Abstract
Aim
To conduct a systematic review of the literature on biosafety with the use of lasers.
Methods
The systematic review of literature was performed using MEDLINE (PubMed), Science Direct and Web of Science databases. The electronic search strategy included terms in the Medical Subject Heading (MeSH) related to biosafety in dentistry and laser, forms of contamination with aerosols, as well as their synonyms. The selected keywords were “aerosol virus transmission dentistry,” “laser‐generated air contaminants,” “biosafety dentistry laser” combined with the terms AND/OR.
Results
A total of 1334 abstracts were reviewed, resulting in inclusion of 23 reviews. The dental surgeons are professionals with a high risk of contamination; high‐power lasers form aerosols that need to be controlled and low‐power lasers must be protected to minimize the risks of cross‐infection.
Conclusion
The biosafety of using lasers is important for professionals can be more oriented as to the correct use of this equipment. This study has the relevance of showing biosafety measures for the professional, staff and patients, as well as suggesting that more studies that are clinical should be conducted in this area.
Keywords: 2019‐nCoV pandemic, aerosols, biosafety, COVID‐19, lasers
1. INTRODUCTION
Recently, the World Health Organization (WHO) has declared a pandemic due to the spread of SARS‐CoV‐2 (severe acute respiratory syndrome of coronavirus), which rapidly infected communities in different countries through sneezing, coughing, and inhalation of droplets or by indirect contact with oral, nasal, and ocular mucous membranes. 1 , 2 , 3 , 4 , 5 Dental professionals play a crucial role in preventing the transmission of this viral infection, as aerosols and droplets are its main means of propagation. 1 , 2 , 4 , 6 , 7 , 8 , 9 Thus, dental offices must be an environment of great control and prevention of microbiological infections in general. Professionals need to adopt individual protection measures, and laser equipment, which are very well indicated in several specialties, also needs to follow biosafety standards.
The oral cavity is the place on the body with the highest concentration of microorganisms, thus exposing the clinical environment to biological risks. 1 , 2 , 5 The clinical routine is characterized by several disease‐transmitting vehicles and high patient turnover, which imply an increasing frequency of handling biological material and the consequent need to keep the equipment and the entire care structure safe. 10 In addition to all the care required to protect the equipment and instruments used during treatment against possible biological risks, the professional and his or her team, as well as the patients, must be protected. The use of appropriate personal protective equipment (PPE) is essential, as are other practical changes in the adoption of biosafety measures, since the SARs‐CoV2 virus is highly transmissible. 6 , 11
With the increasing use of lasers in dental offices and the current panorama of the large number of people affected by SARS‐CoV‐2, it is of utmost importance that more extreme biosafety measures be adopted to prevent cross‐transmission between patients and professionals when using laser equipment. 12 , 13
There are two types of lasers. High‐power lasers have photothermal effects and produce spray and/or ablation plume. 14 , 15 Low‐power lasers have modulating effects on the inflammatory process, accelerating the healing process and analgesia, which, when associated with a photosensitizer, also has antimicrobial effects and the significant advantage of not producing aerosol. 12
Thus, the laser equipment used in dentistry also needs to be used with care, so that the risks of cross‐infection are minimized and even avoided. In this study, a review is performed on the forms of viral transmission in dental offices and on ways to prevent them, with an emphasis on the laser equipment used in dentistry.
2. MATERIALS AND METHODS
2.1. Searching method
Three independent researchers performed an electronic search of articles from January to May 2020, covering the international databases MEDLINE (PubMed) (title/abstract), Science Direct (Title/Abstract/Keyword), and Web of Science (Topic). The electronic search strategy included terms in the Medical Subject Heading (MeSH) related to biosafety in dentistry and laser, forms of contamination with aerosols, and their synonyms. The selected keywords were “aerosol virus transmission dentistry,” “laser‐generated air contaminants,” and “biosafety dentistry laser,” combined with the terms AND/OR. The authors also manually searched the reference lists of the selected articles to identify other possible relevant studies.
2.2. Selection of studies
The researchers independently reviewed the title and abstract of all identified articles and included all that were potentially relevant. The authors obtained full texts of all possibly relevant abstracts and independently reviewed them to identify inclusion/exclusion criteria.
Two independent reviewers screened the full‐text publications against the specified inclusion criteria; disagreements were resolved through discussion.
