Abstract
Viral infections of the lower respiratory tract are considered a public health problem. They affect millions of people worldwide, causing thousands of deaths, and are treated with expensive medicines, such as antivirals or palliative measures. In this study, we conducted a systematic review to describe the use of quercetin‐type flavonols against lower respiratory tract viruses and discussed the preclinical impact of this approach on different signs and clinical mechanisms of infection. The systematic review was performed in PubMed/MEDLINE, Scopus, Scielo, and Biblioteca Virtual de Saúde (BVS). After the database search, 11 relevant studies were identified as eligible. The analysis of these studies showed evidence of antiviral activity of quercetin‐type flavonols with significantly reduced mortality rate (M‐H = 0.19, 95% CI: 0.05 to 0.65, p‐value = 0.008) of infected animals and a reduction in the average viral load (IV = −1.93, 95% CI: −3.54 to −0.31, p‐value = 0.02). Additionally, quercetin and its derivatives reduced the amount of proinflammatory cytokines, chemokines, reactive oxygen species, mucus production, and airway resistance in animals infected with a respiratory virus. Overall, supplementation with quercetin‐type flavonols is a promising strategy for treating viral‐induced lower respiratory tract infections.
Keywords: chemoprophylaxis, flavonoids, influenza virus, quercetin, rhinovirus, virus‐induced lower respiratory tract infections
1. INTRODUCTION
Apitherapy is a traditional medicine practice that uses bee products to prevent and treat many diseases, especially virus‐induced upper and lower respiratory tract infections (Fratellone, Tsimis, & Fratellone, 2016; Lima et al., 2020a; Trumbeckaite, Dauksiene, Bernatoniene, & Janulis, 2015). Some apitherapy products, such as honey, pollen, propolis, and royal jelly, contain compounds (e.g., polyphenols, vitamins, essential oils, and minerals) with significant biological activity (Fratellone et al., 2016). However, the antiviral effect of these bee products is mainly attributed to the presence of phenolic compounds, such as quercetin and its glycosides derivatives (Fratini, Cilia, Mancini, & Felicioli, 2016; Rzepecka‐Stojko et al., 2015; Samarghandian, Farkhondeh, & Samini, 2017; Schnitzler et al., 2010; Siheri, Alenezi, Tusiimire, & Watson, 2017).
The potential antiviral effect of quercetin has been evaluated in in vitro studies and covers mainly respiratory viruses, such as influenza virus (IV), human respiratory syncytial virus, human rhinovirus (RV), human metapneumovirus, parainfluenza, and coronavirus (e.g., SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2) (Batiha et al., 2020; Biancatelli et al., 2020; Kaul, Middleton, & Ogra, 1985). In these studies, quercetin induced a concentration‐dependent reduction in the infectivity of these viruses. Quercetin also affects multiple viral virulence steps essential for the infectious process in lung cells (i.e., viral entry, replication, release, maturation, and protein assembly) (Kaul et al., 1985). Moreover, the in vivo antiviral activity of quercetin and its derivatives is also related to their modulatory effect on immune response mechanisms. For instance, quercetin‐like flavonols are known to upregulate the interferon (IFN)‐γ from T helper‐1 (TH1) lymphocyte, increasing cell‐mediated immunological activity and downregulate IL‐4 release by T helper‐2 lymphocyte (TH2) (Chen et al., 2012; Nair et al., 2002).
However, the evidence of the antiviral activity of quercetin and its derivatives against viral infections of lower airways remains decentralized. In this context, little effort has been made to determine the antiviral activity of these compounds, and the therapeutic effect of supplementation with quercetin‐like compounds in vivo needs to be clarified. Therefore, in this study, we aim to summarize the available data on the antiviral effect of quercetin and its derivatives against viral lower respiratory tract infections. For this, we quantified the magnitude of the in vivo therapeutic effect of these compounds present in many apitherapy products through a systematic review and meta‐analysis.
2. METHODS
The therapeutic effects of quercetin and its derivatives on virus‐induced lower respiratory tract infections in experimental models were evaluated by a systematic review and meta‐analysis performed according to the principles described in the Cochrane Handbook (Higgins & Green, 2011). The search, selection of studies, extraction, analysis, and interpretation of data of interest were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Liberati et al., 2009). To identify studies of interest, we applied the PICOS strategy (Eriksen & Frandsen, 2018) as follows: Population—rodents with virus‐induced lower respiratory tract infections; Intervention—treatment with quercetin or its derivatives; Control—treatment with placebo; Outcomes—mortality and lung viral load; and Study design—in vivo studies conducted in rodents (mice, rats, hamster, or rabbits).
Initially, the systematic search was performed in four databases (PubMed/MEDLINE, Scopus, Biblioteca Virtual em Saúde, and SciELO) using the Medical Subject Heading (MeSH) term “Quercetin*” combined with at least two of the following descriptors: “Virus,” “Viruses,” “Respiratory System,” “Respiratory Tract,” “Lung,” “Low Respiratory Tract.” These descriptors were connected using the connector “AND” between them as in the following example: “Quercetin” AND “Virus” AND “Lower Respiratory Tract.” The search was conducted until April 20th, 2020, restricted to studies written in English, and no date limit was established. A gray literature search of dissertations and thesis was also performed in the databases Thesis and Dissertation Catalog of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Digital Library of Thesis and Dissertations of the Universidade Federal de Minas Gerais (UFMG) and Universidade de São Paulo (USP). In addition, the reference lists of all included articles and relevant narrative reviews were evaluated for any relevant studies.
Subsequently, review articles, notes, correspondences, editorials, and letters were excluded. Furthermore, other studies were excluded based on the following criteria: (a) in vivo studies of virus‐induced upper respiratory tract infections; (b) studies that do not identify the microorganism used in the lower respiratory tract infection; and (c) studies in which none of the primary outcomes (i.e., lung viral load and mortality) was the subject of the study. In cases where the study was in accordance with the inclusion criteria, but the full text was not available, the corresponding author was contacted by e‐mail three times (with 14‐day intervals between them). The articles were included if the authors provided the full paper. The details of the search strategy for each database are shown in Data S1. We did not use any type of research limit on the bases selected. Thus, the usage of the search equations provided in Data S1 guarantees the reproducibility of searches.
In the first phase of study selection, two independent researchers (J.C.M.B. and L.P.B.C.) searched the databases. Duplicated records were deleted using the software EndNote X9 version 19.0.0.12062 (Clarivate Analytics, Sydney, Australia), and titles and abstracts of the selected studies were screened according to the PICOS eligibility criteria. Thereby, studies that evaluated the therapeutic effects of quercetin and its derivatives in rodent models of virus‐induced lower respiratory tract infection were selected and then evaluated by full‐text review. Any discrepancies were resolved by discussion with a third investigator (W.G.L), and the Kappa coefficient (performed with 95% confidence interval) was used to analyze the degree of agreement between the evaluators (Landis & Koch, 1977).
After a full analytical reading, all data of interest were summarized in Table 1 for further analysis and interpretation. Furthermore, the data regarding mortality rate and viral load in the lung of mice with virus‐induced lower respiratory tract infection treated or not with quercetin and its derivatives were pooled to estimate the mean difference and the confidence intervals by meta‐analysis using Review Manager (RevMan)® 5.3 software (Lima et al., 2020b). All pooled data were estimated using a random effect model (Lima, Silva Alves, Sanches, Antunes Fernandes, & de Paiva, 2019; Lima, Souza, Fernandes, Cardoso, & Godói, 2019). Heterogeneity of the primary data was analyzed using the I2 statistic, in which I2 > 50% was considered to have substantial heterogeneity (de Carvalho, Lima, Coelho, Cardoso, & Fernandes, 2020; Lima et al., 2020c). In all procedures, the significance level was 5%. To assess the robustness of our findings, we conducted a sensitivity analysis considering only studies that used the A/PR/8/34 strain of influenza virus H1N1 to induce viral pneumonia in mice.
TABLE 1.
