Abstract
Our hypothesis was that high hemoglobin (Hb) level might be associated with hypercoagulable state and death due to COVID-19. Of the 9467 hospitalized COVID-19 patients, patients were subdivided into 5 groups based on the level of Hb; Hb < 10 g/dL, 10 g/dL ≤ Hb < 12 g/dL, 12 g/dL ≤ Hb < 14 g/dL, 14 g/dL ≤ Hb < 16 g/dL, and Hb ≥ 16 g/dL. Compared to patients with 12 g/dL ≤ Hb < 14 g/dL, patients with Hb ≥ 16 g/dL had significantly higher adjusted in-hospital mortality (OR [95% CI] 1.62 [1.15–2.27], P = 0.005).
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-021-02516-1.
Keywords: COVID-19, Hemoglobin, Mortality
Highlights
We analyzed 9467 patients dividing subgroups based on hemoglobin.
Patients with hemoglobin ≥ 16 g/dL had higher risk adjusted in-hospital mortality.
Treatment strategies based on hemoglobin would be warranted.
Introduction
Anemia is considered to be an independent predictor of mortality due to coronavirus disease 2019 (COVID-19) [1]. However, it remains uncertain if the association between hemoglobin (Hb) level and mortality is linear or non-linear. We hypothesized that high Hb level might be associated with hypercoagulable state and death since COVID-19 increases coagulopathy resulting in systemic thrombosis [2]. We aimed to investigate whether high Hb level was associated with in-hospital mortality among patients hospitalized with COVID-19.
Methods
This retrospective study was conducted by review of the medical records of 9542 hospitalized patients who were discharged between March 1st, 2020 and March 30th 2021, with laboratory confirmed COVID-19 in the Mount Sinai Health System [3]. After excluding patients with Hb < 6 g/dL or > 19 g/dL as outliners, the final cohort of our study included 9467 patients. Patients were subdivided into five groups based on Hb level: Hb < 10 g/dL, 10 g/dL ≤ Hb < 12 g/dL, 12 g/dL ≤ Hb < 14 g/dL, 14 g/dL ≤ Hb < 16 g/dL, and 16 g/dL ≤ Hb [4]. The primary outcome of interest was in-hospital mortality. Secondary outcomes were acute kidney injury and acute venous thromboembolism. Acute kidney injury was defined as a 50% or 0.3 mg/dL increase of creatinine level [5].
A logistic regression model was created to estimate the association between in-hospital mortality and Hb level where the reference level was 12 g/dL ≤ Hb < 14 g/dL. In addition to Hb subgroups, the following variables were used for a logistic regression model: age, sex, asthma, chronic obstructive pulmonary disease, obstructive sleep apnea, obesity, hypertension, diabetes mellitus, human immunodeficiency virus, cancer, atrial fibrillation, coronary artery disease, heart failure, peripheral vascular disease, chronic viral hepatitis, alcoholic/non-alcoholic liver disease, estimated glomerular filtration rate, blood urea nitrogen, white blood cell count, platelet, C reactive protein, d-dimer, vital signs (temperature, heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, oxygen saturation) at admission, endotracheal intubation, intensive care unit admission, treatment with therapeutic anticoagulation, prophylactic anticoagulation, steroid, interleukin-6 inhibitor, remdesivir, and convalescent plasma. The Modification of Diet in Renal Disease equation was used to estimate glomerular filtration rate [6].
Additionally, we checked the association between Hb level and risk-adjusted in-hospital mortality, where Hb was analyzed as a continuous variables using a smooth spline curve. All statistical analyses were performed using R (version 3.6.2, R). P values < 0.05 considered statistically significant.
This study was approved by the institutional review boards of Icahn School of Medicine at Mount Sinai (#2,000,495) and conducted in accordance with the principles of the Declaration of Helsinki. The waiver of patients’ informed consent was also approved by the institutional review boards.
Results
Table 1 shows baseline characteristics stratified by Hb level. Patients with high Hb were younger, more likely to be male, and had fewer comorbidities. Patients with high Hb had relatively lower C reactive protein and d-dimer levels (Table 1). The treatments and in-hospital outcomes by Hb levels were also shown in Table 1. Patients with the lowest and highest Hb had higher in-hospital mortality. The incidences of acute kidney injury were higher in patients with lower Hb. The proportions of acute venous thromboembolism were not different across the Hb groups.