Each paper was given a score accordingly Assessment of Multiple Systematic Reviews (AMSTAR 2) to analyze the quality (high, moderate, low) of the included studies.
2.3. Inclusion/exclusion criteria
The inclusion criteria were articles published in English, Spanish, or Portuguese; full articles portraying the systematic review's theme; and articles published and indexed in the referred databases within the last 10 years.
The exclusion criteria were article published in other languages; articles reporting only a clinical case and articles that did not address the theme of this review.
2.4. Data collection
A data‐extraction form was designed to clarify the details of each study. The researchers independently read the full texts of the included articles and extracted all of the characteristics from each study, such as its study design, population, intervention, and main considerations. The articles’ topics were categorized into three groups: forms of viral transmission in dental offices, forms of contamination from using high‐power lasers and how to minimize risks, and forms of contamination from using low‐power lasers and how to minimize the risks.
3. RESULTS
In total, 1334 articles were found. After reading the abstracts and applying the inclusion and exclusion criteria, only 23 articles were selected for this review (Figure 1). The excluded articles published outside the selected period, are not related to forms of viral transmission in a dental office or were merely the author's comments/opinions and was neither descriptive nor observational. Table 1 summarizes the excluded articles after their full texts read and the respective reasons for exclusion.
FIGURE 1.
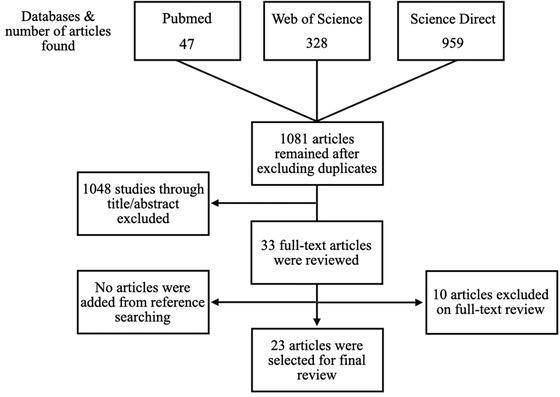
Flowchart representing the article selection
TABLE 1.
Summary of articles excluded after full reading and reasons
| Authors | Periodic (year) | Reason |
|---|---|---|
| Muñoz‐Leyva et al | Can J Anesth. (2020). | This study is a letter to the editor reinforcing the importance of using PPE by dentists. |
| Yousif Ali et al | Int J Surg. (2020). | Letter to the editor reporting on the signs and symptoms of COVID‐19. |
| Nicola et al | Int J Surg. (2020). | Article describing prevalence, signs, symptoms, and forms of transmission, complications and prevention of COVID‐19. |
| Al‐Jabir et al | Int J Surg. (2020). | It presents a guide to treatment with COVID‐19 for patients with some pre‐existing diseases. |
| Pan et al | Microb Infect. (2020). | It portrays lessons about the pandemic caused by COVID‐19 and the economic crisis that will be faced in 2020. |
| Jerrold | American Journal of Orthodont Dentofac Orthoped. (2020). | It raises some questions related to dental care during the pandemic, including the professional's responsibility to care for his patients. A signature on a term does not release you from liability. |
| Yu Fei et al | Microb Infect. (2020). | It reports coronavirus diagnostic measures and how it causes pneumonia. It speaks of the beginning in China and perspectives. |
| Rima B et al | J Gen Virol. (2017). | Explains the taxonomy of the virus of the family Pnemoviridae. |
| Sohrabi et al | Int J Surg. (2020). | This paper reports a review of the literature of the year 2019 related to the virus that causes COVID‐19, its effects, forms of transmission and prevention recommended by WHO. |
| Coulthard | British Dent J. (2020). | It reports the author's opinion about the dental team being familiar with the assessment of the risk of cross‐infection, but should not be placed at risk unnecessarily, as this would be morally unacceptable. |
3.1. Characteristics of the selected studies
All the selected articles were in English and/or Portuguese. The countries where the primary studies included in this review took place were Brazil (3), the Netherlands (2), Italy (1), China (3), Saudi Arabia (2), Pakistan (1), India (2), Canada (1), the USA (6), Germany (1), and the Republic of Korea (1). Tables 2, 3, 4 discuss, respectively, forms of viral transmission in dental offices, forms of contamination when using high‐power lasers, forms of contamination when using low‐power lasers and how to minimize them.
TABLE 2.
