Abstract
Background/Objectives
COVID‐19 has caused significant morbidity and mortality in nursing homes. Vaccination against SARS‐COV‐2 holds promise for reduction in COVID‐19. This operational analysis describes the proportion of SARS‐COV‐2 positive tests before, during, and after vaccination.
Design
Retrospective longitudinal cohort analysis from October 1, 2020 until February 14, 2021.
Setting
A total of 130 Department of Veterans Affairs (VA) Community Living Centers (CLC), analogous to nursing homes.
Intervention
Vaccination for SARS‐CoV‐2.
Measurements
The primary measure is the proportion of SARS‐CoV‐2 positive tests among CLC residents. In a pooled analysis of weekly testing and vaccine data, the proportion of positive tests was compared for the unvaccinated, first dose, and second dose. For each CLC, we identified the week in which 50% of CLC residents were vaccinated (index week). The analysis aligned the index week for CLCs and examined the proportion of SARS‐CoV‐2 positive tests at the CLC level before and after. As a reference, we plotted the proportion of positive tests in nursing homes in the same county as the CLC using publicly reported data.
Results
Within the pooled VA CLCs, the first SARS‐CoV‐2 vaccine dose was delivered to 50% of CLC residents within 1 week of availability and second dose within 5 weeks. Relative to the index week, the risk ratio of SARS‐CoV‐2 positive tests in the vaccinated relative to unvaccinated was significantly lower in Week 4 (relative risk 0.37, 95% confidence interval 0.20–0.68). Throughout the study period, the proportion of SARS‐CoV‐2 positive tests in community nursing homes was higher compared to VA CLC and also declined after vaccine availability.
Conclusion
The proportion of SARS‐CoV‐2 positive tests significantly declined in VA CLCs 4 weeks after vaccine delivery and continued to decline in vaccinated and unvaccinated residents. The results describe the importance of SARS‐CoV‐2 surveillance and vaccination in VA nursing home residents.
Keywords: COVID‐19, nursing home, vaccination
Short abstract
See related editorial by Ouslander et al and related articles by Mor et al, Moore et al, and Domi et al. in this issue.
Key Points
Protection against SARS‐CoV‐2 is vital for nursing home residents.
Once available, Veterans Affairs (VA) Community Living Centers rapidly disseminated the SARS‐CoV‐2.
VA Community Living Centers observed a reduction in the proportion of positive SARS‐CoV‐2 following vaccination.
Why Does this Paper Matter?
By reducing infection, SARS‐CoV‐2 vaccination holds promise as a mechanism to decrease pandemic‐related isolation of nursing home residents.
INTRODUCTION
Nursing homes have borne the brunt of the COVID‐19 pandemic, caused by the SARS‐CoV‐2 virus, with nearly one third of COVID‐19‐related fatalities nationwide. 1 As congregate living facilities for persons requiring skilled care and assistance in daily function, these settings have the potential to rapidly escalate viral transmissions. Nursing home SARS‐CoV‐2 rates fluctuate with the community prevalence and size of the facility. 2
The US Department of Veterans Affairs (VA) operates Community Living Centers (CLC), which are VA‐owned and ‐operated nursing homes. CLCs have a strong focus on social living in a home‐like environment. When COVID‐19 was first reported, the CLC environment transformed with a focus on preventing viral transmission through infection control practices, in line with national guidelines. 3 , 4 As the pandemic heightened, the VA CLCs adapted with protocols for symptom monitoring, resident and staff isolation, quarantine, and diagnostic and screen testing. When vaccines became available, the VA followed CDC guidance and prioritized vaccine delivery and administration to CLC residents and staff. 5
The objective of this analysis is to describe the proportion of SARS‐CoV‐2 positive tests among CLC residents before and after vaccination.
METHODS
Cohort
This is a retrospective analysis of VA operational data. CLCs (n = 130) operating across the United States and Puerto Rico were included. This analysis focuses on the time from October 1, 2020 to February 14, 2021. Consistent with Centers for Medicare and Medicaid Services (CMS) recommendations on hospital‐acquired infection, 6 the analysis excludes residents who tested positive for SARS‐CoV‐2 prior to or within 3 days of CLC admission. Summary characteristics of CLCs residents are provided from VA electronic records. This is a descriptive analysis of VA operational data conducted for nonresearch purposes, thus institutional review board approval was not required.
