Abstract
Background
Co‐infections, secondary bacterial or fungal infections, are important risk factors for poor outcomes in viral infections. The prevalence of co‐infection and secondary infection in patients infected with SARS‐CoV‐2 is not well understood.
Aims
To investigate the role of co‐infections and secondary infections in disease severity of hospitalized individuals with COVID‐19.
Materials and Methods
A retrospective study was carried out between 11 January 2020 and 1 March 2020 among 408 laboratory confirmed COVID‐19 patients in China. These patients were divided into three groups based on disease severity: mild or moderate, severe, or critically ill. Microbiological pathogens in blood, urine, and respiratory tract specimens were detected by the combination of culture, serology, polymerase chain reaction, and metagenomic next‐generation sequencing (mNGS).
Results
The median age of participants was 48 years (IQR 34–60 years). Fifty‐two patients (12.7%) had at least one additional pathogen, 8.1% were co‐infected, and 5.1% had a secondary infection. There were 13 Mycoplasma pneumoniae cases, 8 Haemophilus influenzae cases, 8 respiratory viruses, and 3 Streptococcus pneumoniae cases, primarily detected in mild and moderate COVID‐19 patients. Hospital‐acquired infection pathogens were more common in critically ill patients. Compared to those without additional pathogens, patients with co‐infections and/or secondary infections were more likely to receive antibiotics (p < 0.001) and have elevated levels of d‐dimer (p = 0.0012), interleukin‐6 (p = 0.0027), and procalcitonin (p = 0.0002). The performance of conventional culture was comparable with that of mNGS in diagnosis of secondary infections.
Conclusion
Co‐infections and secondary infections existed in hospitalized COVID‐19 patients and were relevant to the disease severity. Screening of common respiratory pathogens and hospital infection control should be strengthened.
Keywords: co‐infection, COVID‐19, etiology, mNGS, secondary infection
Abbreviations
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- CAP
community‐acquired pneumonia
- CDC
Chinese Centers for Disease Control
- cDNA
complementary DNA
- CRP
C‐reactive protein
- CT
computed tomography
- ICU
intensive care unit
- IL‐6
interleukin‐6
- IQR
interquartile range
- MNCP
micro/nanofluidic chip platform
- mNGS
metagenomic next‐generation sequencing
- NMPA
National Medical Products Administration
- NP
nasopharyngeal
- PCR
polymerase chain reaction
- PCT
procalcitonin
- RSV
respiratory syncytial virus
- RV
respiratory virus
- SARS‐CoV‐2
severe acute respiratory coronavirus‐2
- VAP
ventilator‐associated pneumonia
- WHO
World Health Organization
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is an ongoing pandemic. 1 As of 30 October 2020, a total of 44 888 869 cases have been diagnosed and 1 178 475 deaths have been reported worldwide by the World Health Organization. 2 Although most COVID‐19 cases are asymptomatic or have mild symptoms similar with other respiratory viral infections, 3 severe lower respiratory tract infections have disproportionately affected older populations 4 and individuals with underlying diseases, such as cardiovascular disease and diabetes. 5 Similar to common causes of respiratory viral pneumonia, autopsy of COVID‐19 death 6 revealed signs of acute respiratory distress syndrome, fibrosis, and inflammatory injury.
Co‐infections, especially secondary streptococcal or fungal infections, are important risk factors for poor outcomes of influenza pneumonias. 7 For example, secondary bacterial infections were identified in up to 34% of 2009 H1N1 pandemic influenza cases treated in intensive care units (ICU) and up to 55% of fatal cases. 8 , 9 However, the role of co‐infections and secondary infections in COVID‐19 patients is not well described. Although antibiotics are not effective for SARS‐CoV‐2, which are empirically used in COVID‐19 patients suspected with co‐infection or secondary infection. Therefore, understanding the epidemiological patterns of COVID‐19 with co‐infection and secondary infection is crucial for clinical treatment and to help ensure rational use of antibiotics.
Microbiological testing is rapidly evolving and in many cases, traditional microbiological testing is being eclipsed by molecular methods for the detection of pathogens, allowing for clinically relevant increases in speed and breadth of detection. For example, Xpert® Flu/RSV assay can detect influenza virus A, influenza virus B, and respiratory syncytial virus (RSV) in respiratory specimens within 40 min, 10 while micro/nanofluidic chip platform can detect multiple pathogens within 2 h. 11 Although more time consuming, metagenomic next‐generation sequencing (mNGS) has been proposed as a universal method to detect all pathogens in a clinical sample and is especially suitable for rare, novel, and atypical etiologies of complicated infectious diseases, 12 such as COVID‐19. Although the promise of these tools in the future of infectious disease diagnostics is enormous, their superiority to classical, “gold standard” approaches, such as laboratory cultures, has not been fully validated.
As such, the objective of this study was to describe the clinical and etiological characteristics associated with co‐infections and secondary infections of COVID‐19 patients presenting in Shenzhen, China and to compare the performance of conventional culture with mNGS in the diagnosis of secondary infections.