Summary of the studies included in their aerosol virus transmission dentistry
| Risks and forms of viral transmission in dental offices | |||||
|---|---|---|---|---|---|
| Title | Authors | Periodic (year) | Study/design | Population/intervention | Considerations |
| Biosafety conducts adopted by orthodontists. | Gonçalves C, Monteiro J, Martins M | Dental Press J Orthod (2018). | Observational | It evaluates the biosafety conducts adopted by orthodontists and possible differences regarding training time. | 90 orthodontists answered the questionnaires. 63.23% use an autoclave to sterilize orthodontic pliers. All use an autoclave to sterilize instruments and 95.6% of the interviewees carry out previous cleaning with chemical products. 65.56% sterilize the bands in an autoclave and associate disinfection methods. For items such as rubber bands, accessories, bandages, metal springs and bows, there was a high “nothing” response rate. All respondents wear gloves and masks, while 78.92% wear aprons, 58.92% wear goggles and 50.01% wear hats. |
| Cross‐transmission in the dental office: does this make you ill? | Volgenant CMC, Soet JJ De | Curr Oral Health Rep (2018). | Descriptive | It provides the most recent information on the risks related to the transmission of (pathogenic) microorganisms in the dental office. | The risk of transmission of pathogens in a dental office is still unknown, but it cannot be considered insignificant. Thus, infection control in the dental office must be considered an essential item. |
| The severe acute respiratory syndrome coronavirus‐2 (SARS CoV‐2) in dentistry. Management of biological risk in dental practice. | Giudice R Lo | Int J Environ Res Public Health (2020). | Descriptive | It suggests more appropriate procedures in all aspects of dental practice to reduce the risk of infection. | Considering the route of transmission of the virus, a specific protocol must be applied to reduce the risk of infection, in addition to measures that prevent the spread of infection from a patient to another person or medical tools and equipment (cross‐infection). |
| Saliva is a non‐negligible factor in the spread of COVID‐19. | Li Y, Ren B, Peng X, et al | Mol Oral Microbiol (2020). | Descriptive | It suggests some protective measures that can help to reduce the risk of saliva‐related transmission, in order to prevent the possible spread of SARS‐CoV‐2 among patients, visitors, and dentists. | The control of saliva‐related transmission in the dental clinic is essential, especially in the epidemic period of COVID‐19. |
| COVID‐19: present and future challenges for dental practice. | Odeh ND, Babkair H, Abu‐hammad S, Borzangy S | Int J Environ Res Public Health (2020). | Descriptive | It addresses several issues related to the COVID‐19 pandemic that are directly related to dental practice in terms of prevention, treatment, and orofacial clinical manifestations. | Dentists must always competently follow the cross‐infection control protocols, but especially during this critical period, they must do their utmost to decide on the emergency cases indicated for dental treatment. Dentists should also be updated on how this pandemic is related to their profession, in order to be well oriented and prepared |
| Role of respirators in controlling the spread of novel coronavirus (Covid‐19) among dental health care providers: a review. | F. Umer, Z. Haji KZ | Int Endod J (2020). | Descriptive | Discuss respirators, their purpose, types, clinical efficiency and appropriate placement and removal techniques to prevent infection in times of COVID‐19 pandemic. | As the most common route of transmission is via aerosols and droplet inhalation, it is essential that healthcare professionals have the correct personal protective equipment (PPE), including clothing, masks, and goggles. |
| Precautions and recommendations for orthodontic settings during the COVID‐19 outbreak: a review | Turkistani KA | Am J Orthod Dentofacial Orthop (2020). | Descritivo | It reviews the literature in the period of March 2020, using the word set (CORD‐19 2020), infection control and disease transmission in orthodontics. | Emphasizes the minimization of aerosol production and the reinforcement of strict infection control measures. Reinforces strict infection control measures and minimizes personal contact and aerosol production are essential to avoid contamination in orthodontic configurations. |
| Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents | Kampf G, Todt D, Pfaender S, Steinmann E | J Hosp Infect (2020). | Descriptive | Reviews the literature on all available information on the persistence of human and veterinary coronavirus on inanimate surfaces, as well as inactivation strategies with biocidal agents used for chemical disinfection, for example, in health facilities. | Analysis of 22 studies reveals that viruses can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days, but can be effectively inactivated by surface disinfection procedures with 62‐71% ethanol, 0% hydrogen peroxide, 5% or 0.1% sodium hypochlorite in 1 minute. Other biocidal agents, such as 0.05‐0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective. |
