Summary
Objectives
To observe the role of CSF Gene XPERT (CBNAAT) in diagnosis of tuberculous meningitis (TBM) and determine its sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
Methods
A prospective study was done from October 2017 to March 2020. CSF samples of 55 children diagnosed as tuberculous meningitis as per defined clinical and imaging criteria, were subjected to routine CSF analysis, MGIT culture and CBNAAT. Children on prior anti-tuberculous therapy for more than one month were excluded from study.
Results
Of 55 children, meningeal signs were present in 54.5% children. Neurological deficits were present in 47.3%. Common CT brain findings were communicating hydrocephalus followed by infarct and basal exudates. CSF Gene XPERT (CBNAAT) were positive in 9 (16.4%), of which 6 was also culture positive and 3; negative. Two children were rifampicin resistant. Fifteen (27.3%) children had positive CSF culture. Gene XPERT showed sensitivity, specificity, PPV, NPV and diagnostic accuracy of 40%, 92.5%, 66.7%, 80.4% and 78.2% respectively as compared to culture.
Conclusion
Although sensitivity of CSF CBNAAT is low i.e. 40% but positive result not only confirm bacteriological diagnosis of tuberculous meningitis but also reveal about rifampicin sensitivity and resistance for plan of therapy.
Keywords: Tuberculous meningitis, Cerebrospinal fluid, CBNAAT, Gene XPERT, Sensitivity
1. Introduction
Mycobacterium tuberculosis is presently a leading cause of death worldwide alongside HIV. Among all tuberculosis patients, 25% have extrapulmonary site involvement namely, lymph node, meninges, kidney, spine and growing end of bones [1], [2], [3]. Tuberculous meningitis is the most severe consequence of Mycobacterium tuberculosis infections. There is high mortality and morbidity with severe neurological sequelae among survivals [4], [5]. In India, pediatric tuberculosis has gained major attention in last two decades as health problem. Tuberculous meningitis is second most common cause of extrapulmonary tuberculosis after lymph node involvement [1].
The diagnosis of extrapulmonary tuberculosis is more difficult than pulmonary as direct smear microscopy and Cartridge Based Nucleic Acid Amplification Test (CBNAAT), which provide rapid and confirm bacteriological diagnosis have low yield [6]. The diagnosis of tuberculous meningitis (TBM) in young children is difficult due to the paucibacillary nature of disease in cerebrospinal fluid (CSF) [7]. Other tests for extrapulmonary tuberculosis i.e. tuberculin test and polymerase chain reactions (PCR) assays have been reported to have variable sensitivity and specificity [7]. So, there is need of rapid diagnois of TBM, which is essential for treatment initiation and improvement of outcome [8], [9], [10]. Cerebral imaging also contributes for diagnosis of probable or possible TBM like clinical and laboratory findings. However, discrimination between TBM and another cerebral disease is frequently very difficult. The findings in CT /MRI Brain are hydrocephalus, basal meningeal enhancement, tuberculoma and vasculitis [11], [12], [13].
WHO has reported high sensitivity (59–84%) and specificity (73–89%) of Xpert for CSF than conventional tools. Accuracy of nucleic acid-based amplification (NAA) tests, though better than that of conventional microscopic methods, was not considered completely satisfactory for many years because their low sensitivity and specificity in diagnosis of tuberculous meningitis [14], [15]. There are few studies on CSF Gene Xpert for detection of Mycobacterium tuberculosis in children. Therefore this study was done to observe the sensitivity, specificity, positive predictive value and negative predictive value of CSF CBNAAT for diagnosis of tuberculous meningitis in children.
2. Methods
This study was carried out in the Department of Pediatrics and Microbiology on admitted children from October 2017 to March 2020.This prospective observational study was approved by Ethical committee, Institute of Medical Sciences, Banaras Hindu University, Varanasi. The informed consent was taken from parent or legal guardian of patients before inclusion of the patient for the study.
