Abstract
To date, mother‐to‐fetus transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), responsible for the coronavirus disease 2019 (COVID‐19) pandemic, remains controversial. Although placental COVID‐19 infection has been documented in some cases during the second‐ and third‐trimesters, no reports are available for the first trimester of pregnancy, and no SARS‐CoV‐2 protein has been found in fetal tissues. We studied the placenta and fetal organs from an early pregnancy miscarriage in a COVID‐19 maternal infection by immunohistochemical, reverse transcription quantitative real‐time polymerase chain reaction, immunofluorescence, and electron microscopy methods. SARS‐CoV‐2 nucleocapsid protein, viral RNA, and particles consistent with coronavirus were found in the placenta and fetal tissues, accompanied by RNA replication revealed by double‐stranded RNA (dsRNA) positive immunostain. Prominent damage of the placenta and fetal organs were associated with a hyperinflammatory process identified by histological examination and immunohistochemistry. The findings provided in this study document that congenital SARS‐CoV‐2 infection is possible during the first trimester of pregnancy and that fetal organs, such as lung and kidney, are targets for coronavirus. The infection and multi‐organic fetal inflammation produced by SARS‐CoV‐2 during early pregnancy should alert clinicians in the assessment and management of pregnant women for possible fetal consequences and adverse perinatal outcomes.
Keywords: COVID‐19, fetus, first trimester, miscarriage, placenta, pregnancy
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic is a global public health emergency caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 SARS‐CoV‐2 transmission from the mother to the fetus (termed vertical transmission) remains controversial 2 , 3 , 4 ; even though some cases have documented perinatal transmission assessed by serological analysis of positive neonates. 5 , 6 , 7 , 8 , 9 Accumulating evidence supports the transplacental transmission of SARS‐CoV‐2, with the detection of coronavirus presence in the maternal and fetal sides of the placenta 10 , 11 , 12 , 13 , 14 , 15 (recently reviewed by Kotlyar et al. 16 ). To date, the most highly suggestive evidence for vertical transmission of SARS‐CoV‐2 is three case reports showing viral RNA in the placenta, fetal‐intravascular mononuclear cells, 14 neonatal blood 9 and recently, SARS‐CoV‐2 RNA in fetal lung samples obtained by needle aspiration. 17 But no SARS‐CoV‐2 protein or virus particles have been detected in fetal tissues. Here, we present a case of fetal infection with SARS‐CoV‐2 during the first trimester of pregnancy with virus detection by polymerase chain reaction (PCR), immunofluorescence, and viral replication in fetal organs and placenta, associated with a hyperinflammatory process highly consistent with an in utero transmission of SARS‐CoV‐2.
2. MATERIALS AND METHODS
2.1. Reverse transcription quantitative real‐time polymerase chain reaction (RT‐qPCR) for SARS‐CoV‐2
Tissues previously stored in RNAlater solution were disrupted through mechanical lysis and RNA was purified using the RNeasy Qiagen commercial kit (Qiagen). The SARS‐CoV‐2 RNA was detected following La Charité, Berlin protocol, 18 using TaqPath 1 step RT‐PCR master Mix, CG commercial kit (Thermo Fisher Scientific), and probes and primers designed for RdRP and E viral genes. RNase P human gene was used as RNA isolation control. RT‐qPCR was performed on a StepOnePlus instrument (Applied Biosystems/Thermo Fisher Scientific). Each 20 µl RT‐PCR reaction contained 5 µl enzyme mix, primers, and probes as indicated previously (La Charité) and 5 µl of RNA. Conditions at the thermocycler were previously reported. 18 A cycle threshold (C t) value <38 was considered positive for SARS‐CoV‐2 RNA. C t values were collected using the threshold at 0.035 fluorescence level.