Main characteristics of the included studies
| References | Pneumonia model (Mice lineage, virus strain, and infection protocol) | Treatment (Molecule, dose, and therapeutic regime) | Primary outcomes associated with the treated group |
|---|---|---|---|
| Farazuddin et al., 2018 |
|
|
Attenuation of RV‐induced pulmonary damage (histology) ↓ Viral load in the lung (by measuring viral RNA) ↓ Pulmonary levels of CXCL‐1, CXCL‐10, IL‐17, CCL3, TNF‐α, and IFN‐γ ↓ Infiltration of neutrophils, macrophages, and T cells into pulmonary tissue ↓ Expression of Gob5 and mucin genes in lung Attenuation of RV‐induced airway resistance |
| Choi et al., 2012 |
|
|
↓ Mortality ↓ Lung damage ↓ Viral load in the lung (by CPE50 assay) |
| Fan et al., 2011 |
|
|
Attenuation of FLUAV‐induced pulmonary damage (weight and macroscopic aspects) |
| Y. Kim et al., 2010 |
|
|
↓ Viral load in the lung (by TCID50/ml titers) ↓ Pulmonary levels of IFN‐γ, iNOS, and CCL5 Attenuation of FLUAV‐induced pulmonary damage (histology) |
| Davis et al., 2008 |
|
|
↓ Morbidity ↓ Symptom severity ↓ Mortality |
| Dayem et al., 2015 |
|
|
↓ Viral load in lung (by EID50/ml) ↓ Bodyweight loss ↓ Mortality |
| Savov et al., 2006 |
|
|
↓ FLUAV‐induced oxidative damage in lungs and liver (by TBARS assay) ↑ Level of cytochrome P‐450 in liver ↓ Enzymatic activity of NADPH‐cytochrome c reductase, aminopyrine N‐demethylase and analgin N‐demethylase in liver |
| Kumar et al., 2005 |
|
|
↑ Level of catalase in lung ↑ Level of Superoxide dismutase in lung ↑ GSH/GSSG ratio in the lung Quercetin supplementation does not revert FLUAV‐induced reduction in Vitamin E concentration in lung |
| Kumar et al., 2003 |
|
|
Attenuation of FLUAV‐induced pulmonary damage (histology) ↓ Superoxide production by alveolar macrophages ↓ FLUAV‐induced oxidative damage in lungs (by TBARS assay) |
| Raju et al., 2000 |
|
|
↓ FLUAV‐induced oxidative damage in lungs (by TBARS assay) ↓ Superoxide production by alveolar macrophages Quercetin supplementation does not revert FLUAV‐induced reduction in catalase and superoxide dismutase levels in lung |
| Ganesan et al., 2012 |
|
|
↓ Viral load in lung (by measuring viral RNA) ↓ Pulmonary levels of CXCL‐1, CXCL‐2, MCP‐1, TNF‐α, IFN‐γ, and IFN‐α (only the group treated by 1 day) Attenuation of RV‐induced airway resistance |
Abbreviations: CPE50, 50% cytopathic effect assay; EID50/ml, 50% egg infectious doses per milliliter; GSH, Reduced glutathione; GSSG, Glutathione; i.n., intranasal; i.p., Intraperitoneal administration; i.v., Intravenous administration; PFU, Plate forming unit; p.o., Oral route; TBARS, Thiobarbituric acid reactive species; TCID50/ml, 50% Tissue Culture Infectious Dose per milliliter.
3. RESULTS
3.1. Study inclusion
The electronic search resulted in 139 records, 26 from Pubmed/MEDLINE, 66 from Scopus, 41 from BVS, and 5 from SciELO (Figure 1). None additional study was identified in the gray literature search or through the screening of included studies' reference lists. After excluding duplicated studies, the titles and abstracts of 124 records were screened, resulting in 15 studies that met the inclusion criteria. Therefore, the full text of 15 articles were assessed for eligibility, and 4 of them were excluded for the following reasons: (a) different study design (n = 3) or (b) incomplete text (n = 1) (Figure 1). Finally, 11 studies were selected for the qualitative analysis (Choi, Song, & Kwon, 2012; Davis, Murphy, McClellan, Carmichael, & Gangemi, 2008; Dayem, Choi, Kim, & Cho, 2015; Fan et al., 2011; Farazuddin et al., 2018; Ganesan et al., 2012; Y. Kim, Narayanan, & Chang, 2010; Kumar et al., 2005; Kumar, Sharma, Khanna, & Raj, 2003; Raju, Lakshmi, Anand, Rao, & Sharma, 2000; Savov et al., 2006). Of these, 4 were included in the quantitative studies and used in the meta‐analysis for the outcomes of interest (i.e., mortality [Choi et al., 2012; Davis et al., 2008; Dayem et al., 2015] and lung viral load [Choi et al., 2012; Dayem et al., 2015; Y. Kim et al., 2010]). The degree of agreement between the two researchers was considered substantial (Kappa coefficient of 0.613).
FIGURE 1.
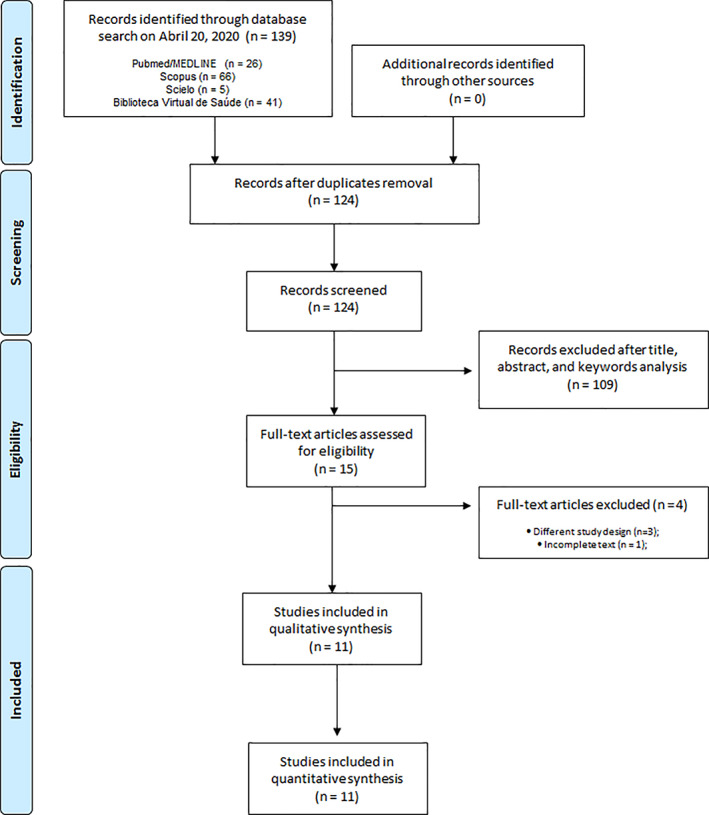
Flowchart of the selection of articles for systematic review according to the PRISMA criteria
3.2. Virus‐induced lower respiratory tract infection model
The viral infection of the lower respiratory tract in the majority of the studies was induced by Influenza virus (IV) (9/11; 81.8%) (Choi et al., 2012; Davis et al., 2008; Dayem et al., 2015; Fan et al., 2011; Y. Kim et al., 2010; Kumar et al., 2003, 2005; Raju et al., 2000; Savov et al., 2006), using the variants H1N1 (5/9; 55.6%) (Choi et al., 2012; Davis et al., 2008; Dayem et al., 2015; Fan et al., 2011; Y. Kim et al., 2010) or H3N2 (4/9; 44.4%) (Kumar et al., 2003, 2005; Raju et al., 2000; Savov et al., 2006). Only two studies induced lung infection with noninfluenza viruses, both of which employed RV (2/11; 18.2%) (Farazuddin et al., 2018; Ganesan et al., 2012). In all studies included, viral infection was performed by intranasal instillation of a viral suspension in phosphate‐buffered saline (PBS) or 0.9% saline (11/11; 100%) (Choi et al., 2012; Davis et al., 2008; Dayem et al., 2015; Fan et al., 2011; Farazuddin et al., 2018; Ganesan et al., 2012; Y. Kim et al., 2010; Kumar et al., 2003, 2005; Raju et al., 2000; Savov et al., 2006). Regarding the animals infected with IV, the average viral load employed was 104 plaque‐forming units (PFU), which is equivalent to ~1–1.5 times the lethal dose for 50% of mice (LD50). The two studies that used RV as the infectious agent employed a viral load of 5 × 106 PFU. Mice were used in all experimental models of viral pneumonia. The strains employed were: BALB/c (4/11; 36.4%) (Choi et al., 2012; Y. Kim et al., 2010; Kumar et al., 2003, 2005), C57BL/6 (3/11; 27.3%) (Dayem et al., 2015; Farazuddin et al., 2018; Ganesan et al., 2012), ICR (2/11; 18.2%) (Davis et al., 2008; Savov et al., 2006), Kunming (1/11; 9.1%) (Fan et al., 2011), or Swiss (1/11; 9.1%) (Raju et al., 2000). Male animals (6/11; 54.5%) (Choi et al., 2012; Davis et al., 2008; Kumar et al., 2003, 2005; Raju et al., 2000; Savov et al., 2006) were preferred over female animals (4/11; 36.4%) (Dayem et al., 2015; Farazuddin et al., 2018; Ganesan et al., 2012; Y. Kim et al., 2010), and a study did not identify the sex of the animals (1/11; 9.1%) (Fan et al., 2011).
