Abstract
Background
Blood usage and collections were impacted throughout 2020 both by the severity of the COVID‐19 pandemic as well as public health decisions affecting hospital operations. We sought to understand the longer‐term effects of the pandemic on blood usage via changes in case volume and clinical intensity as well as whether the blood needs of COVID‐19‐positive patients differed from other transfused patients.
Study design and methods
A single‐center retrospective study of blood use in 2020 as compared to 2014–2019 was conducted at a tertiary care center. Statistical analysis was performed in an R‐based workflow. p values are reported using two‐sided t‐tests for total hospital blood usage and using Mann–Whitney U tests for comparisons of patient blood usage.
Results
Mean monthly red cell usage in 2020 decreased by 11.2% (p = .003), plasma usage decreased by 23.8%, (p < .001) platelet usage decreased by 11.4% (p < .001), and monthly cryoprecipitate use increased by 18% (p = .03). A linear regression model predicted significant associations between total blood usage and the year, number of Medicare eligible discharges, and Case Mix Index. COVID‐19‐positive patients requiring at least one blood product did not use significantly different amounts of red cells, plasma, or platelets from all other transfused patients.
Conclusions
Year 2020 began with decreased blood usage that was normalized by late spring. Reassuringly, transfused COVID‐19‐positive patients in general and those requiring ICU level care do not use significantly increased amounts of blood as compared to similar transfused hospital patients.
Keywords: Blood Management, Transfusion Practices (Adult), Transfusion Service Operations
1. INTRODUCTION
During the first quarter of 2020, the COVID‐19 pandemic disrupted blood collections due to cancellations, increased social distancing, and personal protective equipment requirements, 1 leading to blood shortages. 2 Concurrently and also for pandemic‐related reasons, hospitals canceled elective surgeries, decreasing blood utilization. 3 By late spring, blood usage returned to historical norms. 4 , 5 , 6 , 7 The ongoing pandemic continued to spur shortages of blood components throughout 2020, 8 , 9 especially for patient populations with special needs. 10
Historically, transfusion services have endured blood shortages due to decreases in blood collections around holidays and the summer with the knowledge that they could be mitigated via temporary changes in clinical practices, including cancellation of procedures. 11 , 12 However, the length of the pandemic has made sustained cancellation of all blood intensive procedures unfeasible. Accordingly, blood services have had to massively increase efforts around patient blood management, reducing wastage, and improving inventory management. 13
While some of the improvements in blood inventory management accelerated by the pandemic may have long‐term benefits, the stress of running a transfusion service under this level of uncertainty has been an additional challenge to manage in 2020. Our institution, the University of Maryland Medical Center (UMMC), is a large academic center with a case mix index (CMI) of 2.74 in 2019, the second highest reported case mix index for hospitals in the United States billing over 5000 cases. 14 This is reflected in UMMC's use of over 75,000 blood products in that same year. Supporting trauma, adult and pediatric hematopoietic cell and solid organ transplants, as well as adult and pediatric medical and surgical services throughout 2020 has required intensive coordination between our blood supplier and hospital leadership. We sought to understand these wide swings in blood usage throughout 2020 and correlate them with clinical intensity as measured by admissions and CMI. In addition, we evaluated how these changes had impacted blood expiration and rates of product transfers between hospitals in our health system.
Lastly, we also compared the blood needs of transfused COVID‐19‐positive patients as compared to other transfused patients.
2. MATERIALS AND METHODS
This single‐center retrospective study was conducted at UMMC in downtown Baltimore, a teaching hospital and tertiary care center with 757 beds. UMMC accepts patient and interfacility blood transfers from affiliated hospitals in the University of Maryland Medical System (UMMS). UMMS consists of 13 hospitals, including UMMC as the primary referral center. Critically ill COVID‐19‐positive patients requiring Veno‐Venous Extracorporeal Membrane Oxygenation (VV‐ECMO) were cared for in a specialized Biocontainment Unit (BCU) intensive care setting. Previously, this unit had been known as the Lung Rescue Unit (LRU), an intensive care setting for acute respiratory distress syndrome of other etiologies with patients typically also requiring VV‐ECMO. Blood needs for transfused COVID‐19‐positive patients were compared in general to all transfused hospitalized patients, and transfused BCU patients were compared to LRU patients.
