Abstract
We present the case of a 3-year-old female liver transplant recipient with a history of Caroli disease who presented with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) test and was ultimately diagnosed with multisystem inflammatory syndrome in children (MIS-C) complicated by portal vein thrombosis. To the best of our knowledge, this is the first case report of MIS-C in a pediatric solid organ transplant (SOT) recipient. Based on our patient, MIS-C could be a potential complication of Coronavirus disease 2019 (COVID-19) in SOT recipients and may have a negative outcome on transplant graft function.
KEYWORDS: clinical research/practice, complication, infection and infectious agents - viral, infectious disease, liver transplantation/hepatology, pediatrics
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; EBV, Epstein-Barr virus; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyl transferase; IL, interleukin; INR, international normalized ratio; IVIG, intravenous immunoglobulin; LDH, lactic acid dehydrogenase; MIS-C, multisystem inflammatory syndrome in children; POD, post-operative day; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first emerged in Wuhan, China as the cause of an unusual pneumonia and quickly spread throughout the globe to be declared a global pandemic by the World Health Organization (WHO) on March 11, 2020. Initially, children were reported to be less frequently affected and to have mild respiratory illness without the hyperinflammatory response reported in a subset of adults.1 However, in April 2020, physicians in the United Kingdom reported a cluster of cases of children with a hyperinflammatory syndrome including fever, hypotensive shock, elevated markers of inflammation, gastrointestinal symptoms, and cardiac dysfunction that resembled Kawasaki Disease.2 On May 14, 2020, the United States Center for Disease Control (CDC) published a Health Advisory to clinicians suggesting that this cluster of symptoms occurring 4–6 weeks after COVID-19 infection may be an entity called multisystem inflammatory syndrome in children (MIS-C).3
The MIS-C case definition from the CDC includes age less than 21 years, fever greater than 38.0°C for 24 or more hours, laboratory evidence of inflammation, severe clinical illness requiring hospitalization, multisystem organ involvement, evidence of current or recent SARS-CoV-2 infection, and no alternative plausible diagnosis.3 , 4 Laboratory evidence of inflammation can include elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, D-dimer, ferritin, lactic acid dehydrogenase (LDH) or interleukin 6 (IL-6), neutrophilia, lymphocytopenia, or hypoalbuminemia.3 , 4 Multisystem involvement is defined as two or more organ systems involved including cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurologic.3, 4, 5 Most children with MIS-C are previously healthy and a disproportionate number of cases have been reported in children of Black and Hispanic race/ethnicity.3, 4, 5
COVID-19 in pediatric transplant recipients has been infrequently reported. A report by Goss et al., including five US pediatric transplant centers, describes asymptomatic to mild respiratory COVID-19 illness in 26 solid organ transplant (SOT) recipients.6 Bush et al. also described mild respiratory COVID-19 illness in a pediatric renal transplant recipient.7 To our knowledge, MIS-C has not been reported in pediatric SOT recipients.
2. CLINICAL CASE
Our patient is a 3-year-old African American female liver transplant recipient who presented with a positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) test and concern for MIS-C. The patient had a history of end-stage liver disease secondary to Caroli disease and received a whole organ pediatric liver transplant with Roux-en-Y hepaticojejunostomy 394 days prior to presentation. Her post-transplant course was complicated by right hepatic artery thrombosis on post-operative day (POD) 7, persistent Epstein-Barr virus (EBV) DNAemia, and portal vein thrombosis requiring percutaneous recanalization by interventional radiology with subsequent normal blood flow. She was receiving tacrolimus and enoxaparin at prophylactic dosing and had preserved allograft function with near normal aminotransferases on routine laboratory check 2 weeks prior to presentation.