Table 1.
Baseline characteristics and in-hospital outcomes of patients hospitalized due to COVID-19 infection and stratified by hemoglobin level
| Hb < 10 g/dL N = 1045 | Hb 10- 12 g/dL N = 1871 | Hb 12- 14 g/dL N = 3408 | Hb 14- 16 g/dL N = 2555 | Hb ≥ 16 g/dL N = 588 | P value | |
|---|---|---|---|---|---|---|
| Demographics and comorbidities | ||||||
| Age (years), mean (SD) | 66.9 (16.5) | 67.7 (17.2) | 65.4 (16.7) | 62.5 (16.8) | 60.6 (17.2) | < 0.001 |
| Male, n (%) | 492 (47.1) | 760 (40.6) | 1600 (46.9) | 1834 (71.8) | 498 (84.7) | < 0.001 |
| Race, n (%) | < 0.001 | |||||
| White | 257 (24.6) | 534 (28.5) | 980 (28.8) | 667 (26.1) | 148 (25.2) | |
| African American | 345 (33.0) | 510 (27.3) | 814 (23.9) | 504 (19.7) | 107 (18.2) | |
| Hispanic | 240 (23.0) | 442 (23.6) | 833 (24.4) | 735 (28.8) | 169 (28.7) | |
| Asian | 56 ( 5.4) | 112 ( 6.0) | 203 ( 6.0) | 153 ( 6.0) | 33 ( 5.6) | |
| Others | 147 (14.1) | 273 (14.6) | 578 (17.0) | 496 (19.4) | 131 (22.3) | |
| Comorbidities | ||||||
| COPD, n (%) | 58 ( 5.6) | 109 ( 5.8) | 128 ( 3.8) | 108 ( 4.2) | 18 ( 3.1) | 0.001 |
| Hypertension, n (%) | 525 (50.2) | 837 (44.7) | 1155 (33.9) | 761 (29.8) | 161 (27.4) | < 0.001 |
| Diabetes mellitus, n (%) | 366 (35.0) | 579 (30.9) | 706 (20.7) | 452 (17.7) | 124 (21.1) | < 0.001 |
| Obstructive sleep apnea, n (%) | 31 ( 3.0) | 64 ( 3.4) | 67 ( 2.0) | 54 ( 2.1) | 7 ( 1.2) | 0.002 |
| Obesity, n (%) | 99 ( 9.5) | 182 ( 9.7) | 300 ( 8.8) | 216 ( 8.5) | 37 ( 6.3) | 0.11 |
| Atrial fibrillation, n (%) | 131 (12.5) | 183 ( 9.8) | 229 ( 6.7) | 172 ( 6.7) | 46 ( 7.8) | < 0.001 |
| Heart failure, n (%) | 198 (18.9) | 259 (13.8) | 240 ( 7.0) | 115 ( 4.5) | 32 ( 5.4) | < 0.001 |
| Coronary artery disease, n (%) | 233 (22.3) | 347 (18.5) | 434 (12.7) | 296 (11.6) | 66 (11.2) | < 0.001 |
| Vital signs | ||||||
| Respiratory rate (/min), mean (SD) | 20.4 (4.8) | 20.5 (5.0) | 20.6 (5.1) | 21.0 (6.1) | 21.2 (6.3) | 0.001 |
| O2 Saturation (%), mean (SD) | 82.7 (19.5) | 84.0 (18.1) | 84.8 (16.1) | 83.6 (17.7) | 83.1 (19.0) | 0.003 |
| Blood tests | ||||||
| White blood cell (K/μL), median [IQR] | 7.6 [5.1, 11.2] | 7.4 [5.3, 10.5] | 7.1 [5.3, 10.0] | 7.5 [5.6, 10.4] | 7.9 [5.9, 11.3] | < 0.001 |
| Hemoglobin (g/dL), median [IQR] | 8.9 [8.1, 9.5] | 11.1 [10.6, 11.6] | 13.0 [12.5, 13.5] | 14.7 [14.3, 15.2] | 16.5 [16.2, 17.0] | < 0.001 |
| Platelet (K/μL), median [IQR] | 218.0 [149.0, 305.0] | 211.0 [157.0, 281.0] | 205.0 [157.0, 265.0] | 201.0 [155.0, 256.0] | 183.0 [143.0, 247.0] | < 0.001 |
