal DAAs are a rare but important entity to recognize at cross-sectional imaging. In this pictorial essay, we have shown the typical appearances of neonatal DAAs at echocardiography, as well as CT and MRI, including potential complications such as thrombus, compression of adjacent structures, and rupture in the setting of an underlying connective tissue disorder. Accurate description of neonatal DAAs and their associated complications is crucial to help guide the decision between conservative management versus surgical resection.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: B.B. disclosed no relevant relationships. S.A. disclosed no relevant relationships. S.J.Y. disclosed no relevant relationships. M.S. disclosed no relevant relationships. C.Z.L. disclosed no relevant relationships.
Abbreviation:
- DAA
- ductus arteriosus aneurysm
References
- 1.Tseng JJ, Jan SL. Fetal echocardiographic diagnosis of isolated ductus arteriosus aneurysm: a longitudinal study from 32 weeks of gestation to term. Ultrasound Obstet Gynecol 2005;26(1):50–56. [DOI] [PubMed] [Google Scholar]
- 2.Dyamenahalli U, Smallhorn JF, Geva T, et al. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J Am Coll Cardiol 2000;36(1):262–269. [DOI] [PubMed] [Google Scholar]
- 3.Jan SL, Hwang B, Fu YC, Chai JW, Chi CS. Isolated neonatal ductus arteriosus aneurysm. J Am Coll Cardiol 2002;39(2):342–347. [DOI] [PubMed] [Google Scholar]
- 4.Lund JT, Jensen MB, Hjelms E. Aneurysm of the ductus arteriosus. A review of the literature and the surgical implications. Eur J Cardiothorac Surg 1991;5(11):566–570. [DOI] [PubMed] [Google Scholar]
- 5.Koneti NR, Kanchi V, Kandraju H, Jaishankar S. Symptomatic aneurysm of ductus arteriosus in neonates. Ann Pediatr Cardiol 2011;4(2):159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan GJ, Yim DL, Hayes AM, Martin RP, Hamilton MC, Stuart G. Imaging and percutaneous occlusion of a large aneurysm of the ductus arteriosus in an infant with Loeys-Dietz syndrome. Congenit Heart Dis 2013;8(6):E192–E195. [DOI] [PubMed] [Google Scholar]
- 7.Matsui H, McCarthy K, Ho S. Morphology of the patent arterial duct: features relevant to treatment. Images Paediatr Cardiol 2008;10(1):27–38. [PMC free article] [PubMed] [Google Scholar]
- 8.Kirks DR, McCook TA, Serwer GA, Oldham HN Jr. Aneurysm of the ductus arteriosus in the neonate. AJR Am J Roentgenol 1980;134(3):573–576. [DOI] [PubMed] [Google Scholar]
- 9.Ovalı F. Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front Pediatr 2020;8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda I, Endo M. Coronary artery spasm: a hypothesis on prevention by progesterone. Med Hypotheses 1997;49(2):183–185. [DOI] [PubMed] [Google Scholar]
- 11.Pulkkinen MO, Momma K, Pulkkinen J. Constriction of the fetal ductus arteriosus by progesterone. Biol Neonate 1986;50(5):270–273. [DOI] [PubMed] [Google Scholar]
- 12.Sung RY, Yin JA, Loong EP, Fok TF, Lau J. Topical prostaglandin E2 gel for cervical ripening and closure of the ductus arteriosus in the newborn. Arch Dis Child 1990;65(7 Spec No):703–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danford DA, Rayburn WF, Miller AM, Felix GL, Bussey ME. Effect of low intravaginal doses of prostaglandin E2 on the closure time of the ductus arteriosus in term newborn infants. J Pediatr 1993;122(4):632–634. [DOI] [PubMed] [Google Scholar]
- 14.Goitein O, Fuhrman CR, Lacomis JM. Incidental finding on MDCT of patent ductus arteriosus: use of CT and MRI to assess clinical importance. AJR Am J Roentgenol 2005;184(6):1924–1931. [DOI] [PubMed] [Google Scholar]
- 15.De Freitas S, Connolly C, Neary C, Sultan S. Ductus arteriosus aneurysm presenting as hoarseness: successful repair with an endovascular approach. J Surg Case Rep 2016;2016(4):rjw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh J, Kobayashi D, Chen MY, Anthony EY, Williams DA. Neonatal systemic thromboembolism secondary to ductus arteriosus aneurysm and patent foramen ovale. Congenit Heart Dis 2013;8(1):E5–E9. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan RM, Michelfelder EC, Choe KA, et al. Ductus arteriosus aneurysm with massive thrombosis of pulmonary artery and fetal hydrops. Pediatr Dev Pathol 2012;15(1):79–85. [DOI] [PubMed] [Google Scholar]