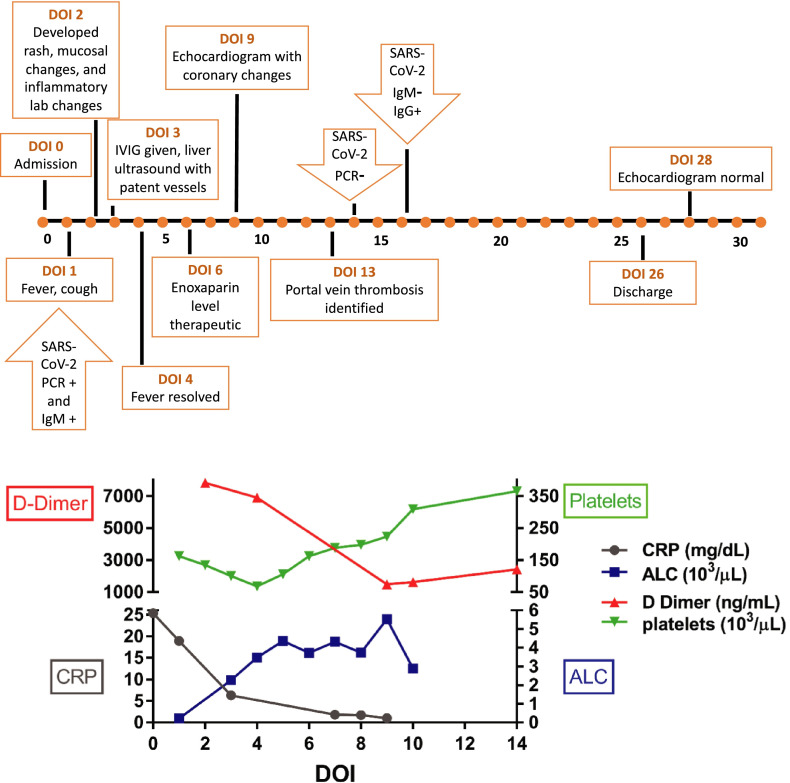
The patient was initially admitted with new onset oliguric renal failure, supratherapeutic tacrolimus levels (15.2 ng/ml), and hyponatremia (serum sodium 126 meq/L). Her enoxaparin level was undetectable consistent with a history of poor adherence. Allograft function was within normal limits (aspartate transaminase [AST] 13 U/L, alanine aminotransferase [ALT] 29 U/L, total bilirubin 0.75 mg/dl, gamma-glutamyl transferase [GGT] 18 U/L, INR 1.42 sec). Hours after admission, she developed fever of 38.2°C ( Figure 1) and was found to be SARS-CoV-2 positive by RT-PCR ([Abbott] test detecting the RdRp and N genes of SARS-CoV-2 virus, sensitive to 100 RNA copies/ml). She had associated cough without respiratory distress or oxygen requirement, but chest radiograph demonstrated bilateral scattered lung opacities within the lower chest. The patient had several family members who were SARS-CoV-2 positive 2 weeks prior to presentation. She was presumed to have primary COVID-19 infection in the setting of chronic immunosuppression. She did not receive COVID-19 directed therapy.
FIGURE 1.
Timeline of key clinical events with associated laboratory evidence of inflammation based on day of illness
On day of illness (DOI) 2, she continued to have fevers and developed an erythematous rash over her trunk, neck and face with desquamation of the perineal area, and oral mucosal changes consistent with a “strawberry tongue”. She was also noted to have periocular swelling and eyelid edema without conjunctivitis. She developed abdominal distention after several episodes of diarrhea. Her evaluation was expanded based on the evolving presentation.
Laboratory testing on DOI 2 revealed lymphopenia (absolute lymphocyte count 210 cells/ul), thrombocytopenia (132 × 103/ul), and systemic inflammation with elevated CRP (18.9 mg/dL), erythrocyte sedimentation rate (25 mm/hr), ferritin (361 ng/ml), and D-dimer (7,822 ng/ml) (Figure 1). B-type natriuretic peptide (BNP) was elevated at 1073 pg/ml. SARS-CoV-2 IgG antibodies were reactive. Although at admission her echocardiogram was reported as normal, a repeat echocardiogram on DOI 7 reveled mild dilation of the left main coronary artery without aneurism (Z-score +2.67) and preserved cardiac function. SARS-CoV-2 IgM testing was not available initially; but on DOI 16, IgM antibodies were not reactive and IgG antibodies remained positive. Given these clinical and laboratory findings, she fulfilled the case definition for MIS-C.