| eGFR (ml/min./1.73m2), median [IQR] | 37.7 [15.4, 75.1] | 55.0 [29.2, 86.5] | 73.2 [49.8, 97.3] | 76.0 [54.2, 96.5] | 73.9 [52.8, 90.7] | < 0.001 |
| C reactive protein (mg/L), median [IQR] | 97.2 [37.0, 183.9] | 82.0 [34.9, 164.1] | 85.7 [36.4, 162.2] | 92.8 [43.1, 171.7] | 83.4 [38.5, 149.6] | 0.001 |
| D-Dimer (μg/mL), median [IQR] | 2.19 [1.18, 3.81] | 1.76 [0.98, 3.09] | 1.24 [0.70, 2.30] | 1.11 [0.65, 2.06] | 1.16 [0.66, 2.47] | < 0.001 |
| Treatment | ||||||
| Therapeutic anticoagulation, n (%) | 411 (39.3) | 705 (37.7) | 1152 (33.8) | 893 (35.0) | 213 (36.2) | 0.004 |
| Prophylactic anticoagulation, n (%) | 465 (44.5) | 981 (52.4) | 1970 (57.8) | 1465 (57.3) | 311 (52.9) | < 0.001 |
| Steroid treatment, n (%) | 426 (40.8) | 900 (48.1) | 1740 (51.1) | 1360 (53.2) | 299 (50.9) | < 0.001 |
| IL-6 inhibitor, n (%) | 16 ( 1.5) | 39 ( 2.1) | 134 ( 3.9) | 115 ( 4.5) | 19 ( 3.2) | < 0.001 |
| Convalescent Plasma, n (%) | 103 ( 9.9) | 212 (11.3) | 416 (12.2) | 310 (12.1) | 69 (11.7) | 0.29 |
| Use of Remdesivir, n (%) | 85 ( 8.1) | 246 (13.1) | 640 (18.8) | 514 (20.1) | 107 (18.2) | < 0.001 |
| In-hospital outcomes | ||||||
| In-hospital mortality | 348 (33.3) | 494 (26.4) | 681 (20.0) | 530 (20.7) | 148 (25.2) | < 0.001 |
| Intensive care unit admission | 234 (22.4) | 363 (19.4) | 632 (18.5) | 530 (20.7) | 140 (23.8) | 0.005 |
| Endotracheal intubation | 176 (16.8) | 242 (12.9) | 413 (12.1) | 336 (13.2) | 87 (14.8) | 0.002 |
| Acute kidney injury | 498 (47.9) | 636 (34.8) | 770 (23.0) | 556 (21.9) | 141 (24.1) | < 0.001 |
| Acute venous thromboembolism | 14 ( 1.3) | 24 ( 1.3) | 42 ( 1.2) | 23 ( 0.9) | 10 ( 1.7) | 0.48 |
COPD chronic obstructive pulmonary disease, eGFR estimated glomerular filtration rate, Hb hemoglobin, IL interleukin, IQR interquartile range, SD standard deviation
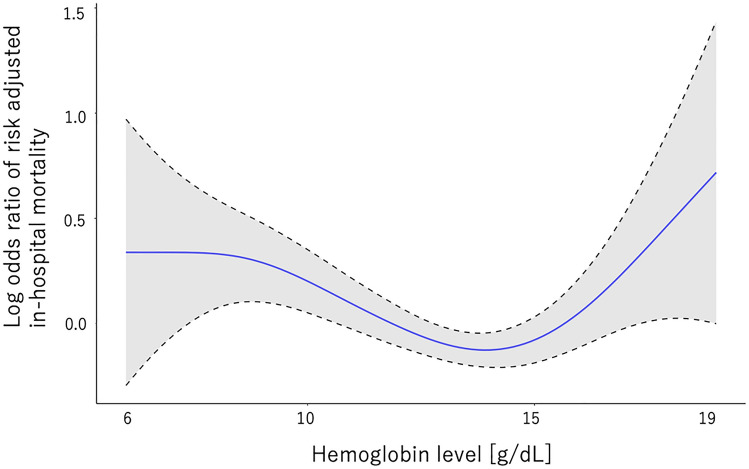
Notably, compared to patients with Hb between 12 and 14 g/dL, patients with Hb ≥ 16 g/dL had significantly higher risk-adjusted in-hospital mortality (odds ratio (OR) [95% confidential interval (CI)] 1.62 [1.15–2.27], P = 0.005) as well as those with Hb < 10 g/dL (OR [95% CI] 1.61 [1.24–2.08], P < 0.001) and those with 10 g/dL ≤ Hb < 12 g/dL (OR [95%CI] 1.30 [1.05–1.60], P = 0.014), but not those with 14 g/dL ≤ Hb < 16 g/dL (OR [95%CI] 1.08 [0.88–1.32], P = 0.48). The smooth spline curve showed the U curve association of Hb level with risk-adjusted in-hospital mortality (Fig. 1). The details of logistic regression models are shown in Supplemental Table 1.