| Transmission routes of 2019‐nCoV and controls in dental practice | Peng X, Xu X, Li Y, et al | Int J Oral Sci (2020). | Descriptive | Recommendations for infection control measures during dental practice to block transmission routes from person to person in dental clinics and hospitals. | The 2019‐nCoV person‐to‐person transmission routes included direct transmission, such as coughing, sneezing, droplet inhalation transmission, and contact transmission, such as contact with the oral, nasal and ocular mucous membranes. 2019‐nCoV can also be transmitted via saliva, and oral‐fetal routes can also be a potential route of transmission from person to person. Dental practice participants are exposed to a tremendous risk of infection by 2019‐nCoV due to face‐to‐face communication and exposure to saliva, blood, and other body fluids and the handling of sharp instruments. |
| Rapid detection of SARS‐CoV‐2 in saliva: can an endodontist take the lead in point‐of‐care COVID‐19 testing? | Sidhartha Sharma, Vijay Kumar, Amrita Chawla AL | Int Endod J (2020). | Descriptive | It exposes the desire for alternative, fast, and prompt diagnostic tools (POC) and sensitive to COVID‐19, which can be used routinely by endodontists, using saliva as a sample before starting an emergency procedure. | There are still few diagnostic tools proposed in the literature. All the methods mentioned, need further research so that their sensitivity and validity are used with a salivary sample. If approved, it can offer an opportunity to allow detection of salivary viruses in an endodontic facility without the need for a complex diagnostic infrastructure. The chair test would help to reduce the waiting period and allow immediate intervention. In addition, patients with a negative test can be treated routinely after the emergency restrictions have ended. |
| Being a front‐line dentist during the Covid‐19 pandemic: a literature review | Fallahi HR, Keyhan SO, Zandian D, et al | Maxillofac Plast Reconstr Surg (2020). | Descriptive | Information collected so far about the virus, according to the guidelines of international health institutions, and provides a comprehensive protocol to manage possible exposure to patients or suspected of having coronavirus. | Face‐to‐face communication and consistent exposure to body fluids, such as blood and saliva, predispose dental care professionals to serious health problems. risk of infection 2019‐nCoV. Dental practice can be a potential risk for the dental team and there is a high risk of cross infection. |
| Possible aerosol transmission of COVID‐19 and special precautions in dentistry. | Ge Z, Yang L, Xia J, et al | J Zhejiang Univ Sci B (2020). | Descriptive | It was a survey of the works showing the forms of viral transmission in the dental offices. | So far, there is no report of infection in dental offices. She reports that the oral cavity is a place of high risk of transmission of the Sars‐Cov‐2 virus and that the best way to avoid cross‐infection is the use of personal protective equipment, washing hands, using absolute isolation, in addition to indicating strategies for some dental specialties. |
| H1N1 influenza: assessment of knowledge and awareness of private dental health professionals of a Tricity. | Singh I, Munjal S, Kumar M, Jha M GR, BT | J Family Med Prim Care (2019). | Observational | Cross‐sectional study among 255 private dentists who work at Tricity. A self‐administered, anonymous and multiple choice questionnaire was applied to collect information. The questionnaire contained 12 questions about knowledge and awareness about the maintenance of swine flu, given the time constraints. | Awareness about the mode of transmission of swine flu was positively reported by 88.5% of individuals. About 24.6% of individuals reported having met a patient with swine flu at their clinic. Preventive measures to prevent the spread of swine flu were known to 71.2% of individuals. |
| A scoping review on bio‐aerosols in healthcare and the dental environment. | Zemouri C, Soet H De, Crielaard W, Laheij A | PLoS One (2017). | Descriptive | Reviews evidence of bio‐aerosols in the health and dental fields. | The search resulted in 5823 studies, of which 62 were included. Dental parts of the hands were found to generate aerosols in dental configurations. Another 30 sources of human activities, interventions and daily cleaning at the hospital also generate aerosols. 55 bacterial species, 45 genera of fungi and 10 viruses in a hospital environment and 16 bacterial and 23 fungal species in the dental environment were identified. Patients with certain risk factors were more likely to acquire Legionella in hospitals. Such infections can lead to irreversible septic shock and death. Only a few studies have found that bio‐aerosol generation procedures have resulted in the transmission of infectious diseases or allergic reactions. |