2.2. Immunofluorescence assays
Tissue Sections (1 µm) were deparaffinized and hydrated using a standard technique, by heating in Tris‐EDTA buffer (10 mM Tris Base, 1 mM, 0.05% Tween 20, pH = 9) for 15 min at 100°C to reverse formaldehyde cross‐link. The samples were incubated with phosphate‐buffered saline (PBS) containing 10% fetal bovine serum (FBS) and 0.2% Triton X‐100 (Sigma‐Aldrich) for 1 h at room temperature (RT). The slides were washed with immunofluorescence buffer (A3059; Sigma‐Aldrich) and incubated overnight at 4°C with mouse monoclonal antibody anti‐SARS‐CoV/SARS‐CoV‐2 Nuclear Protein (40143‐MM05; dilution 1:200 19 , 20 ; Sino Biological) or J2 anti‐double‐stranded RNA (10010200; dilution 1:100 21 , 22 ; Scicons). Detection was performed by incubation for 1 h at RT with secondary antibody anti‐mouse‐IgG H&L conjugated with Alexa Fluor 488 (ab150117; Abcam) or antibody anti‐mouse‐IgG conjugated with Cy3 (A1052; dilution 1:100; Invitrogen). The sections were stained for nuclear DNA with 4ʹ,6‐diamidino‐2‐phenylindole, dilactate (DAPI) (422801; Biolegend) and mounted using Dako Fluorescent mounting medium (S3023; Dako). The samples were analyzed by laser scanning microscopy on an LSM 510 Meta inverted confocal microscope on Axiovert 200M motorized microscope (Carl Zeiss).
2.3. Immunohistochemistry
Post‐mortem fetal tissues were fixed in 10% formalin and embedded in paraffin for further histopathological examination by hematoxylin/eosin stain and immunohistochemistry using monoclonal rabbit antibodies for CD163 (clone EP324, dilution 1:50–1:200) and CD68 (clone KP‐1, dilution 1:100–1:500) (Bio SB Inc.). Tissue Sections (3 μm thick sections) prepared for antibody application were immersed in a citrate buffer and subjected to microwave irradiation. All primary antibodies were incubated with the tissue sections for 2 h at RT. Slides were then developed using an avidin–biotin–peroxidase method (Dako).
2.4. Ultrastructural studies
Samples were fixed in 2.5% glutaraldehyde in the cacodylate buffer (0.1 M) for 24 h at RT and washed three times. The samples were post‐fixed for 1 h in 1% osmium tetroxide and 1.5% ferrous potassium cyanide, stained in the block with 1% aqueous uranyl acetate for 30 min. After, gradual dehydration was performed in ethanol (50%, 60%, 70%, 80%, 96%, and three changes of 100%). Subsequently, two immersions in propylene oxide were carried out (15 min each). The infiltration process started with a 1:1 ratio of Epon‐812 and propylene oxide overnight. After this, 2:1 ratio for 2 h, and a final immersion step in a thin layer of Epon‐812 for 2 h. Finally, the sample was embedded in Epon (EMbed‐812) and polymerized overnight at 60°C. Thin sections (80 nm) of the embedded sample were obtained in Leica EM UC7 ultramicrotome and were placed in copper grids (200 mesh). The obtained sections were stained with 5% uranyl acetate and 0.25% lead citrate. The samples were observed in a Scanning Transmission Electron Microscope (Crossbeam 550; Carl Zeiss) at 15 kV and Jeol 1010.
2.5. Ethics statement
The case study was performed in agreement with the principles of the Declaration of Helsinki and following the CARE guidelines of the Equator Network (https://www.equator-network.org/reporting-guidelines/care/). The patient gave her written informed consent to the inclusion of material for publication, acknowledging they cannot be identified via this manuscript and that they are fully anonymized.