She was treated with intravenous immunoglobulin (IVIG) 2 g/kg once on DOI 3. She tolerated the infusion well and subsequently had resolution of fever on DOI 4. Upon admission, the tacrolimus was held secondary to the supratherapeutic level and subsequently the dose was adjusted to maintain goal trough levels between 5 and 7 ng/ml. Enoxaparin was resumed at admission and a therapeutic level was achieved by DOI 6. Following the repeat echocardiogram, she was also started on high dose aspirin, 30 mg/kg/day for 5 days followed by 3 mg/kg/day, until a repeat echocardiogram on DOI 28, which revealed resolution of the coronary dilation. Her SARS-CoV-2 RT-PCR resulted as negative on DOI 13.
Her hospital course was complicated by increasing liver enzymes (AST 132 U/L, ALT 66 U/L, total bilirubin 0.23 mg/dl, GGT 39 U/L) and liver ultrasound with Doppler showed complete occlusion of the extrahepatic portal vein with lack of blood flow on DOI 13. Notably, a liver ultrasound upon admission had reported patent blood flow in the main portal vein. She underwent re-cannulation of the main portal vein by interventional radiology. The blood flow in the portal vein was re-established and the patient continued to receive enoxaparin therapy. At the time of discharge on DOI 26, all laboratory abnormalities had resolved.
3. DISCUSSION
Multisystem inflammatory syndrome in children (MIS-C), a suspected post-infectious hyperinflammatory response following COVID-19, may present with overlapping symptoms of acute COVID-19 or signs of Kawasaki Disease.3 , 8 Multiple reports during the pandemic have provided clinical features and treatment recommendations, increasing a clinician’s ability to diagnose this novel illness and distinguish it from primary COVID-19.4 However, there is little known about MIS-C and its related complications in pediatric SOT recipients. In fact, it is hypothesized that pediatric SOT patients may have a less robust immune or inflammatory response to COVID-19 given their immunosuppressed status.6 , 9 A single case has been reported of an adult renal transplant patient with a hyperinflammatory response after recovery of COVID-19.10 There have been no reported cases of MIS-C after pediatric SOT. Our case is unique as it describes a young liver transplant recipient who fulfilled the case definition for MIS-C.
Initially, our patient presented with apparent acute COVID-19 illness, as she had respiratory symptoms with a positive RT-PCR for SARS-CoV-2. However, she rapidly developed evidence of inflammation and clinical features concerning for MIS-C, prompting further testing, including antibodies to SARS-CoV-2 and cardiac evaluation. Although respiratory symptoms are not a prominent feature of MIS-C, the presence of respiratory symptoms does not exclude the possibility of MIS-C, particularly as the spectrum of manifestations of MIS-C in immunocompromised patients is unknown.3 Published large MIS-C cohorts indicate that many MIS-C patients present with mild respiratory symptoms, infiltrates on chest radiograph, and positive SARS-CoV-2 nucleic acid tests as noted in our patient.3 , 8 The timing between our patient’s prior exposure to COVID-19 infected family members and her febrile presentation added to her clinical features of rash with desquamation, strawberry tongue, coronary dilation and elevated BNP, favor a diagnosis of MIS-C over primary COVID-19. It is notable that immunosuppressed patients may shed virus and have positive testing for a prolonged time after resolution of acute illness.11 Our patient fulfilled the case definition of MIS-C with features of Kawasaki Disease.3 She had fever, signs of systemic inflammation, clinical features of cardiac, renal, gastrointestinal, dermatologic and hematologic dysfunction with positive SARS-CoV-2 RT-PCR and IgG antibodies. Additionally, while she had findings suggestive of Kawasaki Disease, including strawberry tongue and coronary artery dilation, she did not fulfill the criteria for complete or incomplete Kawasaki Disease. Her gastrointestinal symptoms, thrombocytopenia and positive SARS-CoV-2 antibodies also support the MIS-C diagnosis over Kawasaki Disease.8 In total, her presentation was most consistent with MIS-C.