- 18.Stewart A, Dyamenahalli U, Greenberg SB, Drummond-Webb J. Ductus arteriosus aneurysm with community-acquired methicillin-resistant Staphylococcus aureus infection and spontaneous rupture: a potentially fatal quandary. Pediatrics 2006;117(6):e1259–e1262. [DOI] [PubMed] [Google Scholar]
- 19.Botta AM, Aquino F, Pereira C, et al. Silent patent ductus arteriosus aneurysm. Arq Bras Cardiol 2002;79(3):302–307. [DOI] [PubMed] [Google Scholar]
- 20.Ise H, Akasaka N, Kamiya H, Otani N. Thoracic endovascular aortic repair with perioperative antibiotic therapy for infected ductus arteriosus aneurysm in an adult. J Surg Case Rep 2018;2018(8):rjy229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan ID. Patent arterial duct: when should it be closed? Arch Dis Child 1998;78(3):285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thilén U, Aström-Olsson K. Does the risk of infective endarteritis justify routine patent ductus arteriosus closure? Eur Heart J 1997;18(3):503–506. [DOI] [PubMed] [Google Scholar]
- 23.Huggon IC, Qureshi SA. Is the prevention of infective endarteritis a valid reason for closure of the patent arterial duct? Eur Heart J 1997;18(3):364–366. [DOI] [PubMed] [Google Scholar]
- 24.Shinkawa T, Yamagishi M, Shuntoh K, Fujiwara K, Watanabe T, Yoshida S. Infectious ductal aneurysm after coil embolization in an infant. Ann Thorac Surg 2006;81(1):339–341. [DOI] [PubMed] [Google Scholar]
- 25.Hornung TS, Nicholson IA, Nunn GR, Hawker RE. Neonatal ductus arteriosus aneurysm causing nerve palsies and airway compression: surgical treatment by decompression without excision. Pediatr Cardiol 1999;20(2):158–160. [DOI] [PubMed] [Google Scholar]
- 26.Roughneen PT, Parikh P, Stark J. Bronchial obstruction secondary to aneurysm of a persistent ductus arteriosus. Eur J Cardiothorac Surg 1996;10(2):146–147. [DOI] [PubMed] [Google Scholar]
- 27.Brock J, Nussbaum E, Shows J, Nguyen S, Setty SP. An Unusual Cardiac Cause of Unilateral Neonatal Wheezing. Case Rep Pediatr 2019;20199638518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pontone G, Andreini D, Bartorelli AL, Dainese L, Fusari M, Biglioli P. Incidental detection of a giant ductus arteriosus aneurysm by low-dose multidetector computed tomography in an asymptomatic adult. J Vasc Surg 2010;51(5):1260–1264. [DOI] [PubMed] [Google Scholar]
- 29.Ferlic RM, Hofschire PJ, Mooring PK. Ruptured ductus arteriosus. Aneurysm in an infant. Report of a survivor. Ann Thorac Surg 1975;20(4):456–460. [DOI] [PubMed] [Google Scholar]
- 30.González IA, González C, Cazzaniga M, Fernández L. Spontaneous closure of a large ductus arteriosus aneurysm. Rev Esp Cardiol 2010;63(7):875–876. [DOI] [PubMed] [Google Scholar]
- 31.Meester JAN, Verstraeten A, Schepers D, Alaerts M, Van Laer L, Loeys BL. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann Cardiothorac Surg 2017;6(6):582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crisfield RJ. Spontaneous aneurysm of the ductus arteriosus in a patient with Marfan's syndrome. J Thorac Cardiovasc Surg 1971;62(2):243–247. [PubMed] [Google Scholar]
- 33.Bruinsma GJ, Leicher FG, Haalebos MM. Ruptured aneurysm of a ductus arteriosus diverticulum in an adult. Neth Heart J 2001;9(2):85–86. [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuta M, Horita T, Seko-Nakamura Y, et al. Sudden Death Caused by Rupture of Spontaneous Ductus Arteriosus Aneurysm in an Adult. J Forensic Sci 2020;65(3):1004–1008. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell RS, Seifert FC, Miller DC, Jamieson SW, Shumway NE. Aneurysm of the diverticulum of the ductus arteriosus in the adult. Successful surgical treatment in five patients and review of the literature. J Thorac Cardiovasc Surg 1983;86(3):400–408. [PubMed] [Google Scholar]