3. RESULTS
3.1. Case presentation
On April 8, 2020, a 28‐year‐old patient, gravida 4, para 3, with a 13‐week diamniotic twin pregnancy was admitted at this Level 3 Care Center with a threat of abortion, referred from another tertiary level hospital (Instituto Nacional de Enfermedades Respiratorias, Mexico City). On physical examination at COVID‐19 triage, she presented hyperemic congestive oropharynx, both lungs with a vesicular breath sound, fever (39°C), headache, arthralgias, and fatigue in the past 5 days before hospitalization. She complained of colic pain in the hypogastrium and vaginal bleeding of a dark, almost black shade. Obstetric ultrasound was performed, and both fetuses were found without evidence of heart sound. The pregnancy was ended a few hours later because the patient started labor spontaneously, expelling both fetuses without complications. The RT‐qPCR result for SARS‐CoV‐2 from the nasopharyngeal swabs was positive after an initial negative result. No serological tests could be performed.
The necropsy on both fetuses was performed by experienced perinatal pathologists (M.Y.V.‐V., E.R.M.‐V., and D.L.D.‐P.). Fetus A was 12 cm in length and weighed 37 g (according to 13.3 weeks' gestational age), external habitus without congenital malformations, general edema, and congestive appearance. The weight of the lung was 1.08 g (according to gestational age) and of the heart was 20 mg (according to gestational age). Mild state of maceration. Fetus B appeared with a more evident macerated state. The placenta was bichorial, biamniotic. The placental weight was 25 g with measurements of 8 × 7 × 1 cm with parenchymal infarcts in 25% of the examined surface. Post‐mortem fetal samples of lung, kidney, liver, heart, larynx, trachea, bowel, brain, and placenta were collected, and stored at −80°C until further analysis (several fetal and placental specimens were also stored in RNAlater solution). The woman had an adequate evolution and was discharged 24 h later. The patient was classified as a COVID‐19 case according to a recent classification for maternal SARS‐CoV‐2 infection. 23 Furthermore, laboratory findings in the mother supported this diagnosis since the patient showed an increased number of neutrophils (9 × 103 cells/ml), decreased lymphocyte count (1.2 × 103 cells/ml), and a higher concentration of lactate dehydrogenase (LDH) (260 IU/L). Indeed, COVID‐19 infection in pregnant women has been associated with lymphopenia, a higher neutrophil count and elevated LDH levels compared to nonpregnant patients. 24
3.2. SARS‐CoV‐2 RNA, immunofluorescence and electron microscopy analysis of placenta and fetal tissues
We first determined SARS‐CoV‐2 viral loads in several tissue samples from the placenta and fetus on the basis of RT‐qPCR threshold values. C t values ranged from 31 to 35.6 and samples were considered positive for SARS‐CoV‐2 RNA based on the detection of the RdRP and E viral genes. The viral load for placenta A and fetal A lung and kidney were 35.6, 33.7, and 34, respectively. Placenta B C t value was 33 while fetus B was 31, although this was highly macerated. Fetal tissues and placenta were negative for other viruses and bacteria, such as Staphylococcus aureus, Staphylococcus agalactiae, Klebsiella pneumoniae, Escherichia coli, Mycoplasma genitalium, Listeria monocytogenes, Cytomegalovirus, Parvovirus B19, Herpes simplex, Respiratory Syncytial Virus, as assessed by RT‐qPCR (Supporting Information). The fetus could be classified as having a confirmed congenital infection because of detection of viral genome by PCR in fetal and placenta tissues. 23
We next investigated the viral protein marker presence in three samples that suggest vertical infection: placenta, lung, and fetal kidney of fetus A. The analysis of confocal microscopy showed a positive signal to N viral protein of SARS‐CoV‐2 in the placenta and fetal tissues (Figure 1A). This result suggests that the virus accesses the tissues' cellular components. In an attempt to evaluate wether viral genome replication occurred in these tissues, we performed a stain to identify the replication intermediates of dsRNA viral using a monoclonal J2‐dsRNA‐specific antibody that has been widely used for replication studies, including SARS‐CoV. 21 , 22 Interestingly, the dsRNA signal was detected in the placenta, fetal lung, and kidney tissues indicating viral replication, and suggesting that virus genome synthesis take place in these tissues (Figure 1B). Immunofluorescence in other areas of the fetal organs analyzed revealed that some regions were negative for dsRNA or N protein staining, representing uninfected cells.
Figure 1.