3.3. Treatments: Compounds and scheme used
Most of the included studies used quercetin as a therapeutic or prophylactic agent against viral pneumonia (7/11; 63.6%) (Davis et al., 2008; Farazuddin et al., 2018; Ganesan et al., 2012; Kumar et al., 2003, 2005; Raju et al., 2000; Savov et al., 2006). The other studies included glycosylated derivatives of quercetin (5/11; 45.4%), such as rutin (Savov et al., 2006), isoquercetin (Y. Kim et al., 2010), quercitrin (Choi et al., 2012), or quercetin‐3‐O‐β‐D‐glucuronide (Fan et al., 2011). One study used a nonglycosylated analog of quercetin (1/11; 9%) called isorhamnetin (Dayem et al., 2015), and another one used a combination of quercetin and rutin (Savov et al., 2006). Among the studies that evaluated the antiviral effect of quercetin, six used this flavonoid orally (6/7; 85.7%) at doses ranging from 1 to 12.5 mg/Kg (Davis et al., 2008; Farazuddin et al., 2018; Ganesan et al., 2012; Kumar et al., 2003, 2005; Raju et al., 2000), and one used it intraperitoneally (1/7; 14.3%) at a dosage of 20 mg/Kg alone or combined with rutin (Savov et al., 2006). Quercetin was administered orally as a 0.9% saline solution (5/6; 83.3%) (Davis et al., 2008; Ganesan et al., 2012; Kumar et al., 2003, 2005; Raju et al., 2000) or as a diet containing 0.1% quercetin and offered ad libitum (1/6; 16.7%) (Farazuddin et al., 2018). The quercetin derivatives quercitrin and quercetin‐3‐O‐β‐D‐glucuronide were used orally at doses of 6.25 mg/Kg (Choi et al., 2012) and 3 or 6 mg/Kg (Fan et al., 2011), respectively. Isoquercetin was administered to infected animals at doses of 2 or 10 mg/Kg intraperitoneally (Y. Kim et al., 2010), and the studies on the therapeutic effects of isorhamnetin utilized a local use (intranasal) of this aglycone at 1 mg/Kg (Dayem et al., 2015).
Regarding the treatment time, six studies used the flavonoids after infection (6/11; 54.5%) (Dayem et al., 2015; Fan et al., 2011; Ganesan et al., 2012; Kumar et al., 2003, 2005; Raju et al., 2000), two before infection (prophylaxis) (2/11; 18.2%) (Davis et al., 2008; Farazuddin et al., 2018), and three used the compounds in a combined schedule of pre‐ and posttreatment (3/11; 27.3%) (Choi et al., 2012; Y. Kim et al., 2010; Savov et al., 2006). The average time for studies using pretreatment was 8.5 days, while for those that used posttreatment was 5.2 days. In the schemes that combined pre‐ and posttreatment, the compounds were administered on an average of 2.5 days before infection and maintained for up to 6 days after the start of the treatment.
3.4. Mortality
Three (Choi et al., 2012; Davis et al., 2008; Dayem et al., 2015) out of the 11 studies selected for systematic review evaluated the mortality of mice with a viral infection of the lower respiratory tract that received or not quercetin and its derivatives (isorhamnetin and quercitrin). Together, these studies have a total of 100 animals, 10 of which were treated with quercitrin, 30 with quercetin, 10 with isorhamnetin, and 50 were included in the untreated control group. All studies induced lung infection with IV H1N1. As shown in Figure 2, quercetin‐type flavonols administration significantly reduced the mortality of animals infected with IV (M‐H = 0.19, 95% CI: 0.05 to 0.65, p‐value = 0.008). In this context, in the treated group, the lethality rate was 22% (11/50), while in the placebo group, the mortality was almost twofold higher (58%; 29/50). The heterogeneity of this population was significantly low, as shown by the I2 statistic (28%; p‐value = 0.25). Moreover, sensitivity analysis considering only studies that used the A/PR/8/34 strain of influenza virus H1N1 to induce viral pneumonia in mice generated results in the range of the crude analysis (Crude analysis: n = 100; M‐H = 0.19, 95% CI: 0.05 to 0.65, p‐value = 0.008; heterogeneity: I2 = 28%; p‐value = 0.25 vs. Sensitive analysis: n = 80; M‐H = 0.27, 95% CI: 0.10 to 0.75, p‐value = 0.01; heterogeneity: I2 = 6%; p‐value = 0.30).
FIGURE 2.
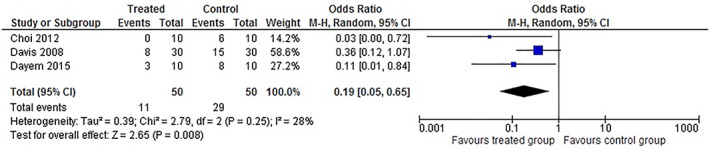
Meta‐analysis of the mortality in animals with virus‐induced lower respiratory tract infections treated with quercetin‐type flavonols
3.5. Lung viral infection
Among the included studies, 5 described the effect of quercetin and its derivatives on the pulmonary viral load of mice infected with respiratory viruses (Choi et al., 2012; Dayem et al., 2015; Farazuddin et al., 2018; Ganesan et al., 2012; Y. Kim et al., 2010). Two of these studies used RV to induce infection and assessed viral load indirectly by quantifying copies of viral RNA by RT‐PCR and, therefore, were excluded from the meta‐analysis (Farazuddin et al., 2018; Ganesan et al., 2012). However, in both studies, quercetin was administered orally and reduced the viral lung load of animals infected with RV, suggesting that it possesses antiviral activity against this RNA virus. The remaining 3 studies evaluated the effect of quercetin derivatives on pulmonary viral load in IV H1N1 models. These studies included 26 animals, 5 of which received quercitrin, 3 isorhamnetin, 5 isoquercetin, and 13 received placebo. Figure 3 shows that animals infected with IV and treated with quercetin derivatives had a significantly lower pulmonary viral load than animals treated with placebo (IV = −1.93, 95% CI: −3.54 to −0.31, p‐value = 0.02). The average viral load of animals treated with these flavonoids was 34% lower than that identified in untreated animals, indicating that these compounds have antiviral effects in vivo. Unlike what was observed in the mortality analysis, the heterogeneity of this animal population was consubstantial (I2 = 93%; p‐value = 0.00001), which may be associated with different ways of quantifying the virus. Although all studies used a direct method to quantify the virus, it is possible to identify 3 different ways of representing the viral load: (a) 50% infectious egg doses per milliliter (EID50/ml), (b) 50% cytopathic effect assay (CPE50), and (c) 50% Tissue Culture Infectious Dose per milliliter (TCID50/ml). However, all of these methods quantify only viable viral particles, while the RT‐PCR technique does not make this distinction. Therefore, we decided to combine them in the meta‐analysis. Similar to lethality, the meta‐analysis of lung viral load showed good performance in sensitivity analysis using only studies that induced viral pneumonia by the IV H1N1 A/PR/8/34 lineage (Crude analysis: n = 26; IV = −1.93, 95% CI: −3.54 to −0.31, p‐value = 0.02; heterogeneity: I2 = 93%; p‐value = 0.00001 vs. Sensitive analysis: n = 16; M‐H = −1.23, 95% CI: −2.69 to 0.22, p‐value = 0.10; heterogeneity: I2 = 30%; p‐value = 0.002).