The study was approved by expedited review of the Institutional Review Boards (IRB).
Blood usage and COVID‐19 testing data were obtained from the Cerner Laboratory Information System using the Discern software package (Cerner Laboratory, Cerner Corporation, North Kansas City, MO) and reports generated from the Epic Electronic Health Record (Epic, Epic Systems, Verona, WI).
An R‐based workflow was written in R Markdown using the tidyverse package. 15 , 16 , 17 Linear regression was performed using generalized linear modeling via the base R stats package. In univariate comparisons, p values are reported for total hospital blood usage as the result of two‐sided t tests and for comparisons of individual patient blood usage as the result of Mann–Whitney U tests, both with a significance threshold of 0.05. Averages of hospital blood usage data are reported as means and standard deviations, and averages of patient blood usage data are reported as medians with interquartile ranges unless specified otherwise.
3. RESULTS
3.1. Institutional blood use
Blood usage in 2020 was decreased in the early parts of the pandemic in March and April as compared to previous years, with return to normal levels by the summer. This decrease was most notable in operating room and intensive care unit usage, whereas trauma and hematology/oncology volumes stayed relatively constant (Figure 1). Total Medicare‐eligible patients discharged from the hospital, used as a measure of case volume, mirrored these trends, decreased from 2184 in March to 1490 in April, rebounding to 1825 in May and 1960 in June. In contrast, CMI increased during the early part of the pandemic from a low of 2.44 in February to a high of 2.88 in June (Figure 2).
FIGURE 1.
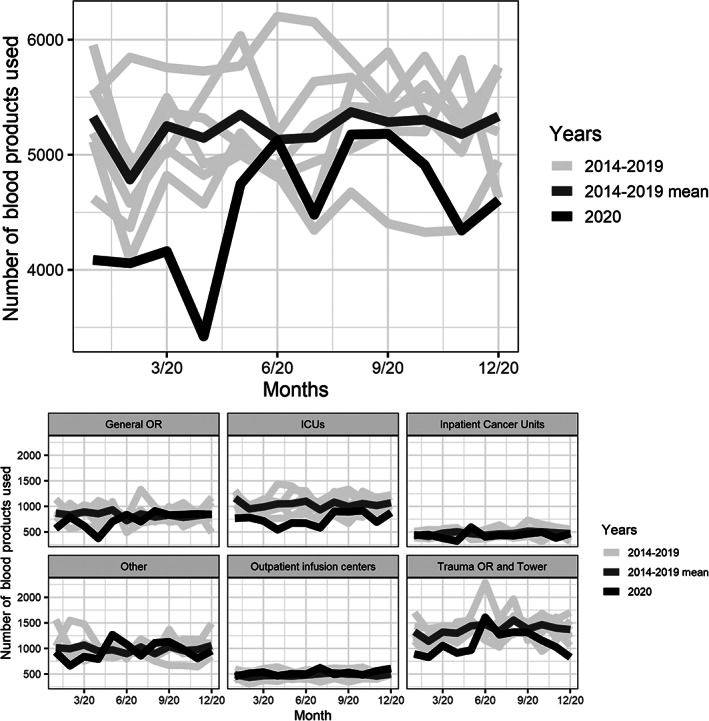
Total blood product usage in 2020 compared to mean usage across 2014–2019 in total and by location. ICUs, intensive care units; OR, operating rooms
FIGURE 2.