Our patient did not have shock as described in the literature in two to three-quarters of MIS-C patients and did not require care in the intensive care unit. She was hemodynamically stable throughout her illness without inotropic support or decreased cardiac function despite elevated BNP and echocardiogram findings of mild dilation of the left coronary artery noted on DOI 7. It is possible that her chronic immune suppression regimen could have contributed to reduce the severity of her MIS-C. True coronary artery aneurysm (CAA) are noted less frequently in MIS-C patients as opposed to Kawasaki Disease in which about one-fourth of untreated patients develop this finding.4
Management strategies of immunosuppression in the setting of acute COVID-19 vary widely.12 Some centers report holding immunosuppression, while others caution discontinuation given the risk for allograft rejection.6 There were no published strategies for transplant immunosuppressive management in MIS-C at the time of this patient’s illness. Given the risk for rejection, we chose to titrate tacrolimus dosing to achieve pre-infection goals of 5 – 7 ng/ml.
Our patient had a history of hepatic artery and portal vein thrombosis that had been treated by endovascular therapies in the early post-transplant period. At the time of this illness, she had Doppler ultrasound assessment on admission with patent blood flow across the portal vein, but developed complete occlusion of the main portal vein after MIS-C despite anticoagulation with enoxaparin at therapeutic levels (0.5–1 IU/ml). Thrombosis is less frequently reported after MIS-C than in adult primary SARS-CoV-2 infection. Feldstein et al., reported 8 of 186 patients with thrombotic complications with MIS-C.8 In a patient with an underlying anatomic risk factor for thrombosis, such as previous history of portal vein thrombosis after liver transplantation, the risk for recurrent thrombus formation and vascular compromise after MIS-C might be increased.
Our case highlights that MIS-C may occur in pediatric posttransplant patients despite immunosuppression and that liver transplant recipients with MIS-C might have an increased risk of thrombotic complications, specifically, portal vein thrombosis with possible compromise of the allograft needing additional interventions. In the context of endemic circulation of SARS-CoV-2, clinicians should be aware of this complication after viral infection and actively evaluate blood flow to the liver allograft in children with MIS-C.13 This is especially relevant in patients with other risk factors for hypercoagulability, and when treatment includes the use of IVIG.
This case illustrates that SOT patients can develop MIS-C after COVID-19 and should be evaluated for complications and potential effects of SARS-CoV-2 infection and inflammation on the allograft in the correct clinical context. This case has led our transplant hepatology team to consider MIS-C in liver transplant recipients presenting with fever and positive SARS-CoV-2 testing and to serially evaluate these patients for thrombotic complications of the allograft.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this manuscript are available from the corresponding author upon reasonable request.
Funding information No financial support.
REFERENCES
- 1.Dong Y, Mo XI, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet (London, England). 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children - United States. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 1. Arthritis & rheumatology (Hoboken. NJ). 2020;72(11):1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet (London, England). 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss MB, Galván NTN, Ruan W, et al. The pediatric solid organ transplant experience with COVID-19: an initial multi-center, multi-organ case series. Pediatr Transplant. 2020:e13868. doi: 10.1111/petr.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush R, Johns F, Acharya R, Upadhyay K. Mild COVID-19 in a pediatric renal transplant recipient. Am J Transplant. 2020;20(10):2942–2945. doi: 10.1111/ajt.16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N England J Medicine. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Peralvarez M, Salcedo M, Colmenero J, Pons JA. Modulating immunosuppression in liver transplant patients with COVID-19. Gut. 2020. [DOI] [PubMed]
- 10.Chavarot N, Burger C, Aguilar C, et al. Ig-responsive relapsing inflammatory syndrome following COVID-19 in a kidney transplant recipient. Kidney Int. 2021;99(3):767–768. doi: 10.1016/j.kint.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Society of Transplantation. 2019-nCoV (coronavirus): FAQs for organ transplantation. https://www.myast.org/sites/default/files/COVID19%20FAQ%20Tx%20Centers%206.18.2020.pdf. Published October 13, 2020. Accessed November 8, 2020.
- 12.Gerussi A, Rigamonti C, Elia C, et al. Coronavirus Disease 2019 (COVID-19) in autoimmune hepatitis: a lesson from immunosuppressed patients. Hepatology communications. 2020;4(9):1257–1262. doi: 10.1002/hep4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler JA, Munoz FM, Goss JA, Miloh TA. Viral upper respiratory infection at pediatric liver transplantation is associated with hepatic artery thrombosis. Liver Transplant. 2017;23(11):1477–1481. doi: 10.1002/lt.24866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this manuscript are available from the corresponding author upon reasonable request.