FIGURE 3.
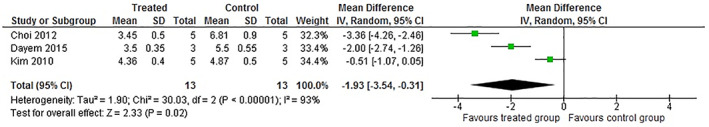
Meta‐analysis of the pulmonary viral load in animals with virus‐induced lower respiratory tract infections treated with quercetin‐type flavonols
3.6. Symptomatology and lung damage
Viral infections of the lower respiratory tract induce important microscopic and macroscopic changes in lung tissues (Herold, Becker, Ridge, & Budinger, 2015; Yoo, Kim, Hufford, & Braciale, 2013). In virtually all studies, the use of quercetin‐type flavonols reduced or prevented the histological and macroscopic pulmonary damage induced by RV or IV (H1N1 and H3N2).
3.7. Airway resistance
Typically, viral infections of the lower respiratory tract induce significant changes in airway dynamics, which can be measured by the reduction in expiratory flow rates, an increase in airway resistance, or disturbances in dynamic lung compliance (Holtzman et al., 2005). Two of the included studies investigated the therapeutic benefits of quercetin's oral use on airway resistance induced by RV. According to Farazuddin et al. (2018), the treatment with quercetin (0.1% quercetin‐containing diet) of RV‐infected mice protected the animals from increased airway responsiveness to methacholine challenge, indicating that the use of this flavonoid attenuates the airway constriction following RV infection. Corroborating with this study, Ganesan et al. (2012) showed that the airway responsiveness to methacholine after 1 day of RV infection was significantly lower in animals that received quercetin orally (0.2 mg) than that observed in untreated mice.
3.8. Inflammatory response
Pulmonary inflammation induced by respiratory viruses is characterized by an intense influx of polymorphonuclear leukocytes in the early stages of infection, culminating in a significant increase in proinflammatory cytokines and chemokines (Yoo et al., 2013). In the included studies, three (Farazuddin et al., 2018; Ganesan et al., 2012; Y. Kim et al., 2010) identified the effect of quercetin and its derivatives on inflammatory parameters associated with lower respiratory tract infection caused by RV or IV. In mice infected with RV, the oral use of quercetin significantly decreased pulmonary inflammation, as shown by the reduction in the levels of interleukin (IL)‐17, tumor necrosis factor‐alpha (TNF‐α), and gamma interferon (IFN)‐γ and IFN‐α (Farazuddin et al., 2018; Ganesan et al., 2012). The cytological analysis revealed an intense infiltration of neutrophils, macrophages, and T cells in the lungs of animals infected with RV, which was partially reduced by quercetin treatment (Farazuddin et al., 2018).
In the study conducted by Y. Kim et al. (2010), the intraperitoneal administration of isoquercetin (2 and 10 mg/Kg), a quercetin monoglycoside heteroside, protected the lungs from the inflammatory damage induced by the IV H1N1. The authors showed that the use of isoquercetin for 8 days (2 days before and 6 after infection) reduced the expression of TNF‐α and monocyte chemoattractant protein‐1 (MCP‐1/CCL‐2) in the lung tissue of infected mice compared to untreated controls. This study also reported that isoquercetin reduces the expression of inducible nitric oxide synthase (iNOS), which is responsible for increasing vascular permeability and stimulating the diapedesis of inflammatory cells.
3.9. Oxidative damage
Four studies (Kumar et al., 2003, 2005; Raju et al., 2000; Savov et al., 2006) investigated the effect of quercetin on ROS‐producing and ROS‐scavenging enzymes induced by IV (H3N2). Studies have shown that the use of quercetin orally or intraperitoneally (combined or not with rutin) reduced the pulmonary oxidative damage induced by the IV (H3N2), as suggested by the reduction in the levels of lipid peroxidation markers (i.e., the reactive substances to thiobarbituric acid [TBARS]) (Raju et al., 2000; Savov et al., 2006). Quercetin has been shown to affect superoxide dismutase production in pulmonary macrophages, reducing ROS generation by immune lung cells (Raju et al., 2000; Savov et al., 2006). The antioxidant effect of this flavonoid was also associated with the modulation of antioxidant enzyme activity. In this context, quercetin increased the catalytic efficiency of catalase and superoxide dismutase in infected BALB/c mice (Kumar et al., 2005), but not in Swiss mice (Raju et al., 2000).
Furthermore, quercetin and rutin act on extra‐pulmonary organs to ensure greater efficiency in removing ROS in animals with viral pneumonia. These flavonoids increased the levels of cytochrome P‐450 and reduced the enzymatic activity of NADPH‐cytochrome c reductase, aminopyrine N‐demethylase, and analgin N‐demethylase in liver tissue (Savov et al., 2006). However, the levels of natural antioxidants that are often reduced due to viral infection, such as vitamin E, were not re‐established after oral administration of quercetin (Kumar et al., 2005).
3.10. Mucus production
Mucus in the respiratory tract is the first interaction that inhaled agents have with the host. Accordingly, this mucus layer can determine the infectivity and transmissibility of respiratory viruses, including IV, respiratory syncytial virus (RSV), and rhinoviruses. One of the included studies (Farazuddin et al., 2018) evaluated the prophylactic effect of quercetin supplementation on mucus production induced by RV. Quercetin reduced the expression of the Gob5 gene involved in mucus production by goblet cells and decreased the expression of genes responsible for mucins synthesis (Farazuddin et al., 2018).
4. DISCUSSION
Quercetin (3,3′, 4′, 5,7‐pentahydroxyflavone) is a natural phenolic compound classified as flavonol that has a flavone backbone (i.e., a three‐ringed molecule with hydroxyl [OH] groups attached) (Figure 4) (Anand David, Arulmoli, & Parasuraman, 2016; Bentz, 2017). Many quercetin derivatives are bound to sugars in their natural state, the O‐glycoside form, where glycosylation can occur at any hydroxyl group (Bentz, 2017). Quercetin and its derivatives are one of the most abundant dietary flavonoids found in fruits (mainly citrus), green leafy vegetables, seeds, buckwheat, nuts, flowers, barks, broccoli, olive oil, apples, onions, green tea, red grapes, red wine, dark cherries, and berries, such as blueberries and cranberries (Anand David et al., 2016; Jan et al., 2010). Furthermore, these flavonoids are the most abundant phenolic compounds in apitherapy products, being recognized as the active component of honey, propolis, royal jelly, beeswax, and pollen (Fratellone et al., 2016; Lima et al., 2020a).
FIGURE 4.