2. METHODS
2.1. Data and sample collection
On 8 January 2020, Shenzhen Centers for Disease Control (CDC) identified the first case of pneumonia with unknown cause. The Shenzhen Third People's Hospital is the only government‐mandated hospital for the treatment of COVID‐19 patients in this metropolis of 20 million people. Between 11 January 2020 and 1 March 2020, a total of 418 patients diagnosed with SARS‐CoV‐2 infections were admitted to our hospital. A total of 408 patients who received at least one microbiological test were enrolled in this study (Figure 1) and their clinical and laboratory findings, microbiological investigation results, and thoracic computed tomography scans were retrospectively collected.
FIGURE 1.
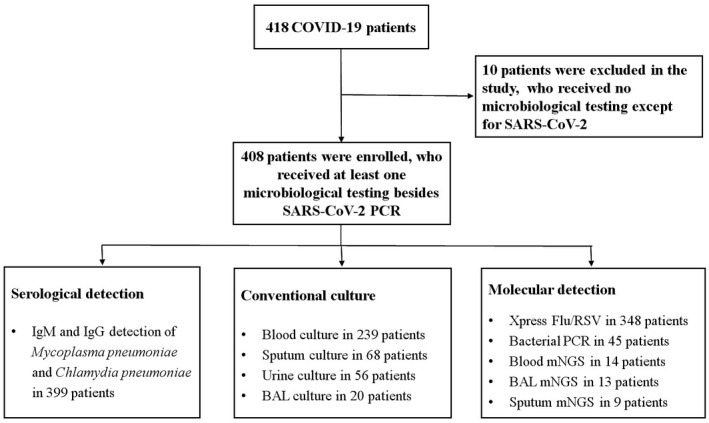
Patients enrollment and microbiological testing
Furthermore, patients were classified into disease groups based on COVID‐19 severity. 13 Mild cases were defined as patients presenting with mild clinical symptoms without pneumonia manifestation on imaging. Moderate cases were patients presenting with a fever and respiratory symptoms, along with radiological findings of pneumonia. Severe cases met any one of the following criteria: respiratory distress, hypoxia (SpO2 ≤ 93%), or abnormal blood gas analysis (PaO2 < 60 mmHg, PaCO2 > 50 mmHg). Lastly, critical cases met any one of the following criteria: respiratory failure requiring mechanical ventilation or shock accompanied by other organ failure that required ICU care. The patients were then divided into three groups: mild and moderate, severe, and critically ill.
Blood samples and nasopharyngeal swabs were routinely collected from each patient, and lower respiratory samples, including sputum and bronchoalveolar lavage fluid (BAL), were collected when available and clinically indicated. Blood cultures were also obtained when clinically indicated. All samples were sent to the clinical laboratory of the hospital for pathogen detection by molecular assay and/or conventional culture. This study was approved by the Institutional Review Board of Shenzhen Third People's Hospital (number 2020‐100).
2.2. Conventional microbiological testing
Microbiological culture was performed on sputum, BAL, urine, and blood at the Clinical Laboratory of our hospital. All sputum samples were examined by microscopy and samples containing a preponderance of leukocytes and a few squamous epithelial cells per 100× magnification field were considered acceptable for culture. Qualitative cultures of blood samples were performed.
2.3. Nucleic acid extraction and molecular detection of SARS‐CoV‐2
Nucleic acid was extracted from respiratory tract samples with the Nucleic Acid Isolation Kit (DA0630, DA’AN, Guangzhou, China) using semi‐automatic Nucleic Acid Extraction System. The real‐time RT‐PCR assay was performed using a SARS‐CoV‐2 Nucleic Acid Detection Kit (DA’AN, Guangzhou, China). Two target sequences in open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) were simultaneously amplified and tested. Amplification of human RNase P gene was used to qualify the sampling process. Positive results were repeated by the local Chinese CDC using different kits approved by the National Medical Products Administration (NMPA).
2.4. Molecular detections of respiratory viruses and bacteria pathogens
We detected influenza virus and RSV using the Xpert® assay (Cepheid, Sunnyvale, CA). 10 Pathogenic Bacterial Nucleic Acid Detection Kit (CapitalBio Technology, Beijing, China) was used to detect 13 bacterial pathogens, including Streptococcus pneumoniae, Staphylococcus aureus, Methicillin‐resistant staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia, Haemophilus influenzae, Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, and Mycobacterium tuberculosis complex.
2.5. PCR and Sanger validation
Clinical isolates with low ID score from MALDI‐TOF‐MS Biotyper were identified by colony PCR combined Sanger sequencing. Briefly, DNA from clinical isolates was extracted, followed by the amplification of bacterial 16S rRNA or fungal ITS regions. 14 , 15 The specific primers used for the gene amplification are as follows: Bact‐F: 5′‐AGAGTTTGATYMTGGCTCAG‐3′ and Bact‐R: 5′‐TACGGYTACCTTGTTACGACT‐3′, ITS‐1:5′‐TCCGTAGGTGAACCTGCGG‐3′ and ITS‐4:5′‐TCCTCCGCTTATTGATATGC‐3′. Subsequently, the PCR products were sequenced and species identification was determined by BLAST with a sequence identity ≥99%.