| Cough aerosol in healthy participants: fundamental knowledge to optimize droplet‐spread infectious respiratory disease management. | Zayas G, Chiang MC, Wong E, et al | BMC Pulm Med (2012). | Observational | It characterizes the aerosol pattern of human cough in order to develop a standard bioaerosol model for human cough for the control of the influenza pandemic. | The voluntary cough generated droplets ranging from 0.1 to 900 microns. Drops smaller than 1 μm represent 97% of the total number of measured droplets contained in the cough aerosol. Age, sex, weight, height, and body mass have no statistically significant effect on the composition of the aerosol in terms of size and number of droplets. |
| Analysis of reported work accidents involving healthcare workers and exposure to biological materials. | Soares RZ, Schoen AS, Da Rocha Gomes Benelli K, et al | Rev Bras Med Trab (2019). | Observational | It presents the epidemiological profile of health professionals victims of accidents involving biological materials in Canoas, Rio Grande do Sul, Brazil, in 2017. | There were 121 occupational accidents with exposure to biological materials in 2017. Accidents prevailed among women (93.4%), whites (69.4%) and workers aged 20 to 30 years (40.5%). Percutaneous exposure was associated with 76.8% of accidents, blood was the most prevalent biological material involved (90%) and hollow needles were the main causative agent (64.5%). Gloves were the most used personal protective equipment (PPE) (75.2%). About 93.4% of the sample was vaccinated against hepatitis B. |
TABLE 3.
Summary of the studies included in on biosafety in the use of low‐power lasers
| Biosafety and low‐power lasers | |||||
|---|---|---|---|---|---|
| Title | Authors | Periodic (year) | Study/design | Population/intervention | Considerations |
| Influence of biosafety materials of the laser output power. | Rodrigues FCN, de Araújo JGL, dos Santos Araújo EM, et al | Lasers Med Sci, 2020. | Observational | Evaluates the interference of protective materials in the light output of low‐power lasers. | All groups had reduced output power after placing protective material when related to the time factor. When compared to the materials used for protection, the protective material containing polyethylene (HDPE) showed a greater reduction in output power than the protective material of polyvinyl chloride (PVC) for red and infrared wavelengths. |
TABLE 4.
Summary of the studies included in on biosafety in the use of high‐power lasers
| Biosafety and high‐power lasers | |||||
|---|---|---|---|---|---|
| Title | Authors | Periodic (year) | Study/design | Population/intervention | Considerations |
| Laser‐generated air contaminants from medical laser applications: a state‐of‐the‐science review of exposure characterization, health effects, and control. | Taylor P, Pierce JS, Lacey SE, et al | J Occup Environ Hyg, 2011. | Descriptive | It analyzes the published literature regarding the chemical and physical composition of the laser‐induced plume, health effects and control methods. | Few studies have attempted to characterize the effects of the type of laser system, power and treated tissue, with regard to exposure to airborne contaminants generated by laser (LGACs). In addition, current control strategies do not seem to be adequate in preventing occupational exposure to LGACs. |
| Application of a two‐zone model to estimate medical laser‐generated particulate matter exposures. | Lopez R, Lacey SE, Jones RM | J Occup Environ Hyg (2015). | Observational | Estimates the exposure of particulate matter to two simulated laser medical procedures using a near‐field/far‐field model. | The results indicate that the concentrations in the simulated scenarios are similar to those obtained in limited field evaluations performed in the hospital's operating rooms. |
| Characterization of size‐specific particulate matter emission rates for a simulated medical laser procedure—a pilot study. | Lopez R, Lacey SE, Lippert JF, et al | Ann Occup Hyg (2015). | Observational | It aims to determine the effect of laser operating parameters on the emission rate of specific mass size of particulate material generated by laser through medical procedures simulated in the laboratory. | Provides a basis for future research to better estimate size specific mass emission rates and particle characteristics for additional laser operating parameters, in order to estimate occupational exposure and inform control strategies |
| Modeled occupational exposures to gas‐phase medical laser‐generated air contaminants. | Taylor P, Lippert JF, Lacey SE, et al | J Ocupe o Environ Hyg (2014). | Observational | Monitors potential exposure to laser‐generated air contaminants (LGAC). | Although the estimated exposures are below a level of health concern, only a subset of LGACs and types of lasers in clinical use have been considered. Particulate matter and other chemical constituents can pose a health risk to medical personnel and patients. Smoke evacuators are recommended and are the most effective tool for controlling surgical smoke, although the frequency of use of the smoke evacuator is as low as 17%, depending on the procedure. |