Variables
CLC testing and vaccination results were summarized weekly at the CLC level. The proportion of positive SARS‐CoV‐2 tests is calculated from both VA and non‐VA facilities as the number of positive tests divided by the number of residents present in the week. Vaccination is reported as the number of residents within a CLC during a week who were administered the first dose, the second dose, and those who were not. For CLCs, the SARS‐CoV‐2 positive rate is summarized weekly for the total population in the CLC that week, and by vaccinated and unvaccinated residents.
For nursing homes in the county of CLCs, the proportion of positive SARS‐CoV‐2 tests were collected from the CMS COVID‐19 Nursing Home Data public website 7 and reported. There are no publicly available data on non‐VA nursing home vaccination at this time.
Analysis
The first analysis uses weekly testing and vaccine data to describe the proportion of SARS‐CoV‐2 positive tests among vaccinated (first dose and second dose) and unvaccinated CLC residents using data pooled across all CLCs.
The second analysis examined whether the proportion of SARS‐CoV‐2 positive tests were lower in the postvaccination periods. The index period was the first week when over 50% of CLC residents received their first dose. For reference, a line representing the proportion of SARS‐CoV‐2 positive tests in community nursing homes in the same county during the same periods has been added. Figure S1 describes sensitivity analysis of using an index date with 25% and 75% vaccinated residents.
After centering on the index date, the third analyses examines the proportion of positive COVID tests in vaccinated residents relative to unvaccinated residents calculated weekly before and after the index date. The weekly calculated relative risks (RRs) with 95% confidence intervals (95% CI) are presented.
RESULTS
During the COVID‐19 pandemic, CLCs (n = 130) cared for an older (mean age 73.5, SD ±3.8 years), male (96.1%), and diverse (69% white, 22% black, 9% other) population with about half having a diagnosis of dementia (52.4%) and the majority residing long‐term (75%). VA CLCs vary in size (mean beds 84, SD ±46). For the reference community nursing homes (n = 4944), there were a mean 40 (SD ±49) nursing homes per county with a CLC.
Figure 1 presents the weekly number of veterans who received vaccination and the proportion of SARS‐COV‐2 positive tests. Pooled analysis of CLCs found that 50% of residents received their first vaccination by December 20, 2020, almost all of these received their second dose by January 17, 2021. As of January 21, 2021, 82% had begun or completed the vaccination process, 13% refused, and 5% were contraindicated.
FIGURE 1.
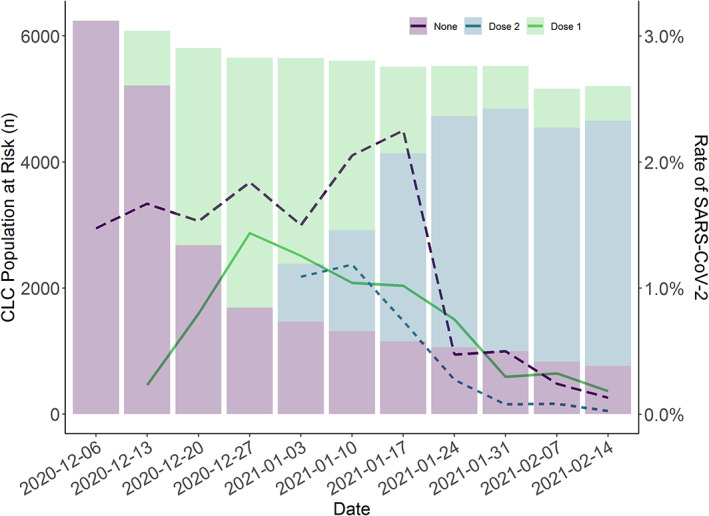
SARS‐CoV‐2 vaccination of Community Living Center (CLC) residents. These results are pooled across 130 CLCs. The line represents the proportion of SARS‐CoV‐2 positive tests (left y‐axis) according to vaccination status. The bars represent the number of residents in the CLCs during the week according to vaccine status (right y‐axis). Vaccines were approved on December 11, 2020 and December 18, 2020 (Week 3 of 2021). The proportion of SARS‐CoV‐2 positive tests declined in the unvaccinated group as well as the vaccinated groups
Figure 2 presents the proportion of SARS‐COV‐2 positive tests in CLCs (n = 130) and community nursing homes in weekly periods before and after the index date. In CLCs, the proportion of positive tests was stable in the weeks prior to and after index week. In community nursing homes, the proportion of positive tests peaked during the index week. Throughout the analysis period, the proportion of SARS‐CoV‐2 positive tests in CLCs is generally below the community nursing homes. The proportion of SARS‐COV‐2 positive tests dropped among all CLC residents at the fourth week after vaccination, including the unvaccinated. There is an approximately 75% drop in the proportion of SARS‐CoV‐2 positive tests in both CLC and community nursing homes after vaccine availability.