2.6. Metagenomic next‐generation sequencing
Nucleic acid of blood (14 patients), BAL (13 patients), and sputum (9 patients) was extracted and complementary DNA (cDNA) was generated from an RNA template by reverse transcription. DNA libraries were constructed through DNA‐fragmentation, end‐repair, adapter‐ligation, and PCR amplification. Quality qualified libraries were sequenced by BGISEQ‐50 platform for 20 million reads. 16 High‐quality sequencing data were generated by removing low‐quality and short (length <35 bp) reads, followed by computational subtraction of human host sequences mapped to the human reference genome (hg19) using Burrows‐Wheeler Alignment. 17 The remaining data were classified by simultaneously aligning to four Microbial Genome Databases, consisting of 4061 viruses, 2473 bacteria, 199 fungi, and 135 parasites. mNGS and conventional culture were compared and assessed only in 13 patients (12 critically ill patients and 1 severe ill patient), since whose specimens (blood, BAL, or sputum) were detected by these two detection assays at the same time.
2.7. Definition of etiology in this study
Co‐infections were considered present at the time of admission (initial 48 h), secondary infections emerged during the course of hospitalization. 1 The definition for pathogen detection was based on positive results from at least one testing method. The etiology was considered definite if one of the following criteria was met: (a) positive growth of a non‐skin flora commensal on one or more blood culture; to define a bloodstream infection as that caused by a common skin colonizer such as coagulase‐negative staphylococci or Corynebacterium, we required two or more blood cultures drawn from different sites and a clinical evaluation from one of our researchers (YYX or ZFJ); (b) ≥104 cfu/ml culture from BAL or urine; (c) positive culture from purulent sputum with compatible findings on gram staining; and (d) a fourfold increase in IgG antibodies of Mycoplasma pneumoniae or Chlamydia pneumoniae between acute and convalescent phase samples. The criteria of probable etiology were: (a) positive IgM antibody for Mycoplasma pneumonia, (b) detection of a respiratory virus or bacterial pathogen in respiratory samples by molecular detection, and (c) number of reads of viruses and conditional pathogens (bacteria and Candida) detected by mNGS was >50, tuberculosis and filamentous fungi was >3. 18
2.8. Statistical analysis
Categorical variables were presented as numbers and percentages. Data for continuous variables were presented as median (IQR). Comparisons of clinical features were conducted between individuals with and without co‐infection using a χ 2 test and the Mann–Whitney test (SPSS 22.0).
3. RESULTS
3.1. Patient characteristics
Of 408 enrolled COVID‐19 patients, 52 (12.7%) patients had at least one additional pathogen detected. The overall fatality rate was 0.7%. As shown in Table 1, more patients with co‐infections or secondary infections had diabetes (p = 0.036), were critically ill (p < 0.001), acquired required oxygen therapy (p = 0.044) and received antibiotic therapy (p < 0.001). Patients with thrombocytopenia (p = 0.032), and higher levels of d‐dimer (p = 0.0012), Interleukin‐6 (IL‐6) (p = 0.0027), and procalcitonin (PCT) (p = 0.0002) were more likely to have an additional pathogen.
TABLE 1.
Demographics features of COVID‐19 patients on admission to hospital
| All patients (n = 408) | At least one additional pathogen (n = 52) | No additional pathogen detection (n = 356) | p value | |
|---|---|---|---|---|
| Median (IQR) age, years | 48.0 (34.0‐60.0) | 54.5 (35.0‐65.25) | 47.0 (34.0‐59.0) | 0.32 |
| Gender, male | 196 (48.0) | 26 (50.0) | 170 (47.8) | 0.76 |
| Comorbidity | 106 (26.0) | 18 (34.6) | 88 (24.7) | 0.13 |
| Hypertension | 53 (13.0) | 10 (19.2) | 43 (12.1) | 0.15 |
| Diabetes | 22 (5.4) | 6 (11.5) | 16 (4.5) | 0.036 |
| Cardiovascular diseases | 15 (3.7) | 3 (5.8) | 12 (3.4) | 0.42 |
| Cancer | 7 (1.7) | 0 (0) | 7 (2.0) | 0.60 |
| Others | 35 (8.6) | 6 (11.5) | 29 (8.1) | 0.41 |
| Symptoms at admission | 357 (87.5) | 47 (90.4) | 310 (87.1) | 0.65 |
| Median (IQR) days from onset to admission | 3 (1‐6) | 3 (2‐5.25) | 3 (1‐6) | 0.33 |
| Severity group | ||||
| Mild and moderate | 319 (78.2) | 30 (57.7) | 289 (81.2) | <0.001 |
| Severe ill | 71 (17.4) | 7 (13.5) | 64 (18.0) | 0.56 |
| Critically ill | 18 (4.4) | 15 (28.8) | 3 (0.8) | <0.001 |