| A pilot study to determine medical laser generated air contaminant emission rates for a simulated surgical procedure. | Lopez R, Lacey SE, Lippert JF, et al | J Occup Environ Hyg (2015). | Observational | It establishes a methodology to estimate the emission rates of laser‐generated air contaminants (LGACs) using an emission chamber and to perform a screening study to differentiate the effects of three laser operating parameters. | Confined to the experimental conditions of this screening study, the results indicated that the beam diameter was statistically influential and the marginal power statistically significant in the CO2 emission rates when using the Ho: YAG laser, but not with the carbon dioxide laser; the pulse repetition frequency (PRF) did not influence the emission rates of these contaminants in the gas phase. |
| An assessment of the occupational hazards related to medical lasers. | Pierce JS, Lacey SE, Lippert JF, et al | J Occup Environ Med (2011). | Descriptive | Performs a bibliographic search on PubMed, and all articles relevant to the risks of medical laser with and without beam. | Eye injuries, skin burns, injuries related to the onset of fires, and electric shock have been reported in relation to the medical use of lasers. It is likely that acute and chronic health effects have been experienced by medical personnel as a result of exposure to laser‐generated air contaminants. |
We identified 33 citations of which none was systematic review. Most of the studies were published in the last five years. The “aerosol virus transmission dentistry” was the most researched intervention, the most common group outcome was “laser‐generated air contaminants” and studies spanned a wide diversity of outcomes, effects, and populations.
4. DISCUSSION
The risk of contamination in dental offices is high, and the dentist is highly potential contamination 1 , 5 , 7 , 9 , 16 , 17 , 18 , 19 because the aerosol produced can contain viral particles. 4 , 5 , 8 , 16 , 20 There is also the risk of accidents leading to exposure to biological materials. 17 Considering the current pandemic context, dentists need to adopt more rigorous biosafety measures in their daily routines since the transmission of the SARs‐CoV2 virus is high and we are not know alright the quality of the vaccine.
Lasers are widely used in daily routines and need to be more widespread, as low‐power ones do not produce aerosols and, in addition to this advantage given the current pandemic, can also be used to modulate the inflammatory process, to accelerate tissue repair, and in analgesia. 12 Though the high‐laser power produce aerosols and it is widely used. Thus, it is extremely important to carry out a bibliographic survey of the various ways of using lasers safely and avoid cross‐contamination in dental offices.
Unfortunately, this manuscript evidenced a lack of research on the subject and, therefore, the classification of quality of the selected articles would be impossible, since during the bibliographic search there was no systematic review. However, we use the adapted AMSTAR2 because it includes other types of studies. The need to conduct clinical and / or cellular studies was realized to strengthen the literature regarding the need for biosafety also in the use of lasers.
For this to be possible, biosafety measures must be disseminated clearly and objectively so that health professionals, especially dentists, are prepared for the new conduct that they must take. Salivary components interact with several viruses, and viral transmission may be closely linked to saliva—not only SARS‐CoV‐21,2 but also Epstein‐Barr virus (EBV); herpes simplex virus (HSV); hepatitis A, B, and C viruses (HAV, HBV, HCV); cytomegalovirus (CMV), human immunodeficiency virus (HIV), Chikungunya virus (CHIKV), and Ebola, among others. 1 According to Table 2, perceived the need for to adopt for measures to protect professionals and patients against contamination through aerosols produced in the offices, which must be met with even more rigor after this pandemic.
Gonçalves et al 10 conducted a questionnaire among orthodontic surgeons and revealed that all of the respondents wore gloves and masks, but only 50% wore surgical caps and approximately 78% wore protection goggles. These data are worrying because of the high risk of contamination when health professionals’ eyes, nose, or mouth are unprotected during the performance of medical/dental procedures that generate aerosol in patients infected with SARS‐CoV‐2, there is a high risk of contamination. 4 , 5 , 6 , 8 , 11 Therefore, use of personal protective equipment, such as plastic visors, goggles, filters for face parts class 2/3 (FFP2/3), or N95 masks, which filter 94%/99% and 95% of airborne particles, respectively, are essential in healthcare environments to prevent further spread of disease. 21 Sharma et al 22 suggested that a rapid test for detecting SARS‐CoV‐2 in saliva is possible and available to dentists to expand dental care during this pandemic period. If this test is feasible, it will be an excellent opportunity to streamline dental care during this period, reassuring both professionals and asymptomatic patients, who may or may not be contaminated.