2.1. Inclusion criteria
Children less than or equal to 18 years diagnosed as tuberculous meningitis based on history, clinical features, CSF laboratory findings and brain imaging were included in the study. Staging of tubercular meningitis was done as per British Medical Council Staging System [16].
2.2. Exclusion criteria
Children who had received prior antituberculous therapy for one month or more were excluded from study.
All demographic details and clinical manifestations were recorded in standard pretested proforma. Complete blood count, renal and liver function test, tuberculin test, ELISA for HIV, chest radiographs and cranial tomography or MRI of brain were done in all patients. Lumbar puncture was done after excluding raised intracranial pressure. The collected cerebrospinal fluid was subjected to routine cytochemical study, Gram’s and AFB staining and aerobic culture. Two mL CSF was collected in two different sterile container and was transported to microbiology laboratory for the Gene Xpert (CBNAAT) and MGIT culture of Mycobacterium tuberculosis. On the basis of clinical features, imaging, cerebrospinal fluid and imaging study children of tuberculous were categorized into three groups [11].
-
1.
Definite TBM: AFB seen on CSF microscopy, positive CSF M. tuberculosis culture, or positive CSF M. tuberculosis commercial NAAT in the setting of symptoms/signs suggestive of meningitis.
-
2.
Probable TBM: total score of ≥12 when neuroimaging available or total score of ≥10 when neuroimaging unavailable.
-
3.
Possible TBM: total score of 6–11 when neuroimaging available or total score of 6–9 when neuroimaging unavailable.
2.3. Procedure of CBNAAT
The collected 2 ml of CSF was centrifuged at 3000g for 15 min. Supernatant is poured off and pellet is resuspended in 0.7 ml of phosphate buffered saline. 1.3 ml of sample reagent (2:1 sample reagent to sample ratio) is added and incubated for 15 min followed by vortex during this time. Than 2 ml is added into the cartridge and loaded in Gene Xpert equipment for analyzing the sample. Results were reported as positive or negative for Mycobacterium tuberculosis. Susceptibility to rifampicin was also reported as resistant or sensitive.
2.3.1. Statistical analysis
The data was analyzed using the SPSS software application (version 23.0: SPSS, Chicago, IL, USA).
3. Results
The present study included 55 cases of tuberculous meningitis admitted in ward based on clinical findings, CSF and imaging study. Out of 55 children with tuberculous meningitis, 14 (25.4%) were of less than 5 years of age, 28 (50.9%) of 5 to 10 years of age; and 13 (23.7%) in 10 to 18 years age group. The most common symptom was fever (for more than15 days) in 43 (78.1%) followed by headache (67.2%) and seizures (67.2%). Neck rigidity and kernig’s sign was present in 30 (54.5%) and Brudzinski sign; 26 (47.3%). Eleven (20%) children had cranial nerve palsy and 26 (47.3%) had motor deficits (Table 1).
Table 1.
Basic characteristics of children with Tuberculous meningitis.
| Characteristics | No. of children (n = 55) | % |
|---|---|---|
| Age | ||
| <5yrs | 14 | 25.4 |
| 5–10 yrs | 28 | 50.9 |
| 10–18 yrs | 13 | 23.7 |
| Fever | 43 | 78.1 |
| Headache | 37 | 67.2 |
| Seizure | 37 | 67.2 |
| Abnormal chest X-ray | 31 | 56.3 |
| Positive Neck rigidity and Kernig’s sign | 30 | 54.5 |
| Brudzinski sign | 26 | 47.3 |
| Vomiting | 28 | 50.9 |
| Papilledema | 26 | 47.3 |
| Cranial nerve palsy | 11 | 20 |
| Motor deficit | 26 | 47.3 |
| Hemiplegia | 16 | 29 |
| Paraplegia | 3 | 5.4 |
| Quadriplegia | 7 | 9.8 |
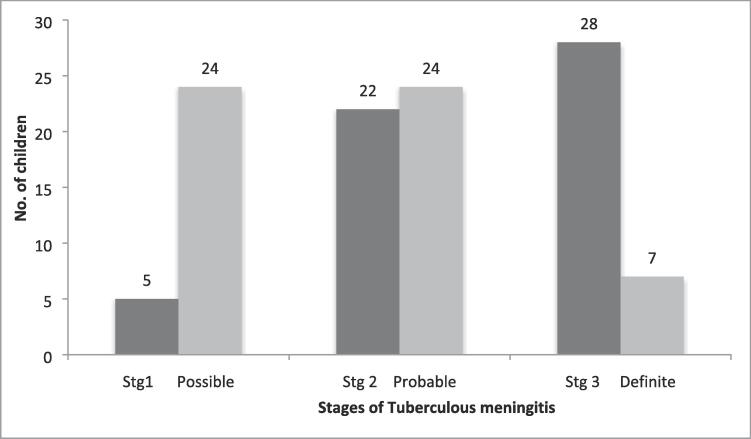
In this study, 28 (50.9%) children were in stage III, followed by stage II in 22 (40%). Only 5 (9.1%) children were in stage I. Seven (12.8%) children were included in definite TBM, 24 (43.6%) children were in probable category, whereas same number of children in possible category (Fig. 1).