Fig. 1.
The association of hemoglobin level and risk adjusted in-hospital mortality (y axis: log odds ratio of adjusted in-hospital mortality, x axis: hemoglobin level [g/dL])
Discussion
Although anemia is already considered to be an independent predictor of mortality due to COVID-19 [1], showing that high hemoblobin is also associated with in-hospital mortality of COVID-19 in our study is valuable. High Hb levels themselves might cause systemic thrombosis. The previous case reports showed that polycythemia vera could be a reason for the thrombosis due to COVID-19 [7, 8]. Although we could not reveal that the incidences of acute venous thromboembolism were different between the Hb subgroups, we assume there might be more microthrombi in lungs among patients with high hemoglobin, which could contribute to hypoxia and death [9].
Our study has limitations. This is a retrospective observational study. Despite rigorous adjustments, unmeasured confounders could not be adjusted. In addition, we did not have the information on the presence of myeloproliferative disease, erythropoietin supplementation, and the causes of death.
Conclusion
High mortality is observed in patients with high Hb levels as well as those with low Hb levels.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CI
Confidential interval
- COVID-19
Coronavirus disease 2019
- Hb
Hemoglobin
- OR
Odds ratio
Authors contribution
TK, MT, NE, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: TK. Data Curation: TK, MT, NE. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: TK. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: TK, MT. Administrative, technical, or material support: NE. Study supervision: NE.
Funding
None.
Declarations
Ethical approval
This study was approved by the institutional review boards of Icahn School of Medicine at Mount Sinai (#2000495) and conducted in accordance with the principles of the Declaration of Helsinki.
Informed consent
The waiver of patients’ informed consent was also approved by the institutional review boards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oh SM, Skendelas JP, Macdonald E, et al. On-admission anemia predicts mortality in COVID-19 patients: a single center, retrospective cohort study. Am J Emerg Med. 2021;48:140–147. doi: 10.1016/j.ajem.2021.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi M, Egorova NN, Kuno T. COVID-19 and influenza testing in New York City. J Med Virol. 2021;93:698–701. doi: 10.1002/jmv.26500. [DOI] [PubMed] [Google Scholar]
- 4.Numasawa Y, Ueda I, Sawano M, et al. Relation of baseline hemoglobin level to in-hospital outcomes in patients who undergo percutaneous coronary intervention (from a Japanese Multicenter Registry) Am J Cardiol. 2018;121:695–702. doi: 10.1016/j.amjcard.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Chandiramani R, Cao D, Nicolas J, Mehran R. Contrast-induced acute kidney injury. Cardiovasc Interv Ther. 2020;35:209–217. doi: 10.1007/s12928-020-00660-8. [DOI] [PubMed] [Google Scholar]
- 6.Acosta-Ochoa I, Bustamante-Munguira J, Mendiluce-Herrero A, Bustamante-Bustamante J, Coca-Rojo A (2019) Impact on outcomes across KDIGO-2012 AKI criteria according to baseline renal function. J Clin Med 8 [DOI] [PMC free article] [PubMed]
- 7.Vervaat FE, Houthuizen P. Case report of SARS Co-V2 infection, acute pulmonary embolism, and right ventricular thrombus. Eur Heart J Case Rep. 2020;4:1–5. doi: 10.1093/ehjcr/ytaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nawazani A, Ghanaim M, Tariq S (2020) Case of COVID-19 infection and polycythaemia presenting with massive acute pulmonary embolism. BMJ Case Rep 13 [DOI] [PMC free article] [PubMed]
- 9.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.