Table 3 shows the scarcity of studies that portrays care with biosafety using low‐power lasers. Low‐power lasers are widely used in several dentistry specialties. They can be used to reduce the microbial load, through photodynamic therapy (aPDT), or even to modulate the inflammatory process, by accelerating tissue repair and promoting analgesia. Both aPDT and photobiomodulation were used during this pandemic period, and they are well indicated as supporting agents in dental treatments because they do not produce aerosols.
Low‐power laser equipment must deliver an adequate dose to irradiated tissues. The amount and quality of irradiation, as well as the coverage area, interfere with how the tissue will react to therapy. 12 In this context, it is necessary to protect the exit area of the laser that will encounter the biological tissue through plastic barriers to prevent cross‐infection. According to Rodrigues et al, 12 these barriers need to be transparent and be well adapted to the tip to prevent significant loss in the laser's power output from interfering in the effects on the target tissues. It is important that more studies be carried out to expand the options for biosafety measures.
On the other hand, high‐power lasers form a spray, ablation plume, or vaporization due to their increased temperature, which produce aerosols. 12 , 13 , 14 , 23 This plume is generated as a result of the irradiated target cells being heated, to the point of causing membranes to rupture, as well as pyrolysis and combustion of the target material. 13 , 23 , 24 The quantity and characteristics of the aerosolized cellular matter are determined by the type of laser used, the dose used, and the type of tissue to be treated. 13 , 24
Several researchers have sought to determine the composition of the laser surgical plume, with specific emphasis on its chemical constituents and particles, including of infectious agents. 13 , 24 The analysis of microorganisms shows bacteria, mycobacteria, fungi, and viruses present in the plume or steam produced by lasers. 13 Table 4 depicts studies performed with these types of equipment.
Unfortunately, there are few existing study, and the most recent are from 2015—that is, they precede the 2020 pandemic. Therefore, no reports exist of the composition of plumes containing SARS‐CoV‐2, but it can be deduced that if lasers are used in patients infected with COVID‐19, the virus will be present in that plume, since Peng et al 1 and Ge et al 5 reported the presence of this virus in the oral cavity. Thus, it is important to use suckers close to the irradiation when using high‐power lasers to remove both the spray and the steam formed during the procedure.
Due to the severity and easily of transmission of the virus that causes COVID‐19, this study brings important data regarding the need to carry out more studies that relates laser and virus, as well as how to prevent this contamination during practices laser clinics. Thus, it is important that preventive measures be adopted to help reduce spread of the SARS‐CoV‐2 virus and others as well.
Lopez et al 15 carried out a pilot study to develop a prototype to decrease the size of particles formed by medical laser equipment, to minimize contamination, estimate occupational exposure, and think about control strategies. Thus, it would be possible to determine the real risks of exposure to professionals and thus to be able to communicate with the health team, so that awareness can be raised about seeking control strategies and devices can be developed to minimize contamination.
Health professionals, including dental surgeons, must be aware that their care routine will never be the same. Professionals who are qualified to use lasers in a practical and safe way need to date on safety measures to employ this technology, in ways that avoid cross‐contamination and reduce aerosols.
5. CONCLUSION
This systematic review demonstrates that there are few studies demonstrate the biosafety care that dentists need with laser equipment. It is extremely important that safe measures are taken to avoid cross‐contamination, since the risk of viral transmission is high in dental offices. It is also necessary that more high‐quality research and the use of standardized methods be carried out for this purpose.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to be disclosed.
ETHICAL CONSIDERATIONS
Not applicable.