| Treatment | ||||
| Oxygen therapy | 138 (33.8) | 24 (46.1) | 114 (32.0) | 0.044 |
| Antibiotic treatment | 60 (14.7) | 34 (65.4) | 26 (7.3) | <0.001 |
| Antiviral treatment | 408 (100) | 52 (100) | 356 (100) | >0.99 |
| Blood routine | ||||
| Leukocyte count (×10⁹/L) | 4.7 (3.8‐5.9) | 4.8 (4.0‐6.3) | 4.6 (3.8‐5.9) | 0.26 |
| >10 × 10⁹/L | 11 (2.7) | 3 (5.8) | 8 (2.2) | 0.15 |
| <4 × 10⁹/L | 122 (29.9) | 13 (25) | 109 (30.6) | 0.40 |
| Lymphocyte count (×10⁹/L) | 1.3 (1.0‐1.8) | 1.2 (0.9‐1.7) | 1.3 (1.0‐1.8) | 0.14 |
| <1.5 × 10⁹/L | 256 (62.7) | 36 (69.2) | 220 (61.8) | 0.31 |
| Platelet count | 186.0 (148.0‐233.0) | 171.0 (128.0‐228.5) | 188.0 (150.0‐234.0) | 0.070 |
| <150 × 10⁹/L | 107 (26.2) | 20 (38.5) | 87 (24.4) | 0.032 |
| Hemoglobin (g/L) | 137.0 (126.0‐147.0) | 135.5 (123.3‐145.0) | 137.0 (127.0‐147.0) | 0.18 |
| Coagulation function | ||||
| d‐dimer (μg/L) | 0.4 (0.3‐0.5) | 0.5 (0.3‐1.0) | 0.4 (0.2‐0.5) | 0.0012 |
| ≥0.5 μg/L | 127 (31.1) | 23 (44.2) | 104 (29.2) | 0.026 |
| Infection‐related biomarkers | ||||
| C‐reactive protein (mg/L) | 9.5 (4.1‐25.1) | 11.2 (3.2‐28.3) | 9.4 (4.3‐25.0) | 0.57 |
| ≥10 mg/L, n = 393 | 197 (48.3) | 27 (51.9) | 170 (47.8) | 0.63 |
| IL‐6 (pg/ml) (n = 348/47/301) | 9.6 (3.9‐19.4) | 19.1 (5.3‐36.1) | 8.8 (3.8‐17.4) | 0.0027 |
| ≥7 pg/ml, n = 326 | 190 (54.6) | 32 (68.1) | 158 (52.5) | 0.046 |
| Procalcitonin (ng/ml) | 0.0 (0.0‐0.1) | 0.1 (0.0‐0.1) | 0.0 (0.0‐0.1) | 0.0002 |
| ≥0.25 ng/ml, n = 385 | 6 (1.5) | 3 (5.8) | 3 (0.8) | 0.03 |
| Clinical outcomes a | ||||
| Remained in hospital | 13 (3.2) | 6 (11.5) | 7 (2.0) | 0.0002 |
| Discharged | 392 (96.1) | 43 (82.7) | 349 (98.0) | <0.001 |
| Median (IQR) duration of hospitalization | 20.0 (15.0‐27.0) | 20.0 (14.0‐27.0) | 20.0 (15.0‐27.0) | 0.84 |
| Died | 3 (0.7) | 3 (5.8) | 0 (0) | 0.002 |
Data are no. (%) and median (IQR).
Clinical outcomes were recorded till 16 March 2020.
Microbial etiology within 48 h after patient's admission was determined in 33 (8.1%) of the 408 COVID‐19 patients (Table 2), among which 29 patients of them have mild and moderate disease. Viral co‐infections were detected in eight patients (2.3%), including RSV (four patients), Influenza virus B (three patients), and Influenza virus A (one patient). Thirteen patients (3.2%) were positive with Mycoplasma pneumoniae IgM. The most common bacterial co‐infections were Haemophilus influenzae (n = 8), Streptococcal pneumoniae (n = 3), Staphylococcus aureus (n = 3), and Klebsiella pneumoniae (n = 2). Four patients had more than one co‐infected bacterium.
TABLE 2.
Distribution of co‐infections within 48 h after admission
| Pathogen identified | No. (%) of patients (n = 408) | Mild and moderate (n = 319) | Severe ill (n = 71) | Critically ill (n = 18) |
|---|---|---|---|---|
| Respiratory virus (n = 348) | 8 (2.3) | |||
| Respiratory syncytial virus | 4 | 4 | ||
| Influenza virus B | 3 | 3 | ||
| Influenza virus A | 1 | 1 | ||
| IgM testing of atypical bacteria (n = 399) | 13 (3.2) | |||
| Mycoplasma pneumoniae | 13 | 13 | ||
| Bacterial PCR (n = 45) | 12 (26.7) | |||
| Haemophilus influenzae | 6 | 4 | 2 | |
| Klebsiella pneumoniae | 2 | 1 | 1 | |
| Streptococcus pneumoniae | 1 | 1 | ||
| Staphylococcus aureus + Streptococcus pneumoniae | 1 | 1 | ||
| Staphylococcus aureus + Haemophilus influenzae | 1 | 1 | ||
| Methicillin‐resistant staphylococcus aureus + Haemophilus influenzae + Streptococcus pneumoniae | 1 | 1 | ||
| Total | 33 (8.1) | 29 (9.1) | 3 (4.2) | 1 (5.6) |
Twenty‐one (5.1%) patients experienced secondary bacterial and fungal infections, among those 5 and 15 patients were, respectively, classified as severe and critically ill groups (Table 3). Bacterial pathogens included Acinetobacter baumannii (n = 7), Stenotrophomonas maltophilia (n = 6), Klebsiella pneumoniae (n = 5), Pseudomonas aeruginosa (n = 5), and Enterobacter aerogenes (n = 4). Fungal pathogens were comprised of Aspergillus fumigatus (n = 2), Aspergillus flavus (n = 1), Penicillium rolfsii (n = 1), and Candida parapsilosis (n = 1). Furthermore, four filamentous fungi were isolated from three patients. Although the physician prescribed antifungal medication (Voriconazole) for treatment, the two patients still died, indicating that the fatality of the patients with fungal secondary infection may be relatively high. Herpes simplex virus‐1 was detected in five critically ill patients.