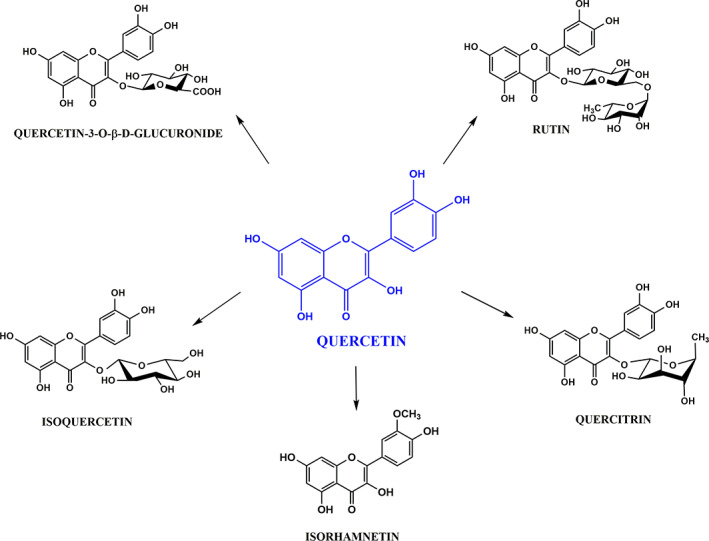
Chemical structure of quercetin and its glycosylated (rutin, isoquercetin, and quercetin‐3‐O‐β‐D‐glucuronide) and nonglycosylated (quercetin and isorhamnetin) derivatives
Among the main clinical applications of bee products, therapeutic and prophylactic use against respiratory diseases of viral etiology stands out. In this context, a study showed that the frequency of flu during an influenza outbreak was significantly lower (3.7% incidence) among patients who used an apicomplex composed of honey, royal jelly (2%), pollen (3%), and propolis (1%) in comparison to untreated patients (38% incidence) (Bratko & Miha, 1976). Several studies report that quercetin and its derivatives are the main active ingredients in these bee products responsible for their antiviral activity (Lima et al., 2020a; Mohamed, Hassan, Hammad, Amer, & Riad, 2015; Schnitzler et al., 2010; Watanabe, Rahmasari, Matsunaga, Haruyama, & Kobayashi, 2014). Moreover, many in vitro assays confirmed the activity of flavonol against respiratory viruses of medical importance (Jan et al., 2010; Kaul et al., 1985).
The data about the in vivo effectiveness of quercetin‐type flavonols against viral respiratory tract infections have reinforced the clinical application of these phenolic compounds. However, the data available are decentralized, and an overall analysis of results obtained using these models is still needed. Thus, we aim to summarize the information available in the biomedical literature regarding the antiviral potential of quercetin and its derivatives against viral pneumonia. In summary, this study showed that the administration of quercetin‐type flavonols significantly reduced the mortality (M‐H = 0.19, 95% CI: 0.05 to 0.65, p‐value = 0.008) and lung viral load (IV = −1.93, 95% CI: −3.54 to −0.31, p‐value = 0.02) of animals infected with IV. These meta‐analyses are in the range of the sensitivity analysis considering only studies that used the IV H1H1 A/PR/8/34 strain to induce viral pneumonia in mice, emphasizing the robustness of our findings. The included studies also showed that the use of quercetin or its derivatives prevented lung damage, reduced inflammation, and oxidative stress, and minimized viral infection effects on respiratory dynamics (Figure 5).
FIGURE 5.
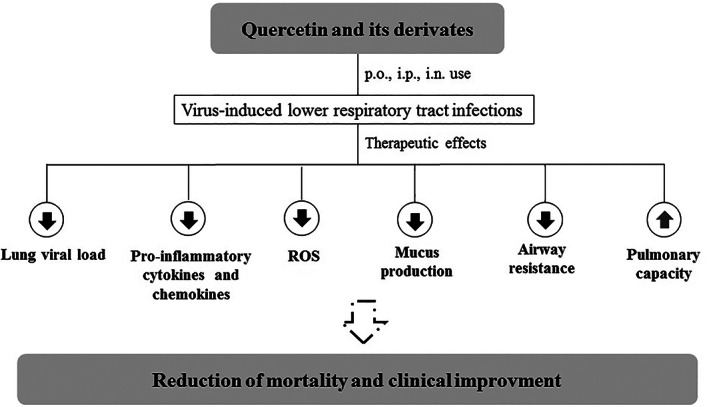
Schematic summary of the clinical activity of quercetin‐type flavonols in animals with virus‐induced lower respiratory tract infections
The antiviral activity of quercetin and its derivatives is mainly due to the inhibition of virus entry into the host cell, a crucial stage during viral infections (Biancatelli et al., 2020; Ganesan et al., 2012; Wu et al., 2015). Some studies have demonstrated that quercetin effectively inhibits IV (Wu et al., 2015) and RV (Ganesan et al., 2012) infection when added during the virus entry stage, while the inhibitory effects of other stages were less clear. In an in vitro model of H1N1 and H3N2 influenza infection of Madin Darby Canine Kidney (MDCK) cells, quercetin reduced the cytopathic effect when administered during viral entry, which was dependent on its binding to hemagglutinin proteins (HA) (Wu et al., 2015). The binding of IV HA to sialic acids presented by cellular receptors triggers virus cell entry by clathrin‐mediated endocytosis (Benton et al., 2018). Thus, the interaction of quercetin with this molecular target justifies its suggested mechanism of action.
Moreover, an in silico study suggested that quercetin interacts with neuraminidase (NA) and acts in the late stages of the viral life cycle. The binding of quercetin to the active site of the H1N1 NA crystallographic structure was more stable (binding energy of −6.8 Kcal/mol) than that observed for oseltamivir (binding energy of −6.8 Kcal/mol), a potent and selective inhibitor of IV NA enzymes (Sadati, Gheibi, Ranjbar, & Hashemzadeh, 2019). NA is essential in the final stage of infection and removes sialic acids from both cellular receptors and newly synthesized HA and NA on nascent virions, enabling an efficient release of new viral particles (McAuley, Gilbertson, Trifkovic, Brown, & McKimm‐Breschkin, 2019).
Immunomodulatory properties are also well reported for quercetin and contribute to its therapeutic effect in animals with viral pneumonia. For instance, a study showed that the antiviral activity of TNF against vesicular stomatitis virus and encephalomyocarditis virus is greatly enhanced by quercetin in WISH cells. However, polyclonal antibodies to interferon completely blocked this effect, suggesting that the antiviral activity of quercetin may be mediated by interferon induction (Ohnishi & Bannai, 1993). Nair et al. (2002) confirmed that the antiviral activity of quercetin is mediated by IFN‐γ production. In this study, quercetin at a concentration of 5 μM, similar to plasma levels, stimulated T‐helper cells to produce and secrete (Th‐1)‐derived IFN‐γ, enhancing cell‐mediated antiviral immunity (Nair et al., 2002). Dietary intake of quercetin has also been shown to increase antiviral immune tone. Mice feed with a diet rich in polyphenols (gallic acid, catechin, p‐hydroxybenzoic acid, vanillic acid, p‐coumaric acid, sinapic acid, ferulic acid, quercetin, and rutin) for 5 weeks showed an increase in macrophage chemotaxis, phagocytosis, microbicidal activity, interleukin‐2 release, natural killer activity, and lymphoproliferative response to concanavalin A and lipopolysaccharide compared to animals that received control diet (Álvarez et al., 2006). Thus, the combination of direct (i.e., inhibition of the entry and release of viral particles) and indirect antiviral effects (i.e., increased antiviral immune tone) of quercetin contribute to the reduction of the pulmonary viral load observed in the meta‐analysis. Due to the direct relationship between viral load and disease lethality (Ngaosuwankul et al., 2010), these data also support the decrease in mortality found among animals that received supplementation with quercetin or its glycosylated derivatives.
Respiratory virus infections change the neural control of the airway's smooth muscle and are associated with an increase in air flux resistance (Farazuddin et al., 2018; Fryer & Jocoby, 1991; Rynko, Fryer, & Jacoby, 2014). Physiologically, parasympathetic nerves release acetylcholine (ACh) onto M3 muscarinic receptors on airway smooth muscle, causing muscle contraction, and bronchoconstriction. ACh also activates M2 muscarinic receptors on postganglionic nerves, inhibiting further ACh release, and limiting bronchoconstriction (Fryer & Jacoby, 1998). However, in viral infections of airways, the M2 receptors in parasympathetic nerves are dysfunctional, which cause loss of M2 receptor‐mediated negative feedback and increase ACh release onto airway smooth muscle, producing intense bronchoconstriction (Farazuddin et al., 2018; Fryer & Jocoby, 1991; Rynko et al., 2014). Parainfluenza virus, for example, decreases M2 receptor mRNA expression in parasympathetic ganglia extracted from infected animals (Rynko et al., 2014) and impairs the bronchodilatation effect of pilocarpine, an agent that stimulates inhibitory M2 muscarinic receptors on parasympathetic nerves (Fryer & Jocoby, 1991). Quercetin and its derivatives are known to reduce airway resistance induced by viral infections by reducing virus‐induced M2 receptor dysfunction (Farazuddin et al., 2018; Ganesan et al., 2012). A study showed that the treatment with quercetin reverts the significant reduction in expression of M2 and M3 receptors in the transverse colon induced by the use of loperamide in vivo (J. E. Kim et al., 2018). Furthermore, quercetin reduced Gα expression, PI3K phosphorylation, and PKC phosphorylation in the primary smooth muscle of rat intestine cells (pRISMCs), suggesting an inhibition of the M3 receptor or activation of M2 (J. E. Kim et al., 2018). Therefore, quercetin has a general anticholinergic effect, contributing to bronchodilation and the consequent reduction in virus‐induced airway resistance.