Fig. 1.
Tuberculin test was positive in 22 (40%) children and 31 (56.3%) had chest radiograph suggestive of tuberculosis (milliary tuberculosis, cavity and hilar/paratracheal lymph nodes). The most common CT brain findings were communicating hydrocephalus in 23 (41.8%) followed by infarct; 16 (21.9) and basal exudates; 10 (18.2%). Tuberculoma was observed in 4 children only.
CSF routine cytochemical study of CSF showed lymphocytic predominance with low glucose along with high protein counts. Nine (16.4%) out of 55 cases showed positive CSF CBNAAT for Mycobacterial tuberculosis, of which 2 were rifampicin resistant (Table 2). MGIT culture was positive in 15 (27.3%) children. Six children, who are positive for CBNAAT, were also positive in CSF culture. Gastric aspirates were positive for Mycobacterial tuberculosis in 11 children. CSF CBNAAT was positive in 3,5 and one cases of definite, probable and possible category of tuberculous meningitis.
Table 2.
Cerebrospinal fluid analysis of children with Tuberculous meningitis.
| CSF routine microscopy | Mean ± Standard deviation |
|---|---|
| TLC (/micro liter) | 149.78 ± 26.5 |
| Neutrophils (%) | 33 ± 10.8 |
| Lymphocytes (%) | 55.11 ± 10.00 |
| Glucose (mg/dL) | 57.22 ± 14.7 |
| Protein (mg/dL) | 106.33 ± 28.4 |
| CBNAAT positive | 9(16.4%)* |
| Rifampicin sensitive | 7(77.8%) |
| Rifampicin resistant | 2(22.2%) |
| CBNAAT negative | 46(83.7%) |
| MGIT culture positive | 15(27.3%) |
CSF CBNAAT was positive in 3, 5 and one cases of definite, probable and possible category of tuberculous meningitis.
The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of CBNAAT are 40%(CI:22.9–52.2), 92.5%(CI:86.1–97.1), 66.7%(CI:38.2–87), 80.4%(CI:74.9–84.4), and 78.2%(CI68.9–84.8) as compared to CSF culture for Mycobactium tuberculosis (Table 3).
Table 3.
CSF CBNAAT for diagnosis of Tuberculous meningitis in children.