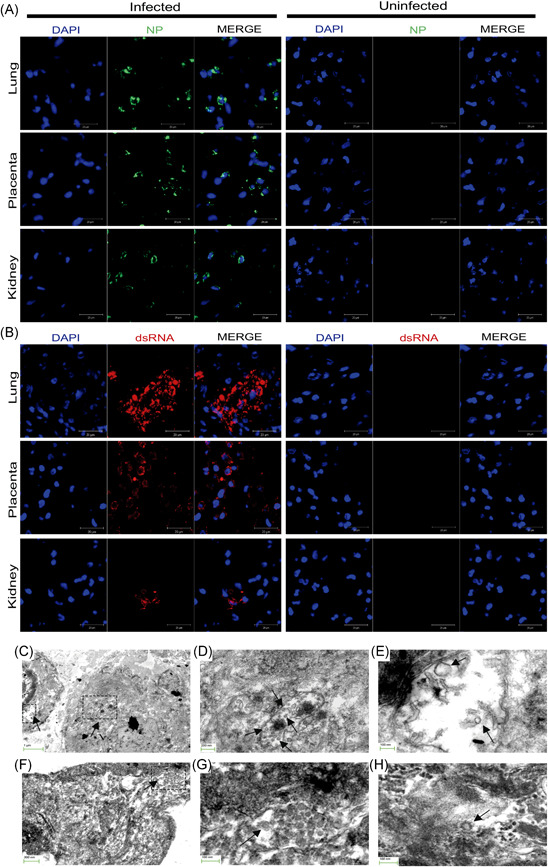
Presence of SARS‐CoV‐2 nucleocapsid protein, viral replicative intermediate dsRNA and virions across fetal and placental tissues. (A) Double immunofluorescence for SARS‐CoV‐2 N viral protein (green) and DAPI (blue) shows strong positivity for nucleocapsid expression (NP) in fetal lung, kidney, and placenta from SARS‐CoV‐2‐infected tissues but not for uninfected samples. (B) Double stain for dsRNA (red) and DAPI (blue) indicates RNA viral replication exclusively in fetal and placenta tissues infected with SARS‐CoV‐2. Merged images show localization of both stains. (×40 magnification, scanning zoom 3×). Scale bar represents 20 µm. (C–H) Scanning Electron Microscopy with transmitted electrons detector mode images of coronavirus particles in fetal lung and placental parenchyma. (C–E) Lung tissue with the presence of virions particles inside a vacuole. The area outlined on the upper right corner shows a large cytoplasmic vacuole with virions inside (E). (F–H) The virion particles also were present in the placental parenchyma. (G) Higher magnification of the rectangle marked in (F). The arrows point to particles consistent with the typical morphological features of coronavirus. Although postmortem changes made the good preservation of cell organelles difficult, parts of the nucleus (Nu) and the Golgi apparatus are seen. DAPI, 4ʹ,6‐diamidino‐2‐phenylindole, dilactate; dsRNA, double‐stranded RNA; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
We carried out the scanning electron microscopy with transmitted electrons detector mode (STEM) characterization of a fragment of fetus A lung and placenta tissues (Figure 1C–H). Unfortunately, postmortem alterations prevented optimal preservation of cell organelles. However, ensembled virus‐like particles were found inside cytoplasmic vacuoles of lung cells and the placenta (labeled with arrows). These virions were again visualized under higher magnification (Figures 1E and 1H) revealing a size between 60 nm and 110 nm and surface spike protein projections, consistent with the size and appearance of SARS‐CoV‐2. 25 , 26
3.3. Histological and immunohistochemical analysis of fetal tissues and placenta
We performed histological examination on paraffin‐embedded tissues from fetus A organs and placenta A carried out with hematoxylin/eosin stain and immunohistochemistry to detect CD68+ and CD163+ inflammatory cells. The fetal heart showed striking evidence of endarteritis in small arteries (Figure 2A) with CD68+ interstitial inflammatory mononuclear cells (Figures 2B and 2E), edema between cardiomyocytes and ischemic changes; the great vessels (pulmonary artery and aorta) showed no alterations. Both lungs were in the pseudoglandular stage, presenting reactive bronchial epithelium and hypercellularity composed of CD68+ inflammatory macrophages (Figures 2C and 2F). These were also seen in interstitium and pleura. Noteworthy, myositis was observed as a mononuclear inflammation between the fascicles of striated muscle cells of the neck, extremities and diaphragm, causing severe damage in fibers with apoptotic cells, atrophy, and intercellular edema (Figure 2H). Both kidneys were found intraluminal with scattered cellular detritus and mild interstitial inflammation with an enhanced infiltration of CD68+ cells (Figure 2I). The histological analysis of the placenta exhibited placental infarction, with diffuse perivillous fibrin, active chronic intervillositis, and subchorial inflammation (Figure 2D). The observed immunophenotypes were of CD163 positivity in the villous stroma and intervillous space (Figure 2G).