FIGURE 2.
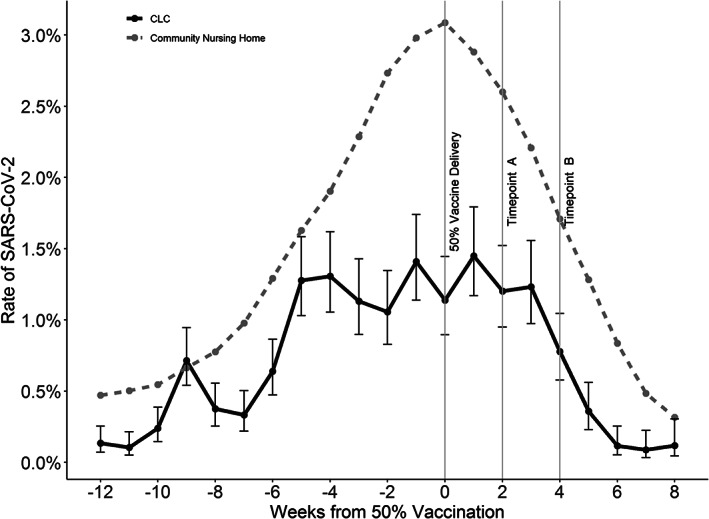
The proportion of SARS‐CoV‐2 positive tests in Community Living Centers (CLCs) relative to 50% vaccination. For each CLC the time of 50% first dose vaccination is determined and used to center the CLCs on the graph (index Week 0). The proportion of positive tests in community nursing homes in the same counties of CLCs is displayed in as a dashed line
Table 1 displays the RR of SARS‐CoV‐2 positive tests in the vaccinated relative to unvaccinated for the weeks surrounding the index. The RR of SARS‐CoV‐2 vaccinated to unvaccinated is significantly reduced in the index week (RR 0.28, 95% CI 0.17–0.46), is not significant in Weeks 1–3, and returns to a significant reduction in Week 4 (RR 0.37, 95% CI 0.20–0.68).
TABLE 1.
Proportion of positive SARS‐CoV‐2 tests and relative risk (RR) for weeks around vaccination (Week 0 represents 50% vaccination within a Community Living Center)
| Week | Vaccinated | Unvaccinated | RR | 95% CI | ||
|---|---|---|---|---|---|---|
| Positives, n | At risk, n | Positive, n | At risk, n | |||
| −2 | <10 | 243 | 68 | 5842 | 0.71 | 0.17, 2.87 |
| −1 | <10 | 996 | 75 | 4966 | 0.60 | 0.30, 1.19 |
| Index | 25 | 3952 | 41 | 1845 | 0.28 | 0.17, 0.47 |
| 1 | 54 | 4169 | 29 | 1567 | 0.70 | 0.45, 1.10 |
| 2 | 49 | 4239 | 19 | 1415 | 0.86 | 0.51, 1.46 |
| 3 | 48 | 4331 | 21 | 1267 | 0.67 | 0.40, 1.11 |
| 4 | 25 | 4364 | 18 | 1165 | 0.37 | 0.20, 0.68 |
Abbreviations: CI, confidence interval.
DISCUSSION
This analysis described the delivery of SARS‐COV‐2 vaccination to nursing home residents and the change in the proportion of SARS‐CoV‐2 positive tests. We observed a decrease in the proportion of SARS‐CoV‐2 among veterans 4 weeks following the first dose, which continued through the second dose. The proportion of SARS‐CoV‐2 positive tests in CLCs trended with community nursing homes. Clinically, these analyses highlight the importance of SARS‐CoV‐2 testing and vaccination in CLCs.