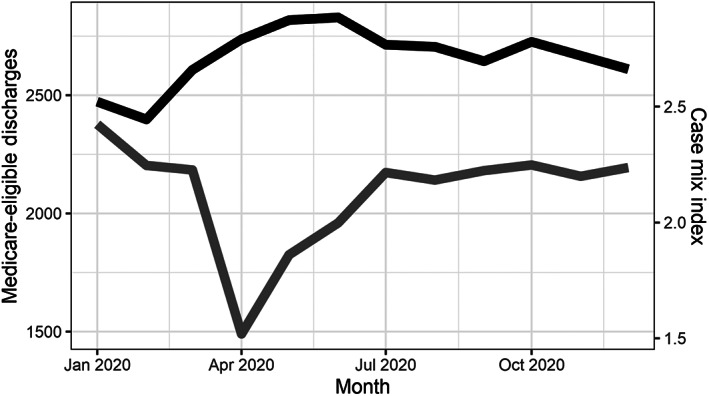
Monthly case mix index (black) and Medicare‐eligible discharges (gray) during 2020
Mean monthly red cell usage in 2020 as compared to 2014–2019 decreased by 11.2% (p = .003), plasma usage decreased by 23.8% (p < .001), and platelet usage decreased by 11.4% (p < .001). In contrast, monthly cryoprecipitate use increased by 18% (p = .03) and mean CMI increased from 2.45 to 2.71 (p < .01). [Table 1]. A linear regression model of monthly blood usage predicted significant associations with year, number of cases, and CMI (model p value < .001, R2 = 0.52) [Table 2].
TABLE 1.
Mean monthly product usage and case mix index (CMI) by years, standard deviations in parentheses
| RBC | Plasma | Platelets | Cryo | CMI | |
|---|---|---|---|---|---|
| 2020 | 2601 (302) | 916 (167) | 836 (71) | 171 (35) | 2.71 (.13) |
| 2014–2019 | 2927 (248) | 1202 (242) | 943 (86) | 145 (39) | 2.45 (.19) |
| p value | .003 | <.001 | <.001 | .03 | <.01 |
Note: p values calculated by t‐test.
Abbreviations: Cryo, cryoprecipitate; RBC, red blood cells.
TABLE 2.
Linear regression model for monthly total blood product usage
| Coefficient | Standard error | p value | |
|---|---|---|---|
| Intercept | −315 | 1604 | .84 |
| Year | −255 | 46 | <.001 |
| Cases | 0.94 | 0.32 | .005 |
| CMI | 1700 | 513 | .001 |
| Adjusted R squared | .52 | ||
| Model p value | <.001 |
Abbreviation: CMI, case mix index.
Bold for P values less than 0.05.
3.2. Blood wastage and transfers
Prior to March, UMMC maintained a monthly expiration rate of 2% or less for red cells, plasma, and platelets. Between March 20th and 23rd after the cancellation of elective surgeries, a spike in plasma wastage was seen to 5.6%. Interfacility transfers between UMMS hospitals of blood products had previously averaged a median of five red cells and seven platelets per month. This increased during March and April and continued into the summer [Figure 3]. During the months of March–July 2020, 300 red cells and 40 apheresis platelet units were transferred from UMMS hospitals to UMMC.
FIGURE 3.
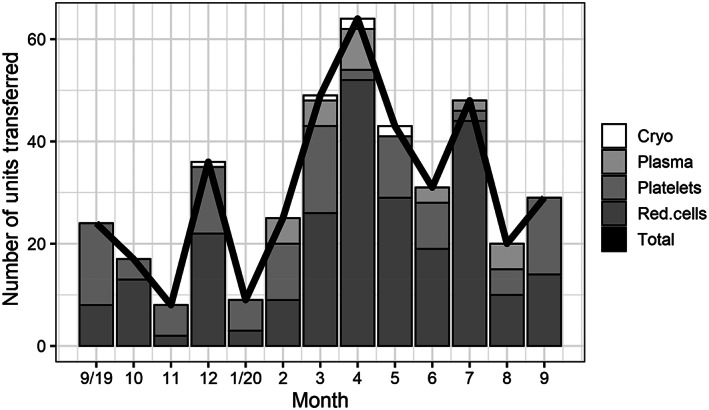
Blood units transferred from system hospitals to University of Maryland Medical Center, September 2019 through September 2020
3.3. COVID‐19 patient blood use
During 2020, 99 patients with COVID‐19 received at least one blood product at UMMC. As compared to COVID‐19 negative or untested patients who received at least one blood product, there were no significant differences in the amounts of red cells, plasma, or platelets transfused to either group (p = .81 for red cells, 0.56 for plasma, 0.22 for platelets) (Table 3).