Respiratory viruses primarily infect and replicate in airway epithelial cells. During the replication process, the cells release antiviral factors and cytokines that alter local airway inflammation. Viral pneumonia usually involves a severe inflammatory process in the lung tissue, contributing to high morbidity and mortality rates. Quercetin improves virus‐induced lung inflammation, resulting in a reduction in the release of proinflammatory cytokines, amelioration of pulmonary tissue lesions (e.g., fibrosis, necrosis, and pulmonary edema), and a reduction in the clinical symptoms of infected animals (Farazuddin et al., 2018; Ganesan et al., 2012; Y. Kim et al., 2010). Quercetin acts directly on inflammation by inhibiting TNF‐α and interrupts the activation of ERK, JNK, and NF‐κB, which are potent inducers of the expression of inflammatory genes and protein secretion. This flavonoid also acts indirectly by increasing the activity of the receptor C activated by the peroxisome proliferator (PPARγ) and/or activating protein‐1 (AP‐1). These two transcriptional factors affect NF‐κB actions (Li et al., 2016). On the other hand, quercetin can also inhibit the proliferation of cells that synthesize IL‐4, IL‐5, IL‐6, IL‐10, and IL‐13 by inducing the production of Th‐1‐derived IFN‐γ (Li et al., 2016; Nair et al., 2002). Another characteristic of quercetin is the negative regulation of the vascular cell adhesion molecule 1 (VCAM‐1) and the expression of CD80 (Yang et al., 2015). VCAM‐1 is usually expressed on the membrane of lung endothelial cells and is involved in the adhesion and migration of monocytes, lymphocytes, eosinophils, and basophils to the pulmonary tissue (Muller, 2011). Furthermore, quercetin also inhibits the production of inflammation‐producing enzymes, such as cyclooxygenase‐2 and lipoxygenase, by blocking the activation of PI3K. Thus, it compromises the synthesis of inflammatory mediator prostaglandin E2 (Li et al., 2016; Xiao et al., 2011).
Markers of redox misbalance in blood and lung are often taken into account in viral pneumonia. In general, lung infections caused by respiratory viruses are associated with cytokine production, inflammation, cell death, and other pathological processes, which could be triggered by enhanced reactive oxygen or nitrogen species (ROS and RNS) production. Quercetin has been shown to act against lung oxidative stress caused by the virus through various mechanisms, such as reducing the generation of ROS (Raju et al., 2000; Savov et al., 2006), increasing the expression of antioxidant enzymes (Kumar et al., 2005), and inducing the metabolism of compounds with oxidative proprieties (Savov et al., 2006). Interestingly, Raju et al. (2000) showed that supplementation with quercetin alone in healthy mice did not significantly affect lipid peroxidation. However, after the viral infection, it showed a significant decrease in the lipid peroxide level. Together, these studies demonstrate that supplementation with quercetin alleviates the toxic effects of free radicals induced during viral infection by influencing signal transduction pathways that modulate the antioxidant properties of organisms, thereby preventing disease development.
Mucus is a viscoelastic fluid produced by mucous membranes composed of glycoproteins and proteoglycans with a crucial protective function (Williams, Sharafkhaneh, Kim, Dickey, & Evans, 2006). However, in respiratory infectious disease, the production and secretion of mucus are markedly upregulated, and this excess can reach the back of the throat and lungs and enter the trachea. Therefore, it is important to control and reduce this exacerbated mucus production in respiratory tract infections because it is associated with airway obstruction and mucociliary clearance impairment, which results in the development of debilitating airflow limitation and particulate/pathogen retention, respectively. Mucus hypersecretion may result from an increase in the steady‐state levels of mucin production, mucin exocytosis, or both. Mucin is produced by the Gob5 (or mCLCA3) protein, a member of the calcium‐dependent chloride channel 1 (CLCA1) family of proteins considered a new therapeutic target to treat hypersecretory airway diseases. Quercetin downregulated the Gob5 gene in mice infected with IV, justifying the reduction in mucus production in these animals. It is also in accordance with the improvement of airway resistance observed by some authors. Thus, these studies show that quercetin reduces mucus production by decreasing the synthesis at the gene level. However, the effect of these flavonoids on the exocytosis of mucus‐containing vesicles must be determined in future studies.
This meta‐analysis has some limitations. First, multiple variables influence the overall therapeutic effect of the studied compounds, such as the wide variety of animals, scheme of treatment used (dose, period, administration via), and type of derivative used. Second, the included studies evaluated the effect of quercetin and its derivatives mostly against IV and HR, which limits the extrapolation of the associations shown here for other respiratory viruses, such as human respiratory syncytial virus, human metapneumovirus, parainfluenza, and coronavirus (e.g., SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2). Third, none of the studies identified the limitations of the experimental design employed, which may compromise the observed correlations. Fourth, the heterogeneity of the studies analyzed in this paper is considerably high, which tends to weaken the robustness of our findings. Fifth, almost all articles included represented the various factors evaluated graphically (e.g., cytokine levels, airway resistance rate, markers of oxidative damage, and mucus content), which restricted our meta‐analysis only to the lethality curve and viral lung load. Sixth, the protocol of this review was not preregistered; consequently, it is not easily available for access, which may introduce a potential additional bias in the study. Finally, it is worth emphasizing that the correlation found in the meta‐analysis does not imply any causation, and there is always the possibility of residual confounding in the included studies.
5. CONCLUSION
Supplementation with quercetin and its glycosylated or aglycone derivatives by oral, local, or parenteral routes reduces the lethality and pulmonary viral load of mice infected with IV. Moreover, these flavonols reduce the inflammatory process, oxidative damage, airway resistance, mucus hypersecretion, and tissue necrosis associated with the respiratory virus. These findings support the potential of quercetin and its derivatives as a curative agent against viral pneumonia. Furthermore, due to their lack of severe side effects and low cost, quercetin may be developed as a safer and cheaper option for the prophylaxis of seasonal or emerging respiratory viral infections, helping to control outbreaks, epidemics, and/or pandemics.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
W.G.L. is grateful to Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) for a PhD fellowship.