TABLE 3.
Distribution of secondary infection pathogens
| Pathogen identified | No. (%) of patients (n = 408) | Mild and moderate (n = 319) | Severe ill (n = 71) | Critically ill (n = 18) |
|---|---|---|---|---|
| Bacterial pathogens | ||||
| Acinetobacter baumannii | 7 | 1 | 2 | 4 |
| Stenotrophomonas maltophilia | 6 | 0 | 1 | 5 |
| Pseudomonas aeruginosa | 5 | 0 | 1 | 4 |
| Klebsiella pneumoniae | 5 | 0 | 0 | 5 |
| Enterobacter aerogenes a | 4 | 0 | 0 | 4 |
| Ralstonia mannitolilytica | 3 | 0 | 0 | 3 |
| Burkholderia multivorans | 3 | 0 | 0 | 3 |
| Escherichia coli | 2 | 0 | 1 | 1 |
| Pseudomonas putida | 2 | 0 | 0 | 2 |
| Enterococcus faecium | 2 | 0 | 0 | 2 |
| Staphylococcus aureus | 1 | 0 | 0 | 1 |
| Enterococcus faecalis | 1 | 0 | 0 | 1 |
| Sphingomonas Paucimobilis | 1 | 0 | 0 | 1 |
| Bacteroides ovatus | 1 | 0 | 0 | 1 |
| Pediococcus lactis | 1 | 0 | 0 | 1 |
| Fungal pathogens | ||||
| Aspergillus fumigatus | 2 | 0 | 0 | 2 |
| Aspergillus flavus | 1 | 0 | 0 | 3 |
| Penicillium rolfsii | 1 | 0 | 0 | 3 |
| Candida parapsilosis | 1 | 0 | 0 | 1 |
| Viral pathogens | ||||
| Herpes simplex virus‐1 b | 5 | 0 | 0 | 5 |
| Patients with secondary infection | 21 (5.1) | 1 (0.3) | 5 (7.0) | 15 (83.3) |
Data are no. (%) of the patients.
The only carbapenem‐resistant enterobacteriaceae (CRE) in this study, isolated from BAL of patient No. 44.
The pathogen only detected by mNGS in blood or BAL. Two patients had co‐infection and secondary infection
Both co‐infection and secondary infection occurred in two patients: one severe ill patient was co‐infected with Haemophilus influenzae, and secondary infected with Pseudomonas aeruginosa, who was discharged after 49 days treatment; and one critically ill patient was co‐infected with Klebsiella pneumoniae, and secondary infected with Aspergillus flavus and Aspergillus fumigatus, was discharged after 45 days treatment.
3.2. Etiological yield of different detection assays
As shown in Table 4, molecular and serological testing for RVs identified 15 mild and moderate patients with co‐infections. A total of 12 (26.7%) patients were positive for bacterial PCR. The positivity rate of conventional culture of BAL, sputum, and urine was 65%, 22.1%, and 5.4%. Because mNGS was only performed in less than 14 severe and critically ill patients, positivity obtained from mNGS measures was 28.6% for blood, 92.3% for BAL, and 66.7% for sputum.
TABLE 4.
Etiological yield among three groups according to different testing
| Microbiological testing | No. (%) of patients | Mild and moderate | Severe ill | Critically ill |
|---|---|---|---|---|
| Xpress Flu/RSV | 8/348 (2.3) | 8/276 (2.9) | 0/59 (0) | 0/13 (0) |
| Serological testing | 13/399 (3.3) | 13/314 (4.1) | 0/69 (0) | 0/16 (0) |
| Bacterial PCR | 12/45 (26.7) | 8/14 (57.1) | 3/16 (18.8) | 1/15 (6.7) |
| Blood culture | 8/239 (3.3) | 0/171 (0) | 1 a /51 (2.3) | 7/17 (41.2) |
| BAL culture | 13/20 (65.0) | 0/1 (0) | 0/3 (0) | 13/16 (81.3) |
| Sputum culture | 15/68 (22.1) | 1 b /23 (4.3) | 1 c /27 (3.7) | 13/18 (72.2) |
| Urine culture | 3/56 (5.4) | 0/22 (0) | 1 d /18 (5.6) | 2/16 (12.5) |
| Blood mNGS | 4/14 (28.6) | NA | 0/1 (0) | 4/13 (30.8) |
| BAL mNGS | 12/13 (92.3) | NA | NA | 12/13 (92.3) |
| Sputum mNGS | 6/9 (66.7) | NA | NA | 6/9 (66.7) |
Data are no. positive/tested cases (%) of patients. NA: not performed.