TABLE 3.
Comparison of blood product usage between COVID‐19 NAT‐positive patients receiving at least one blood product and all transfused hospitalized patients
| COVID‐19 patients | Other transfused patients | p value | |
|---|---|---|---|
| Total Patients | 99 | 5226 | — |
| RBC | 3 (2–6) | 3 (1–6) | .81 |
| Plasma | 3 (2–13) | 3 (2–7) | .56 |
| Platelets | 1 (1–4) | 2 (1–5) | .22 |
Note: p values calculated by Mann–Whitney U test.
Abbreviation: RBC, red blood cell.
In comparison with patients receiving VV‐ECMO in the LRU, there were no significant differences in the use of red cells, plasma, or platelets (p = .34 for red cells, 0.08 for plasma, 0.43 for platelets) (Table 4).
TABLE 4.
Comparison of patient characteristics and blood product usage between biocontainment unit (BCU) and lung rescue unit (LRU) patients receiving at least one blood product
| BCU | LRU | p value | |
|---|---|---|---|
| Patients | 21 | 15 | — |
| Age | 44 (30–49) | 35 (30–53.25) | — |
| Sex | 8 M/12 F | 9 M/6 F | — |
| VV ECMO | 21 (100%) | 15 (100%) | — |
| RBC | 4.5 (3–6.25) | 5.5 (4–10.25) | .34 |
| Plasma | 2 (1–3) | 12 (11–13) | .08 |
| Platelets | 1 (1–3) | 2 (1.5–4) | .43 |
Note: p values calculated by Mann–Whitney U test.
Abbreviations: RBC, red blood cell; VV ECMO, Veno‐venous extracorporeal membrane oxygenation.
4. DISCUSSION
At the onset of the pandemic, fears of a shortage in the blood supply had to be adjusted by a possible decrease in blood utilization due to the deferral of elective surgeries to protect high‐risk patients from contracting the infection while hospitalized for the procedure. The current data show a sharp decrease in overall blood utilization in the surgical and intensive care areas. Blood transfusions also decreased in trauma services, whereas the cancer center patients continued to use blood at the same pace as before the pandemic. This decrease was significant but temporary, with services returning to normal activity levels and blood use for the majority of 2020.
Dealing with uncertainty about both supply and demand is challenging for the blood collection centers that may face wastage of blood while coordinating high numbers of blood drives to mitigate shortages. Our institution was mobilized on both fronts, first by coordinating blood drives on the University hospital campus to allow eligible staff to donate blood and secondly by implementing a blood conservation policy that was more stringent than previous blood management guidelines (Table 5). It is possible that this policy impacted blood usage in 2020 in addition to the significant associations shown with CMI and case volume. However, blood usage returned to normal levels by June despite continued application of this policy. Determining the individual contributions of these policy changes remains an ongoing area of investigation at our institution to balance strict patient blood management with effective use of resources.
TABLE 5.
Blood conservation policy instituted at the start of the pandemic
|
|
|
|
|
|
|
|
Abbreviations: OR, operating room; UMMS, University of Maryland Medical System.
At our institution, the elective surgery schedule was pared down to a minimum for 3 months from Mid‐March to Mid‐June 2020. This 3‐month period of the first surge of the pandemic corresponds exactly on Figure 1 to the drop in blood usage which returned to high levels by the end of June, concurrent with a return to normal levels of surgical volume that persisted through the end of 2020.
In a linear regression model of blood use, later years were significantly associated with less blood use. This is both consistent with the significant decrease in blood use seen for RBCs, plasma, and platelets in (Table 1) as well as reflecting continued efforts in patient blood management. Both total discharged cases and CMI were significantly associated with increasing blood use. During the peak of pandemic‐related changes in blood use in April and May, CMI increased as total cases dropped. This may be due to increased severity of COVID‐19‐related illnesses during this period, as well as a relative decrease in less sick patients due to cancellation of less urgent procedures. The adjusted R squared value for the model was only 0.52, indicating that a little over half of variation in blood usage was predicted by year, cases, and CMI alone.