Brito JCM, Lima WG, Cordeiro LPB, da Cruz Nizer WS. Effectiveness of supplementation with quercetin‐type flavonols for treatment of viral lower respiratory tract infections: Systematic review and meta‐analysis of preclinical studies. Phytotherapy Research. 2021;35:4930–4942. 10.1002/ptr.7122
Júlio César Moreira Brito and William Gustavo Lima contributed equally to this work and should be considered cofirst authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Álvarez, P. , Alvarado, C. , Puerto, M. , Schlumberger, A. , Jiménez, L. , & De la Fuente, M. (2006). Improvement of leukocyte functions in prematurely aging mice after five weeks of diet supplementation with polyphenol‐rich cereals. Nutrition, 22(9), 913–921. 10.1016/j.nut.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Anand David, A. V. , Arulmoli, R. , & Parasuraman, S. (2016). Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacognosy Reviews, 10(20), 84–89. 10.4103/0973-7847.194044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha, G. E. , Beshbishy, A. M. , Ikram, M. , Mulla, Z. S. , Abd El‐Hack, M. E. , Taha, A. E. , … Ali Elewa, Y. H. (2020). The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods, 9(3), 374. 10.3390/foods9030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton, D. J. , Nans, A. , Calder, L. J. , Turner, J. , Neu, U. , Lin, Y. P. , … Skehel, J. J. (2018). Influenza hemagglutinin membrane anchor. Proceedings of the National Academy of Sciences of the United States of America, 115(40), 10112–10117. 10.1073/pnas.1810927115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz, A. B. (2017). A review of quercetin: Chemistry, antioxidant properties, and bioavailability. Journal of Young Investigators. Retrieved from https://www.jyi.org/2009‐april/2017/10/15/a‐review‐of‐quercetin‐chemistry‐antioxidant‐properties‐and‐bioavailability [Google Scholar]
- Biancatelli, R. M. L. C. , Berrill, M. , Catravas, J. D. , & Marik, P. E. (2020). Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS‐CoV‐2 related disease (COVID‐19). Frontiers in Immunology, 11, 1451. 10.3389/fimmu.2020.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratko, F. , & Miha, L. (1976). Clinical value of royal jelly and propolis against viral infections. In Filipic B. (Ed.), Interferon scientific memoranda (pp. 18–32). Bufallo, NY: Calspan Corporation. [Google Scholar]
- Chen, C. , Jiang, Z. Y. , Yu, B. , Wu, X. L. , Dai, C. Q. , Zhao, C. L. , … Chen, X. Y. (2012). Study on the anti‐H1N1 virus effects of quercetin and oseltamivir and their mechanism related to TLR7 pathway. Journal of Asian Natural Products Research, 14(9), 877–885. 10.1080/10286020.2012.702108 [DOI] [PubMed] [Google Scholar]
- Choi, H. J. , Song, J. H. , & Kwon, D. H. (2012). Quercetin 3‐rhamnoside exerts antiinfluenza A virus activity in mice. Phytotherapy Research, 26(3), 462–464. 10.1002/ptr.3529 [DOI] [PubMed] [Google Scholar]
- Davis, J. M. , Murphy, E. A. , McClellan, J. L. , Carmichael, M. D. , & Gangemi, J. D. (2008). Quercetin reduces susceptibility to influenza infection following stressful exercise. American Journal of Physiology ‐ Regulatory Integrative and Comparative Physiology, 295(2), R505–R509. 10.1152/ajpregu.90319.2008 [DOI] [PubMed] [Google Scholar]
- Dayem, A. A. , Choi, H. Y. , Kim, Y. B. , & Cho, S.‐G. (2015). Antiviral effect of methylated flavonol isorhamnetin against influenza. PLOS ONE, 10(3), e0121610. 10.1371/journal.pone.0121610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho, L. G. F. , Lima, W. G. , Coelho, L. G. V. , Cardoso, V. N. , & Fernandes, S. O. A. (2020). Circulating leptin levels as a potential biomarker in inflammatory bowel diseases: A systematic review and meta‐analysis. Inflammatory Bowel Diseases, 27(2), 169–181. 10.1093/IBD/IZAA037 [DOI] [PubMed] [Google Scholar]
- Eriksen, M. B. , & Frandsen, T. F. (2018). The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. Journal of the Medical Library Association, 106(4), 420–431. 10.5195/jmla.2018.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, D. , Zhou, X. , Zhao, C. , Chen, H. , Zhao, Y. , & Gong, X. (2011). Anti‐inflammatory, antiviral and quantitative study of quercetin‐3‐O‐ β‐D‐glucuronide in Polygonum perfoliatum L. Fitoterapia, 82(6), 805–810. 10.1016/j.fitote.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Farazuddin, M. , Mishra, R. , Jing, Y. , Srivastava, V. , Comstock, A. T. , & Sajjan, U. S. (2018). Quercetin prevents rhinovirus‐induced progression of lung disease in mice with COPD phenotype. PLoS ONE, 13(7), e0199612. 10.1371/journal.pone.0199612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratellone, P. M. , Tsimis, F. , & Fratellone, G. (2016). Apitherapy products for medicinal use. The Journal of Alternative and Complementary Medicine, 22(12), 1020–1022. 10.1089/acm.2015.0346 [DOI] [PubMed] [Google Scholar]
- Fratini, F. , Cilia, G. , Mancini, S. , & Felicioli, A. (2016). Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiological Research, 192, 130–141. 10.1016/j.micres.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Fryer, A. D. , & Jocoby, D. B. (1991). Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the Guinea‐pig. British Journal of Pharmacology, 102(1), 267–271. 10.1111/j.1476-5381.1991.tb12164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer, A. D. , & Jacoby, D. B. (1998). Muscarinic receptors and control of airway smooth muscle. American Journal of Respiratory and Critical Care Medicine, 158(5 III), S154–S160. 10.1164/ajrccm.158.supplement_2.13tac120 [DOI] [PubMed] [Google Scholar]
- Ganesan, S. , Faris, A. N. , Comstock, A. T. , Wang, Q. , Nanua, S. , Hershenson, M. B. , & Sajjan, U. S. (2012). Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Research, 94(3), 258–271. 10.1016/j.antiviral.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, S. , Becker, C. , Ridge, K. M. , & Budinger, G. R. S. (2015). Influenza virus‐induced lung injury: Pathogenesis and implications for treatment. European Respiratory Journal, 45(5), 1463–1478. 10.1183/09031936.00186214 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Green, S . (2011). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Retrieved from www.handbook.cochrane.org
- Holtzman, M. J. , Tyner, J. W. , Kim, E. Y. , Lo, M. S. , Patel, A. C. , Shornick, L. P. , … Zhang, Y. (2005). Acute and chronic airway responses to viral infection: Implications for asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society, 2(2), 132–140. 10.1513/pats.200502-015AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan, A. T. , Kamli, M. R. , Murtaza, I. , Singh, J. B. , Ali, A. , & Haq, Q. M. R. (2010). Dietary flavonoid quercetin and associated health benefits‐An overview. Food Reviews International, 26(3), 302–317. 10.1080/87559129.2010.484285 [DOI] [Google Scholar]
- Kaul, T. N. , Middleton, E. , & Ogra, P. L. (1985). Antiviral effect of flavonoids on human viruses. Journal of Medical Virology, 15(1), 71–79. 10.1002/jmv.1890150110 [DOI] [PubMed] [Google Scholar]
- Kim, J. E. , Lee, M. R. , Park, J. J. , Choi, J. Y. , Song, B. R. , Son, H. J. , … Hwang, D. Y. (2018). Quercetin promotes gastrointestinal motility and mucin secretion in loperamide‐induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharmaceutical Biology, 56(1), 309–317. 10.1080/13880209.2018.1474932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Narayanan, S. , & Chang, K. O. (2010). Inhibition of influenza virus replication by plant‐derived isoquercetin. Antiviral Research, 88(2), 227–235. 10.1016/j.antiviral.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Kumar, P. , Khanna, M. , Srivastava, V. , Tyagi, Y. K. , Raj, H. G. , & Ravi, K. (2005). Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Experimental Lung Research, 31(5), 449–459. 10.1080/019021490927088 [DOI] [PubMed] [Google Scholar]
- Kumar, P. , Sharma, S. , Khanna, M. , & Raj, H. G. (2003). Effect of quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. International Journal of Experimental Pathology, 84(3), 127–134. 10.1046/j.1365-2613.2003.00344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, J. R. , & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174. [PubMed] [Google Scholar]