Stenotrophomonas maltophilia.
Acinetobacter baumannii.
Pseudomonas aeruginosa.
Escherichia coli.
3.3. Diagnostic yield between culture and mNGS
As illustrated in Table 5, the common bacteria and Candida pathogen could be detected by both of culture and mNGS. All four filamentous fungi (Aspergillus fumigatus [n = 2], Aspergillus flavus [n = 1], and Penicillium rolfsii [n = 1]) could be isolated by culture, however, only two cases of Aspergillus fumigatus were detected by mNGS. Thirteen patients whose specimens (blood, BAL, or sputum) were detected by both of mNGS and conventional culture at the same time were compared, including 12 critically ill patients and 1 severe ill patient. And, consistent results were obtained in BAL and sputum samples (Table 6).
TABLE 5.
Distribution of pathogens and the yield of conventional culture and mNGS
| Pathogen identified | patients with positive findings (n = 408) | Blood culture (n = 239) | BAL culture (n = 20) | Sputum culture (n = 68) | Urine culture (n = 56) | Blood mNGS (n = 14) | BAL mNGS (n = 13) | Sputum mNGS (n = 9) |
|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 7 (1.7) | – | 3 | 7 | – | – | 2 | – |
| Stenotrophomonas maltophilia | 6 (1.5) | 3 | 6 | 6 | – | – | 5 | 1 |
| Klebsiella pneumoniae | 5 (1.2) | – | 3 | 4 | – | – | 3 | 2 |
| Pseudomonas aeruginosa | 5 (1.2) | – | 3 | 4 | – | – | 2 | 1 |
| Enterobacter aerogenes | 4 (1.0) | – | 4 | 3 | – | – | 2 | 1 |
| Ralstonia mannitolilytica | 3 (0.7) | – | 2 | 3 | – | – | 2 | – |
| Burkholderia multivorans | 3 (0.7) | – | 2 | 2 | – | – | 2 | 1 |
| Escherichia coli | 2 (0.5) | – | – | 1 | 1 | – | – | – |
| Pseudomonas putida | 2 (0.5) | – | 1 | 2 | – | – | – | – |
| Enterococcus faecium | 2 (0.5) | 2 | – | – | – | – | – | – |
| Staphylococcus aureus | 1 (0.2) | – | – | 1 | – | – | – | – |
| Enterococcus faecalis | 1 (0.2) | 1 | – | – | – | – | – | – |
| Sphingomonas Paucimobilis | 1 (0.2) | – | – | 1 | – | – | – | – |
| Pediococcus lactis a | 1 (0.2) | 1 | – | – | – | – | – | – |
| Bacteroides ovatus | 1 (0.2) | 1 | – | – | – | 1 | – | – |
| Aspergillus fumigatus b | 2 (0.5) | – | 3 | 2 | – | 1 | 1 | 1 |
| Aspergillus flavus | 1 (0.2) | – | 1 | 1 | – | – | – | – |
| Penicillium rolfsii b , c | 1 (0.2) | – | 1 | 1 | – | – | – | – |
| Candida parapsilosis | 1 (0.2) | 1 | – | – | 1 | – | – | – |
| Herpes simplex virus‐1 | 5 (1.2) | – | – | – | – | 2 | 4 | – |
Data are no. positive (%) of patients. “−” : negative.
The bacterium was identified by 16S sequencing shown in supplementary file.
Fragment hyphae with 45‐degree branches seen under gram staining of sputum or BAL (1000× magnification).
Penicillium rolfsii was identified by ITS sequencing shown in supplementary file.
TABLE 6.
Concordance of pathogen results between conventional culture and mNGS
| Culture | ||||||||
|---|---|---|---|---|---|---|---|---|
| Blood | BAL | Sputum | ||||||
| Positive | Negative | Positive | Negative | Positive | Negative | |||
| mNGS | Blood | Positive | 2 | 2 a | – | – | – | – |
| Negative | 5 | 4 | – | – | – | – | ||
| BAL | Positive | – | – | 12 | 0 | – | – | |
| Negative | – | – | 0 | 1 | – | – | ||
| Sputum | Positive | – | – | – | – | 6 | 0 | |
| Negative | – | – | – | – | 0 | 1 | ||
HSV‐1 was detected by mNGS with 54 and 68 reads in blood.
4. DISCUSSION
This study summarized co‐infections and secondary infections among 408 hospitalized COVID‐19 patients in Shenzhen, China. A total of 8.1% of participants (mainly mild or moderate patients, 29/33) were co‐infected with common community‐acquired pathogens, while 5.1% (mainly severe and critically ill patients, 20/21) had a secondary infection with hospital‐acquired pathogens. The study also compared the clinical characteristics and outcomes of patients infected with at least one additional pathogen to those with no additional pathogen detection.