The prioritization of SARS‐CoV‐2 vaccine to nursing home residents within VA and non‐VA is critically important. 8 Temporally, the decline in the proportion of positive SARS‐CoV‐2 tests among vaccinated was evident prior to the second vaccine dose and continued afterward. Among CLC residents without vaccination, the proportion of positive tests also declined. During this time, there was a decline in SARS‐CoV‐2 positive tests among non‐VA nursing home residents in counties of CLCs and the US general population. 9 It is possible that with a sufficient proportion of both CLC residents and staff vaccinated, herd immunity was reached. However, the vaccination threshold necessary for herd immunity is unknown. The decline in the proportion of positive tests within the community nursing homes and general population during this time limits the herd immunity interpretation.
The COVID‐19 pandemic was stressful on all within long term care—residents, staff, clinicians, and administrators. As an integrated federal health system, VA CLCs were able to navigate the pandemic better than smaller nursing home systems. 10 CLCs had access to infection control experts and epidemiologists. The first VA guidance on infection control was disseminated to CLCs on March 5, 2020—less than a week after the first reported nursing home case in King County, Washington. 11 VA CLCs operate with an employed staff and restricted those staff to the CLC. Further, CLCs restricted the entrance of nonessential staff to the CLC. This action limited exposure to other high‐risk environments such as other nursing homes or inpatient units by the CLC staff and limited the potential introduction of the virus by staff who had previously been in these other environments. 12 As a large integrated healthcare delivery system, VA managed (and continues to manage) personal protective equipment, virus testing, and vaccination on a system level ensuring that adequate supply availability to the CLCs. Finally, with a common electronic medical record, VA was able to identify, advise, and assist CLCs with multiple cases. While VA navigated the course, there are still profound system impacts from COVID‐19.
The infection control measures necessary to protect nursing home residents have reversed decades of work to transform the nursing facility environment into the resident's home. While this transformation was necessary, vaccination offers hope that residents may again return to a home‐like environment. While vaccination may provide real protection against SARS‐CoV‐2, new variants require continued vigilance. More importantly, this pandemic reinforces the need to be vigilant for all infectious diseases in the vulnerable nursing home population.
This analysis has strengths and limitations. The VA COVID‐19 surveillance system allows analyses to demonstrate the reduction in the proportion of positive SARS‐CoV‐2 tests with vaccine delivery. However, individual resident‐level data would allow more detailed analysis of individual effects from vaccine and infection. In addition, resident‐level data that incorporated prior testing and vaccination would enable reactions to vaccine after COVID‐19 infection. Similarly, the community nursing home data are self‐reported through CMS, but do not report on the testing and vaccination practices of nursing homes, which likely differ from CLCs. There are also major differences in patient populations between VA CLCs and CMS‐certified nursing homes. Additionally, we lack staff vaccination data for this analysis which may be a critical factor in the decline among CLCs and nursing homes. The large geographic spread of the VA system allows a nationwide sampling, but limits the detail on local variation in practices.
The proportion of SARS‐CoV‐2 positive tests decreased in VA CLC residents after vaccination beginning with the first dose and impacting all residents with time. These findings highlight the push to vaccinate nursing home residents and staff. Systems developed for widespread monitoring are important for continued vigilance as COVID variants develop. The long‐term impact of COVID‐19 and vaccination on safety, quality, and cost of long‐term care is yet undescribed and additional study will be required.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Study concept and design: James L. Rudolph, Kevin McConeghy, Orna Intrator, Mary K. Goldstein; acquisition of data: Scotte Hartronft, Michael Kennedy, Orna Intrator, Lisa Minor, Terrence L. Hubert, Mary K. Goldstein; analysis and interpretation of data: James L. Rudolph, Scotte Hartronft, Kevin McConeghy, Michael Kennedy, Orna Intrator, Lisa Minor, Terrence L. Hubert, Mary K. Goldstein; and preparation of manuscript: James L. Rudolph, Scotte Hartronft, Kevin McConeghy, Michael Kennedy, Orna Intrator, Lisa Minor, Terrence L. Hubert, Mary K. Goldstein.