Even prior to the COVID‐19 pandemic, transfusion services have had to balance on‐hand inventory and usage trends while ensuring blood component wastage was minimal. In mid‐March 2020, our plasma wastage rate rose as surgeries were canceled and pre‐thawed inventory was not utilized. As blood usage continued to drop, we anticipated that blood needs would be higher at UMMC than our other UMMS hospitals. To mitigate wastage across our health system, blood inventory was moved from UMMS hospitals to UMMC at an increased rate during the pandemic.
We continue to accept products to reduce wastage at UMMS hospitals and increase inventory at UMMC. The majority of hospitals reporting in the AABB COVID‐19 blood services survey noted increased wastage during week seven, between May 4 and 7, 2020. 18 Our highest wastage was seen prior to week one of the AABB survey responses, but with the quick return to normal usage seen in May, we had the capacity to support UMMS hospitals by accepting expiring blood products and reducing their wastage.
When examining the blood usage of COVID‐19‐positive patients who required transfusion versus all other transfused inpatients, there were no significant differences in use of red cells, plasma, or platelets. As the pandemic increased in severity, COVID‐19‐positive patients were well represented across the spectrum of medical need. This more general comparison of transfused patients captures this diversity in both COVID‐19‐positive and ‐negative patients needing blood.
In a narrower comparison of patients transfused in the BCU versus in the LRU, there were no differences in blood usage between COVID‐19‐positive patients requiring VV ECMO versus other patients requiring VV ECMO support in the LRU. Anemia and thrombocytopenia among patients admitted with COVID‐19 are uncommon, even among those admitted to an ICU environment. 19 , 20 Although severe COVID‐19 is associated with high concentrations of fibrin degradation products, this coagulation abnormality has not been associated with expanded indications for or increased use of blood products. 21 Our results are consistent with these findings, suggesting that COVID‐19 patients in general do not use significantly different amounts of blood than similar patients, either at an institutional level or for those requiring ICU level care. The current data are also in line with a review of the impact of the COVID‐19 pandemic on blood usage. 22 Taken together, the lack of significant differences between COVID‐19‐positive patients requiring transfusion and other hospitalized patients means that contingency planning surrounding blood use can focus on known predictors of heavier blood use, such as total case volume and CMI as seen in this study.
This is a single center, retrospective, observational study, with the inherent limitations that come from assessing only one large academic center's blood use. However, this is mitigated by UMMC's wide variety of patient populations—adult, pediatric, oncologic, trauma, transplant, and surgical. More data on blood use from institutions with different practice settings, especially regarding other state mandates that affected hospital operations, would be helpful in assessing how volume, clinical intensity, and public health measures affect blood needs.
In comparing these results to our anecdotal experiences during 2020, the return to continued high usage of blood was extremely challenging during severe shortages throughout the year. 8 , 9 , 10 Even with a mature PBM program and close communications between our organization and blood supplier, continuing operations during the pandemic means daily ad hoc decisions regarding supply and patients with special needs. We continue to seek additional tools to predict institutional blood needs, such as machine learning models to predict blood usage, 23 , 24 with the understanding that models trained in non‐pandemic environments will require optimization with this kind of data for continued accuracy. As the public health and blood supply landscape of 2021 continues to provide unpredictability and challenges, transfusion medicine services must continue to balance the need to be prepared to provide a lifesaving intervention with the duty to prevent wastage of a precious resource.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
ACKNOWLEDGMENTS
Jeffrey Sholander provided and consulted on data for number of cases and Case Mix Index.
Murphy C, Fontaine M, Luethy P, McGann H, Jackson B. Blood usage at a large academic center in Maryland in relation to the COVID‐19 pandemic in 2020. Transfusion. 2021;61:2075–2081. 10.1111/trf.16415
REFERENCES
- 1. American Red Cross . What to know about the coronavirus and blood donation. https://www.redcrossblood.org/donate-blood/dlp/coronavirus-covid-19-and-blood-donation.html. Accessed 4 Feb 2021.
- 2. American Red Cross . American Red Cross faces severe blood shortage as coronavirus outbreak threatens availability of nation's supply. American Red Cross Press Release, March 17, 2020.