- Li, Y. , Yao, J. , Han, C. , Yang, J. , Chaudhry, M. T. , Wang, S. , … Yin, Y. (2016). Quercetin, inflammation and immunity. Nutrients, 8(3), 1–14. 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. A. , … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6(7), e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, W. G. , Brito, J. C. M. , Cardoso, B. G. , Cardoso, V. N. , de Paiva, M. C. , de Lima, M. E. , & Fernandes, S. O. A. (2020c). Rate of polymyxin resistance among Acinetobacter baumannii recovered from hospitalized patients: A systematic review and meta‐analysis. European Journal of Clinical Microbiology and Infectious Diseases, 39(8), 1427–1438. 10.1007/s10096-020-03876-x [DOI] [PubMed] [Google Scholar]
- Lima, W. G. , Brito, J. C. M. , & da Cruz Nizer, W. S. (2020a). Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID‐19 (SARS‐CoV‐2). Phytotherapy Research, 35, 743–750. 10.1002/ptr.6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, W. G. , Pessoa, R. M. , Vital, K. D. , Takenaka, I. K. T. M. , Cardoso, V. N. , & Fernandes, S. O. A. (2020b). Effect of probiotics on the maintenance of intestinal homeostasis after chemotherapy: Systematic review and meta‐analysis of pre‐clinical studies. Beneficial Microbes, 11, 1–14. 10.3920/bm2019.0142 [DOI] [PubMed] [Google Scholar]
- Lima, W. G. , Silva Alves, G. C. , Sanches, C. , Antunes Fernandes, S. O. , & de Paiva, M. C. (2019). Carbapenem‐resistant Acinetobacter baumannii in patients with burn injury: A systematic review and meta‐analysis. Burns, 45, 1495–1508. 10.1016/j.burns.2019.07.006 [DOI] [PubMed] [Google Scholar]
- Lima, W. G. , Souza, N. A. , Fernandes, S. O. A. , Cardoso, V. N. , & Godói, I. P. (2019). Serum lipid profile as a predictor of dengue severity: A systematic review and meta‐analysis. Reviews in Medical Virology, 29(5), e2056. 10.1002/rmv.2056 [DOI] [PubMed] [Google Scholar]
- McAuley, J. L. , Gilbertson, B. P. , Trifkovic, S. , Brown, L. E. , & McKimm‐Breschkin, J. L. (2019). Influenza virus neuraminidase structure and functions. Frontiers in Microbiology, 10, 39. 10.3389/fmicb.2019.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A. F. , Hassan, M. , Hammad, K. M. , Amer, M. A. , & Riad, S. A. (2015). Monitoring of the antiviral potential of bee venom and wax extracts against Adeno‐7 (DNA) and Rift Valley fever virus (RNA) viruses models. Journal of the Egyptian Society of Parasitology, 45(1), 193–198. 10.12816/0010865 [DOI] [PubMed] [Google Scholar]
- Muller, W. A. (2011). Mechanisms of leukocyte transendothelial migration. Annual Review of Pathology: Mechanisms of Disease, 6(1), 323–344. 10.1146/annurev-pathol-011110-130224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, M. P. N. , Kandaswami, C. , Mahajan, S. , Chadha, K. C. , Chawda, R. , Nair, H. , … Schwartz, S. A. (2002). The flavonoid, quercetin, differentially regulates Th‐1 (IFNγ) and Th‐2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochimica et Biophysica Acta ‐ Molecular Cell Research, 1593(1), 29–36. 10.1016/S0167-4889(02)00328-2 [DOI] [PubMed] [Google Scholar]
- Ngaosuwankul, N. , Noisumdaeng, P. , Komolsiri, P. , Pooruk, P. , Chokephaibulkit, K. , Chotpitayasunondh, T. , … Puthavathana, P. (2010). Influenza A viral loads in respiratory samples collected from patients infected with pandemic H1N1, seasonal H1N1 and H3N2 viruses. Virology Journal, 7(1), 75. 10.1186/1743-422X-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, E. , & Bannai, H. (1993). Quercetin potentiates TNF‐induced antiviral activity. Antiviral Research, 22(4), 327–331. 10.1016/0166-3542(93)90041-G [DOI] [PubMed] [Google Scholar]
- Raju, T. A. N. , Lakshmi, A. N. V. , Anand, T. , Rao, L. V. , & Sharma, G. (2000). Protective effects of quercetin during influenza virus‐induced oxidative stress. Asia Pacific Journal of Clinical Nutrition, 9(4), 314–317. 10.1046/j.1440-6047.2000.00162.x [DOI] [PubMed] [Google Scholar]
- Rynko, A. E. , Fryer, A. D. , & Jacoby, D. B. (2014). Interleukin‐1β mediates virus‐induced M2 muscarinic receptor dysfunction and airway hyperreactivity. American Journal of Respiratory Cell and Molecular Biology, 51(4), 494–501. 10.1165/rcmb.2014-0009OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepecka‐Stojko, A. , Stojko, J. , Kurek‐Górecka, A. , Górecki, M. , Kabała‐Dzik, A. , Kubina, R. , … Iriti, M. (2015). Polyphenols from Bee Pollen: Structure, absorption, metabolism and biological activity. Molecules, 20(12), 21732–21749. 10.3390/molecules201219800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadati, S. M. , Gheibi, N. , Ranjbar, S. , & Hashemzadeh, M. S. (2019). Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidas. Biomedical Reports, 10(1), 33–38. 10.3892/br.2018.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian, S. , Farkhondeh, T. , & Samini, F. (2017). Honey and health: A review of recent clinical research. Pharmacognosy Research, 9(2), 121–127. 10.4103/0974-8490.204647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savov, V. M. , Galabov, A. S. , Tantcheva, L. P. , Mileva, M. M. , Pavlova, E. L. , Stoeva, E. S. , & Braykova, A. A. (2006). Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Experimental and Toxicologic Pathology, 58(1), 59–64. 10.1016/j.etp.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Schnitzler, P. , Neuner, A. , Nolkemper, S. , Zundel, C. , Nowack, H. , Sensch, K. H. , & Reichling, J. (2010). Antiviral activity and mode of action of propolis extracts and selected compounds. Phytotherapy Research, 24(S1), S20–S28. 10.1002/ptr.2868 [DOI] [PubMed] [Google Scholar]
- Siheri, W. , Alenezi, S. , Tusiimire, J. , & Watson, D. G. (2017). The chemical and biological properties of propolis. In Alvarez‐Suarez J. M. (Ed.), Bee products ‐ Chemical and biological properties (pp. 137–178). Cham, Switzerland: Springer. Retrieved from 10.1007/978-3-319-59689-1_7 [DOI] [Google Scholar]
- Trumbeckaite, S. , Dauksiene, J. , Bernatoniene, J. , & Janulis, V. (2015). Knowledge, attitudes, and usage of apitherapy for disease prevention and treatment among undergraduate pharmacy students in Lithuania. Evidence‐Based Complementary and Alternative Medicine, 2015, 1–9. 10.1155/2015/172502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K. , Rahmasari, R. , Matsunaga, A. , Haruyama, T. , & Kobayashi, N. (2014). Anti‐influenza viral effects of honey in vitro: Potent high activity of manuka honey. Archives of Medical Research, 45(5), 359–365. 10.1016/j.arcmed.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Williams, O. W. , Sharafkhaneh, A. , Kim, V. , Dickey, B. F. , & Evans, C. M. (2006). Airway mucus: From production to secretion. American Journal of Respiratory Cell and Molecular Biology, 34(5), 527–536. 10.1165/rcmb.2005-0436SF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W. , Li, R. , Li, X. , He, J. , Jiang, S. , Liu, S. , & Yang, J. (2015). Quercetin as an antiviral agent inhibits influenza a virus (IAV) entry. Viruses, 8(1), 1–18. 10.3390/v8010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X. , Shi, D. , Liu, L. , Wang, J. , Xie, X. , Kang, T. , & Deng, W. (2011). Quercetin suppresses cyclooxygenase‐2 expression and angiogenesis through inactivation of P300 signaling. PLoS One, 6(8), e22934. 10.1371/journal.pone.0022934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D. , Liu, X. , Liu, M. , Chi, H. , Liu, J. , & Han, H. (2015). Protective effects of quercetin and taraxasterol against H2O2‐induced human umbilical vein endothelial cell injury in vitro. Experimental and Therapeutic Medicine, 10(4), 1253–1260. 10.3892/etm.2015.2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, J. K. , Kim, T. S. , Hufford, M. M. , & Braciale, T. J. (2013). Viral infection of the lung: Host response and sequelae. Journal of Allergy and Clinical Immunology, 132(6), 1263–1276. 10.1016/j.jaci.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