| Sensitivity % | Specificity % | PPV % | NPV % | Diagnostic accuracy% | |
|---|---|---|---|---|---|
| Present Study | 40 | 92.5 | 66.78 | 80.4 | 78.2 |
| Vadvai et al. [19] | 29 | – | – | – | – |
| Bhatia et al. [20] | 38.24 | – | – | – | – |
| Ratageri et al. [17] | 46.15 | 100 | --- | – | – |
| Solomon et al. [18] | 26 | 100 | 100 | 53 | – |
| Banker et al. [22] | 84.9 | 86.7 | 33.09 | 98.6 | 86.6 |
| Xiaocui et al. [21] | 34 | 96 | 98.6 | 15.4 | – |
4. Discussion
Childhood tuberculosis comprises 10%–20% of all cases in India and contributes about 8%–20% of deaths related to TB [1]. Tuberculous meningitis (TBM) is a major cause of mortality and morbidity due to diagnostic delay in children [5]. There is also lack of available tests with good sensitivity and specificity. So, there is need of test to diagnose tuberculous meningitis earlier with good accuracy. The present study was conducted to observe the role of CBNAAT in diagnosis of TBM. Out of 55 cases, only 9 (16.4%) were Gene Xpert positive, of which two were rifampicin resistant. Maximum numbers of children were in 5–10 years of age group i.e. 28 (50.9%) as against Ratageri et al. [17] and Solomons etal [18].
On physical examination, Neck rigidity and kernig’s sign was present in 30(54.5%) and Brudzinski sign; 26(47.3%). Eleven (20%) children had cranial nerve palsy and 26(47.3%) had motor deficits in form of hemiplegia, quadriplegia and paraplegia.
As there is no standard diagnostic criteria for TBM, we classified suspected TBM patients into definite (12.8%), probable (43.6%) and possible (43.6%) as per consensus case definition. Gene Xpert was endorsed by WHO in 2013 for extrapulmonary specimens. In our study sensitivity is 40%. This result is comparable to study of Vadvai et al. who reported sensitivity for CSF of 29% [19]. Bhatia et al., 2016 has also reported sensitivity of 29% [20] but Nguyen et al. [14], (2014) in adult has observed higher sensitivity i.e.59.3% of CBNAAT in cerebrospinal fluid in diagnosis of tuberculous meningitis (Table 3).
In present study, sensitivity, specificity , positive predictive value, negative predictive value and diagnostic accuracy of CBNAAT is 40%, 92.5%, 66.7%, 80.4% and 78.2% respectively as reported by Ratageri et al. [17] and Xiaocui et al. [21] There is higher positivity of CBNAAT of CSF in definite criteria. Nguyen et al., in adults reported sensitivity of 59.3%, specificity of 99.5%, negative predictive value is 72.5% and positive predictive value is 99.1% [14].
An outcome of children with tuberculous meningitis depends on many factors such as GCS, stage of TBM, raised intra cranial pressure. In our study, 14 children got successfully discharged without morbidity, 29 (52.7%) with morbidity (paresis, cranial nerve palsy, deafness, visual disturbances, cognitive defects) and 12 (21.8%) had died.
Although CSF detection by CBNAAT is low (16.4%) in present study with small sample size of children, positive result not only confirm diagnosis of tuberculous meningitis but also reveal about rifampicin sensitivity and resistance. Therefore CBNAAT also helps in planning of therapy.
The small sample size, small volume i.e. only 2 ml of cerebrospinal fluid used for CBNAAT and paucibacillary nature of the disease are limitations of the present study.
CRediT authorship contribution statement
Annapurna Rai: Data curation, Formal analysis. Rajniti Prasad: Conceptualization, Supervision. B.K. Das: Conceptualization, Supervision. Shampa Anupurba: Investigation. Utpal Kant Singh: Methodology.
Acknowledgements
We thank all patients and their parents for giving us consent for lumbar puncture and technical staffs of Gene XPERT laboratory for doing CBNAAT and culture.
References:
- 1.World Health Organization (2017). Global tuberculosis report 2017. Geneva, Switzerland.