- 3. Marín‐Mori K, Marín G‐GY, Foncillas‐García M‐Á, Muñoz‐Novas C, Infante M, Churruca‐Sarasqueta J, et al. Blood transfusion activity in a general hospital during the COVID‐19 pandemic. Vox Sang. 2021. 10.1111/vox.13024. [DOI] [PubMed] [Google Scholar]
- 4. Pagano MB, Hess JR, Tsang HC, Staley E, Gernsheimer T, Sen N, et al. Prepare to adapt: blood supply and transfusion support during the first 2weeks of the 2019 novel coronavirus (COVID‐19) pandemic affecting Washington state. Transfusion. 2020;60(5):908–911. 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 5. Murphy C, Jackson B, Fontaine M. Tools for rapid analysis of blood usage and inventory during the COVID‐19 pandemic. Transfusion. 2020;60(10):2199–2202. [DOI] [PubMed] [Google Scholar]
- 6. Pagano MB, Cataife G, Fertrin KY, Gernsheimer T, R Hess J, Staley E, et al. Blood use and transfusion needs at a large health care system in Washington state during the SARS‐CoV‐2 pandemic. Transfusion. 2020;60(12):2859–66. [DOI] [PubMed] [Google Scholar]
- 7. DeSimone RA, Costa VA, Kane K, Sepulveda JL, Ellsworth GB, Gulick RM, et al. Blood component utilization in COVID‐19 patients in New York City: transfusions do not follow the curve. Transfusion. 2021;61(3):692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Red Cross . Red Cross urges blood, platelet donations to prevent another blood shortage. American Red Cross Press Release, May 27, 2020.
- 9. AABB , American Red Cross , America's Blood Centers . Joint industry statement on our nation's blood supply. American Red Cross Press Release, October 19, 2020.
- 10. American Red Cross . Red Cross has urgent need for more black blood donors to help sickle cell patients. American Red Cross Press Release, September 01, 2020.
- 11. Mccarthy LJ. How do I manage a blood shortage in a transfusion service? Transfusion. 2007;47(5):760–2. [DOI] [PubMed] [Google Scholar]
- 12. Stanger SH, Yates N, Wilding R, Cotton S. Blood inventory management: hospital best practice. Transfus Med Rev. 2012;26(2):153–63. [DOI] [PubMed] [Google Scholar]
- 13. Cohn CS, Pagano MB, Allen ES, Frey KP, Gniadek T, Lokhandwala PM, et al. How do I manage long‐term blood component shortages in a hospital transfusion service? Transfusion. 2020;60(9):1897–904. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Medicare & Medicaid Services. FY . Final rule and correction notice data files. Baltimore, MD: Centers for Medicare & Medicaid Services; 2019.p. 2020. [Google Scholar]
- 15. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.R-project.org/. Accessed 4 Feb 2021. [Google Scholar]
- 16. Allaire J, Xie Y, McPherson J, Luraschi J, Ushey K, Atkins A, Wickham H, Cheng J, Chang W, Iannone R. Rmarkdown: dynamic documents for R. R package version 2.1. 2020. https://github.com/rstudio/rmarkdown. Accessed 4 Feb 2021.
- 17. Wickham H, Averick M, Bryan J, Chang W, D'Agostino McGowan L, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. 10.21105/joss.01686. [DOI] [Google Scholar]
- 18. AABB . AABB COVID‐19 hospital transfusion services survey: Week of December 14. 2020: 1–4.
- 19. Guan W‐J, Ni Z‐Y, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid‐19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, et al. Effects of the COVID‐19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7(10):e756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guan L, Tian X, Gombar S, Zemek AJ, Krishnan G, Scott R, et al. Big data modeling to predict platelet usage and minimize wastage in a tertiary care system. Proc Natl Acad Sci USA. 2017;114(43):11368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitterecker A, Hofmann A, Trentino KM, Lloyd A, Leahy MF, Schwarzbauer K, et al. Machine learning‐based prediction of transfusion. Transfusion. 2020;60(9):1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