Lago ADN, Cordon R, Gonçalves LM, et al. How to use laser safely in times of COVID‐19: Systematic review. Spec Care Dentist. 2021;41:463–473. 10.1111/scd.12593
REFERENCES
- 1. Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019‐nCoV and controls in dental practice. Int J Oral Sci. 2020;3(12):9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Ren B, Peng X, Hu T, Li J, Gong T, et al. Saliva is a non‐negligible factor in the spread of COVID‐19. Mole Oral Microbiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sohrabi C, Alsafi Z, O'Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umer F, Haji Z, Zafar K. Role of respirators in controlling the spread of novel coronavirus (COVID‐19) amongst dental healthcare providers: a review. Int Endodontic J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. yu GeZ, ming YangL, jia XiaJ, hui FuX, zhen ZhangY. Possible aerosol transmission of COVID‐19 and special precautions in dentistry. J Zhej Univ: Sci B. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lo Giudice R. The severe acute respiratory syndrome coronavirus‐2 (SARS CoV‐2) in dentistry. Management of biological risk in dental practice. Int J Environ Res Public Health. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Odeh ND, Babkair H, Abu‐Hammad S, Borzangy S, Abu‐Hammad A, Abu‐Hammad O. COVID‐19: present and future challenges for dental practice. Int J Environ Res Public Health. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turkistani KA. Precautions and recommendations for orthodontic settings during the COVID‐19 outbreak: a review. Am J Orthod Dentofaci Orthoped. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fallahi HR, Keyhan SO, Zandian D, Kim S‐G, Cheshmi B. Being a front‐line dentist during the Covid‐19 pandemic: a literature review. Maxill Plastic Reconstruct Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monteiro CGJ, Martins E Martins M, de Cury‐Saramago A A, Teixeira HP. Biosafety conducts adopted by orthodontists. Dent Press J Orthod. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volgenant CMC, de Soet JJ. Cross‐transmission in the dental office: does this make you ill? Curr Oral Health Rep. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodrigues FCN, de Araújo JGL, Dos Santos Araújo EM, Lago ADN, Mantilla TF, de Freitas PM. Influence of biosafety materials of the laser output power. Lasers Med Sci [Internet]. 2020. http://link.springer.com/10.1007/s10103‐020‐03030‐1. Available from. [DOI] [PubMed] [Google Scholar]
- 13. Pierce JS, Lacey SE, Lippert JF, Lopez R, Franke JE, Colvard MD. An assessment of the occupational hazards related to medical lasers. J Occup Environ Med. 2011. [DOI] [PubMed] [Google Scholar]
- 14. Pierce JS, Lacey SE, Lippert JF, Lopez R, Franke JE. Laser‐generated air contaminants from medical laser applications: a state‐of‐the‐science review of exposure characterization, health effects, and control. J Occup Environ Hyg. 2011. [DOI] [PubMed] [Google Scholar]
- 15. Lopez R, Lacey SE, Lippert JF, Liu LC, Esmen NA, Conroy LM. Characterization of size‐specific particulate matter emission rates for a simulated medical laser procedure—a pilot study. Ann Occup Hyg. 2015. [DOI] [PubMed] [Google Scholar]
- 16. Zayas G, Chiang MC, Wong E, et al. Cough aerosol in healthy participants: fundamental knowledge to optimize droplet‐spread infectious respiratory disease management. BMC Pulm Med. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soares RZ, Schoen AS, Da Rocha Gomes Benelli K, Araújo MS, Neves M. Analysis of reported work accidents involving healthcare workers and exposure to biological materials. Revista Brasileira de Medicina do Trabalho. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh I, Munjal S, Kumar M, Jha M, Gambhir R, Talukdar B. H1N1 Influenza: assessment of knowledge and awareness of private dental health professionals of a Tricity. J Fam Med Prim Care. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zemouri C, De Soet H, Crielaard W, Laheij A. A scoping review on bio‐aerosols in healthcare & the dental environment. PLoS ONE. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali Y, Alradhawi M, Shubber N, Abbas AR. Personal protective equipment in the response to the SARS‐CoV‐2 outbreak—a letter to the editor on World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg. 2020;76:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma S, Kumar V, Chawla A, Logani A. Rapid detection of SARS‐CoV‐2 in saliva: can an endodontist take the lead in point‐of‐care COVID‐19 testing? Int Endodontic J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez R, Lacey SE, Jones RM. Application of a two‐zone model to estimate medical laser‐generated particulate matter exposures. J Occup Environ Hyg. 2015. [DOI] [PubMed] [Google Scholar]
- 24. Lippert JF, Lacey SE, Jones RM. Modeled occupational exposures to gas‐phase medical laser‐generated air contaminants. J Occup Environ Hyg [Internet]. 2014;11(11):722‐727. http://www.tandfonline.com/doi/abs/10.1080/15459624.2014.916810. Available from. [DOI] [PubMed] [Google Scholar]