SPONSOR'S ROLE
This is an operational analysis of the US Department of Veterans Affairs (the sponsor). The VA built and provided the COVID‐19 data for operational purposes. VA employees meeting authorship criteria are included. The Veterans Health Administration reviewed and approved the manuscript for publication. The opinions expressed are those of the authors and do not reflect the official policies of the US Government or the US Department of Veterans Affairs.
Supporting information
Figure S1 CLC vaccination level sensitivity analysis for determining index
ACKNOWLEDGMENTS
The authors are appreciative of the analytic core of the VA Long Term Services and Supports Center of Innovation for their work on the analyses. James L. Rudolph, Kevin McConeghy are supported by VA Health Services Research and Development (CIN 13‐419; C19 20‐213) and the National Institute of Aging (3P01AG027296‐11S2). All the authors are VA employees. The opinions expressed are those of the authors and do not reflect the official policies of the US Government or the US Department of Veterans Affairs.
Rudolph JL, Hartronft S, McConeghy K, et al. Proportion of SARS‐CoV‐2 positive tests and vaccination in Veterans Affairs Community Living Centers. J Am Geriatr Soc. 2021;69:2090–2095. 10.1111/jgs.17180
See related editorial by Ouslander et al and related articles by Mor et al, Moore et al, and Domi et al. in this issue.
Funding information Health Services Research and Development, Grant/Award Numbers: C19‐20‐213, CIN 13‐419; National Institute on Aging, Grant/Award Number: 3P01AG027296‐11S2
REFERENCES
- 1. Chidambaram P, Garfield R, Neuman T, Kaiser Family Foundation . COVID‐19 has claimed the lives of 100,000 long‐term care residents and staff. 2020. https://www.kff.org/policy‐watch/covid‐19‐has‐claimed‐the‐lives‐of‐100000‐long‐term‐care‐residents‐and‐staff/
- 2. Bagchi S, Mak J, Li Q, et al. Rates of COVID‐19 among residents and staff members in nursing homes—United States, May 25–November 22, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Center for Clinical Standards and Quality/Survey & Certification Group . Interim Final Rule (IFC), CMS‐3401‐IFC. Volume QSO‐20‐38‐NH. Baltimore, MD: Centers for Medicare and Medicaid Services; 2020. [Google Scholar]
- 4. Center for Clinical Standards and Quality/Quality SOG . Upcoming Requirements for Notification of Confirmed COVID‐19 (or COVID19 Persons under Investigation) among Residents and Staff in Nursing Homes. Volume QSO‐20‐26‐NH. Baltimore, MD: Centers for Medicare & Medicaid Services; 2020. [Google Scholar]
- 5. Dooling K, McClung N, Chamberland M, et al. The advisory committee on immunization practices' interim recommendation for allocating initial supplies of COVID‐19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services . Conditions of Participation. 85 CFR 54873. CMS‐3401‐IFC. Baltimore, MD: Centers for Medicare & Medicaid Services; 2020. [Google Scholar]
- 7. Centers for Medicare & Medicaid Services . COVID‐19 Nursing Home Data. Baltimore, MD: Centers for Medicare & Medicaid Services; 2021. [Google Scholar]
- 8. National Academy of Sciences . A Framework for Equitable Allocation of Vaccine for the Novel Coronavirus. Washington (DC): National Academies Press (US); 2020. https://www.ncbi.nlm.nih.gov/books/NBK564091/. Accessed April 22, 2021. [Google Scholar]
- 9. Johns Hopkins Coronavirus Resource Center . United States Coronavirus Trends; Baltimore, MD USA: Johns Hopkins University; 2021:2021. https://coronavirus.jhu.edu/data. Accessed April 22, 2021. [Google Scholar]
- 10. Clancy C, Boyd T, Hartronft S. Vigilance at VA community living centers in the time of COVID‐19. Federal Medicine . 2020.
- 11. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS‐CoV‐2 infections in residents of a long‐term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kosar CM, White EM, Feifer RA, et al. COVID‐19 mortality rates among nursing home residents declined from March to November 2020. Health Aff. 2021;40:655–663. 10.1377/hlthaff.2020.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 CLC vaccination level sensitivity analysis for determining index