- 2.Jones B.E., Young S.M., Antoniskis D. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 3.Nelson L.J., Wells C.D. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8:636–647. [PubMed] [Google Scholar]
- 4.Brancusi F., Farrar J., Heemskerk D. Tuberculous meningitis in adults: a review of a decade of developments focusing on prognostic factors for outcome. Future Microbiol. 2012;7:1101–1106. doi: 10.2217/fmb.12.86. [DOI] [PubMed] [Google Scholar]
- 5.Thwaites G.E. Advances in the diagnosis and treatment of tuberculous meningitis. Curr Opin Neurol. 2013;6:295–300. doi: 10.1097/WCO.0b013e3283602814. [DOI] [PubMed] [Google Scholar]
- 6.Lawn S.D., Zumla A.I. Diagnosis of extrapulmonary tuberculosis using the Xpert MTB/RIF assay. Expert Rev Anti Infect Ther. 2012;10:631–635. doi: 10.1586/eri.12.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drobniewski F., Nikolayevskyy V., Maxeiner H. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med. 2013;11:190. doi: 10.1186/1741-7015-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachow A., Clowes P., Saathoff E. Increased and expedited case detection by xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clin Infect Dis. 2012;54(10):1388–1396. doi: 10.1093/cid/cis190. [DOI] [PubMed] [Google Scholar]
- 9.Marais S., Pepper D.J., Schutz C., Wilkinson R.J., Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra U.K., Kalita J., Roy A.K., Mandal S.K., Srivastava M. Role of clinical, radiological, and neurophysiological changes in predicting the outcome of tuberculous meningitis: a multivariable analysis. J Neurol Neurosurg Psychiatr. 2000;68:300–303. doi: 10.1136/jnnp.68.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marais S., Thwaites G.E., Schoeman J.F. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 12.Ozateş M., Kemaloglu S., Gürkan F., Ozkan U., Hoşoglu S., Simşek M.M. CT of the brain in tuberculous meningitis. A review of 289 patients. Acta Radiol. 2000;41:13–17. doi: 10.1034/j.1600-0455.2000.041001013.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalita J., Misra U.K., Ranjan P. Predictors of long-term neurological sequelae of tuberculous meningitis: A multivariate analysis. Eur J Neurol. 2007;14:33–37. doi: 10.1111/j.1468-1331.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T.Q.N., Heemskerk D. Evaluation of Xpert MTB/RIF for the diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52:226–233. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (2008). Molecular line probe assays for rapid screening of patients at risk of multi-drug resistant tuberculosis (MDR-TB) 2008. Geneva, Switzerland.
- 16.British Medical Research Council Streptomycin treatment of tuberculous meningitis. BMJ. 1948;1:582–597. [Google Scholar]
- 17.Ratageri V.H., Illalu S., Fattepur S.R., Wari P.K. The utility of CSF Xpert /RIF in diagnosis of Tubercular Meningitis in Children. Indian J Pediatr. 2019;86(12):1089–1093. doi: 10.1007/s12098-019-03032-0. [DOI] [PubMed] [Google Scholar]
- 18.Solomons R.S., Visser D.H., Donald P.R., Marais B.J., Schoeman J.F., van Furth A.M. The diagnostic value of cerebrospinal fluid chemistry results in childhood tuberculous meningitis. Child Nerv Syst. 2015;31(8):1335–1340. doi: 10.1007/s00381-015-2745-z. [DOI] [PubMed] [Google Scholar]
- 19.Vadwai V., Boehme C., Nabeta P., Shetty A., Alland D., Rodrigues C. Xpert MTB/RIF: A new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49:2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatia R., Dayal R., Jindal S., Agarwal D., Goyal A. GeneXpert for Diagnosis of Tubercular Meningitis. Indian Journal of Pediatr. 2016;83:1353–1355. doi: 10.1007/s12098-016-2096-0. [DOI] [PubMed] [Google Scholar]
- 21.Wua X., Tanb G., Gaoa R., Yaoc L., Bid D., Guoa Y. Assessment of the Xpert MTB/RIF Ultra assay on rapid diagnosis of extrapulmonary tuberculosis. Int J Infect Dis. 2019;81:91–96. doi: 10.1016/j.ijid.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 22.Bankar S., Set R., Sharma D., Shah D., Shastri J. Diagnostic accuracy of Xpert MTB/RIF assay in extrapulmonary tuberculosis. Indian J Med Microbiol. 2018;36(3):357–363. doi: 10.4103/ijmm.IJMM_18_173. [DOI] [PubMed] [Google Scholar]