Consistent with previous clinical findings, 1 , 19 408 COVID‐19 patients in our study also had lymphopenia (62.7%) and elevated levels of C‐reactive protein (48.3%), IL‐6 (54.6%), and d‐dimer (31.1%) on admission. As a result of active case detection, individuals were hospitalized early in their illness course, with a median duration of 3 days (IQR 1–6) compared to 7 days (IQR 4–8) in early cases in Wuhan. 20 In this study, more COVID‐19 patients with co‐infections/secondary infections had diabetes (p = 0.036) and higher levels of IL‐6 (p = 0.0027) and d‐dimer (p = 0.0012), which were defined as potential risk factors to identify patients with poor prognosis at an early stage by Zhou et al. 21 Correspondingly, a higher fatality rate was also observed in co‐infections/secondary infection group (5.8%, p = 0.002).
Co‐infections were common in community‐acquired pneumonia patients. 22 Regarding COVID‐19, we do not know whether SARS‐CoV‐2 could outcompete other respiratory pathogens. In the first two studies in China on 41 and 99 COVID‐19 patients, no positive results had been reported though several common community‐acquired pathogens had been detected. 1 , 23 Recently, Lansbury et al. reported 1%–20% of bacterial co‐infection in China, and summarized pooled population of 1014 patients with an estimated 3% (95% CI: 1%–6%) of viral co‐infection. 24 Bacterial co‐infection was reported in 4.9% (95% CI: 2.6%–7.1%) of COVID‐19 patients from latest updates by the Toronto Antimicrobial Resistance Research Network (TARRN) website (https://www.tarrn.org/covid). Our results are concordant with the above‐mentioned reports, as 8.1% of the patients had viral and bacterial co‐infection. We found co‐infections of common community‐acquired pathogens (including Mycoplasma pneumoniae, RSV and influenza viruses, Haemophilus influenzae, and Streptococcus pneumoniae) mainly in mild and moderate patients (9.1%, Table 2). These results suggested that co‐infections should not be ignored in COVID‐19 patients and the screening of such infections at admission should be strengthened.
A latest study reported hospital‐acquired infection in 4.7% of COVID‐19 patients, 25 which is comparable to 5.1% of secondary infection in our cohort. Although previous studies had estimated a prevalence of bacterial and fungal secondary infections as from 4% to 14.3% in COVID‐19 patients. 1 , 25 , 26 It is noteworthy that the etiological results could be influenced by different diagnostic method and specimens types used in the studies, as well as seasonal factor. Here, benefit from the utility of culture, PCR, and mNGS, more pathogens were able to be detected, including anaerobic bacteria in blood, and HSV‐1 in blood and BAL specimens (HSV‐1 could be reactivated in critical care patients and cause ventilator‐associated pneumonia. 27 Our results also show that a total of 60 patients have received antibiotic treatment, including 26 of patients without any additional pathogen detections (Table 1). As antibiotics likely provide limited benefit on COVID‐19 treatment and contribute to the development of drug tolerance and resistance and other adverse consequences, 26 it is necessary to detect bacterial infections in the context of COVID‐19 which may provide more implications for antibiotic stewardship. Most secondary infections occurred in critically ill patients. The possible underlying reason may be that most critically ill patients were associated with longer invasive ventilation which could provide the increased chance for hospital‐acquired infections. 28 , 29 Although the physicians prescribed sensitive antifungal (Voriconazole) for treatment, the two patients still died, indicating that the fatality of the patients with fungal secondary infection was relatively high. These two patients had received methylprednisolone for 6 days (60 mg for 3 days, followed by 40 mg for 3 days), 2‐3 days before positive results of filamentous fungi culture. It has been reported that filamentous fungal infections in viral pneumonia may be related to corticoid use. 30 Thus, further research on the relationship between corticosteroid treatment and the risk of filamentous fungal infections may be warranted in a large number of COVID‐19 patients.
From the limited cases reviewed, culture measures had higher yield with further information during antimicrobial treatment sensitivity, though more cases would help to guide further, suggesting mNGS would not replace the conventional culture techniques.
Limitations to this study exist, which the authors have tried to minimize. First, this is a retrospective study of patients infected with COVID‐19 and as such, our sample was likely imbalanced between the study arms. However, the Shenzhen Third People's Hospital is the only government‐mandated hospital for the treatment of COVID‐19 patients in the region. As such, patients presenting to the hospital would likely be representative of other infected patients. Additional analysis with a larger cohort could help to improve the analysis conducted and further provide insights into the impact of co‐infections on the progression of COVID‐19. Second, given the high infectivity of SARS‐CoV‐2, not all of patients received the same microbiological testing. The testing depended on the physician's decision, which could produce bias in that positive results are more likely to be obtained from clinically suspected patients. Similarly, we only detected the three most common respiratory viruses in patients; other respiratory viruses (e.g., adenovirus, rhinovirus, parainfluenza virus, etc) were not included. Therefore, the co‐infection rate of bacteria and respiratory viruses would be underestimated to some extent. Third, the numbers in determining mNGS compared to standard culture methods is too low to make any definitive conclusions, so this part of the study is descriptive only. Furthermore, the use of IgM/IgG to support diagnosis of Mycoplasma infection has been shown in other research to overestimate the number of true cases, which may have biased our results. Additionally, follow‐up testing of samples or comparative analyses to similar patients in China would add further support to our findings.
5. CONCLUSIONS
In this single‐center study in China, co‐infections and secondary infections existed in hospitalized COVID‐19 patients, and were relevant to the disease severity. In the small number of cases observed, fungal secondary infections associated with fatal cases are presented. Surveillance for common respiratory pathogens in those with COVID‐19 will be helpful to protect against co‐infections and secondary infections and provide suggestions for antimicrobial stewardship and hospital infection control.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board of Shenzhen Third People's Hospital (number 2020‐100). Written informed consent was provided by all adults and the legal representatives of patients aged <18 years.
CONSENT TO PUBLISH
All of the authors are consent to publish on this journal.
COMPETING INTERESTS
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Jiuxin Qu and Lei Liu had roles in the study design and data interpretation. Jiuxin Qu, Shuyan Chen, and Qing Zhu wrote the manuscript. Shuyan Chen, Yanyu Xiao, and Zhaofang Jiang performed the microbiological testing. Shuyan Chen, Chi Wu, and Qing Zhu were responsible for data collection and analysis.
ACKNOWLEDGMENTS
The authors greatly thank Helen Chu from University of Washington for their guidance on the study design and manuscript writing. The authors are grateful to Zhifeng He, Feidi Ye, Tianpin Li, Kai Luo, Chen Chen, Zhenshuo Peng Shijin Yang, Xiaoyong Li, Zhiyong Yu, Ning Lv, Guobin Zhang, Qian Pu, and Shan Chen for collecting the specimens and data.
Chen S, Zhu Q, Xiao Y, et al. Clinical and etiological analysis of co‐infections and secondary infections in COVID‐19 patients: An observational study. Clin Respir J. 2021;15:815–825. 10.1111/crj.13369
Shuyan Chen, Qing Zhu, and Yanyu Xiao contributed equally to this work.
[Correction added on 24 April 2021, after first online publication: Grant numbers have been added to the Funding Information]
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Weekly operational update on COVID‐19 ‐ 30 October 2020. Available at: https://www.who.int/publications/m/item/weekly‐operational‐update‐‐‐30‐october‐2020
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8):782‐793. [DOI] [PubMed] [Google Scholar]
- 4. Koff WC, Williams MA. Covid‐19 and immunity in aging populations—a new research agenda. N Engl J Med. 2020;383(9):804‐805. [DOI] [PubMed] [Google Scholar]
- 5. Alcendor DJ. Racial disparities‐associated COVID‐19 Mortality among minority populations in the US. J Clin Med. 2020;9(8):2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza‐associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309(3):275‐282. [DOI] [PubMed] [Google Scholar]
- 9. Gill JR, Sheng Z‐M, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. To K, Yip C, Lai C, et al. Saliva as a diagnostic specimen for testing respiratory virus by a point‐of‐care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25(3):372‐378. [DOI] [PubMed] [Google Scholar]
- 11. Zhang G, Zheng G, Zhang Y, Ma R, Kang X. Evaluation of a micro/nanofluidic chip platform for the high‐throughput detection of bacteria and their antibiotic resistance genes in post‐neurosurgical meningitis. Int J Infect Dis. 2018;70:115‐120. [DOI] [PubMed] [Google Scholar]
- 12. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next‐generation sequencing in infectious disease diagnostics. mBio. 2015;6(6):e01888‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qu J, Wu C, Li X, et al. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(16):2255‐2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monis PT, Giglio S, Saint CP. Comparison of SYTO9 and SYBR Green I for real‐time polymerase chain reaction and investigation of the effect of dye concentration on amplification and DNA melting curve analysis. Anal Biochem. 2005;340(1):24‐34. [DOI] [PubMed] [Google Scholar]
- 15. Chowdhary A, Agarwal K, Kathuria S, et al. Clinical significance of filamentous basidiomycetes illustrated by isolates of the novel opportunist Ceriporia lacerata from the human respiratory tract. J Clin Microbiol. 2013;51(2):585‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeon YJ, Zhou Y, Li Y, et al. The feasibility study of non‐invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PLoS ONE. 2014;9(10):e110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Durbin R. Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics. 2009;25(14):1754‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next‐generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl. 2):S231‐S240. [DOI] [PubMed] [Google Scholar]
- 19. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;58(4):711‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qu J‐X, Gu LI, Pu Z‐H, et al. Viral etiology of community‐acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis. 2015;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lansbury L, Lim B, Baskaran V, et al. Co‐infections in people with COVID‐19: a systematic review and meta‐analysis. J Infect. 2020;81(2):266‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia‐Vidal C, Sanjuan G, Moreno‐García E, et al.; COVID‐19 Researchers Group . Incidence of co‐infections and superinfections in hospitalized patients with COVID‐19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langford BJ, So M, Raybardhan S, et al. Bacterial co‐infection and secondary infection in patients with COVID‐19: a living rapid review and meta‐analysis. Clin Microbiol Infect. 2020;26(12):1622‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouza E, Giannella M, Torres MV, et al. Herpes simplex virus: a marker of severity in bacterial ventilator‐associated pneumonia. J Crit Care. 2011;26(4):432.e1‐432.e6. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Zhang YI, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):1958‐1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu Y, Yang Q, Xu M, et al. Secondary bacterial infections in critical Ill patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(6):ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee N, Leo Y‐S, Cao B, et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J. 2015;45(6):1642‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