Figure 2.
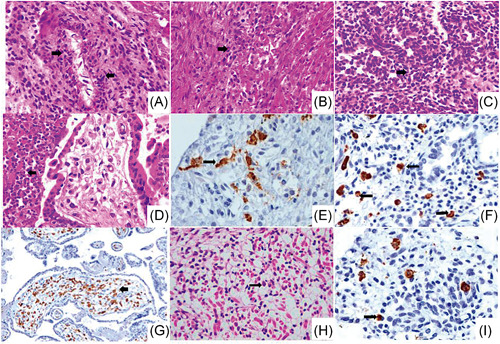
Multi‐organic fetal and placental inflammation. Histological sections with haematoxylin/eosin and immunohistochemistry from the postmortem study of fetus A and placenta showing severe inflammation. (A and B) Arrows point to inflammatory cells (macrophages and neutrophils) in the intima, media and adventitia of the arterial vessels of the heart as well as between the fascicles of the cardiac muscle cells, with several CD68‐positive macrophages (E, brown stain) (×20). (C) Fetal lung shows mild interstitial hypercellularity with inflammatory cells which express CD68 (F, arrows, brown stain) (×20). (D) Chorionic villi with active chronic intervillositis and abundant perivillous neutrophils (arrow) (×10). (G) Section of the placenta exhibiting severe inflammation with Hofbauer cells in the villous stroma strongly positive for CD163 cells (arrow, brown stain) (×10). (H) Striated muscle with inflammatory infiltrates of mononuclear cells and neutrophils, as well as edema, apoptosis, and diffuse myocyte atrophy (arrow, ×40). (I) Immunohistochemical staining for CD68 shows interstitial macrophages infiltrate in the kidney (brown stain) (×40)
4. DISCUSSION
We present novel evidence in support of intrauterine transmission of SARS‐CoV‐2 during early pregnancy (13 weeks), associated with miscarriage, from a COVID‐19‐infected woman. We detected SARS‐CoV‐2 N‐protein and RNA as well as viral replication in fetal lungs and kidneys, and the placenta. Furthermore, viral particles consistent with coronavirus were observed by electron microscopy in fetal lung tissue adjacent to the alveolus region, within the cytosol of apoptotic and not well‐preserved cells, and the placenta parenchyma. The size of these virions' structures was consistent with the size and appearance of SARS‐CoV‐2. 25 , 26 As recently discussed, it is difficult to distinguish virus particles from normal subcellular organelles. Virions can be mistakenly identified with clathrin‐coated vesicles or with heterogeneous vesicles in multivesicular bodies. 27 However, we are confident that the particle aggregates inside vesicles correspond to coronavirus as we have carefully compared them with normal cell organelles. In addition, histopathological findings of fetal and placenta tissues showed a hyperinflammatory process, which most likely led to miscarriage. Our findings at the histopathological level in the placenta are similar to those reported by Schwartz and Morotti. 28 The latter study defined that SARS‐CoV‐2 generates a common characteristic placental damage, which is due to the presence of a chronic histiocytic intervillositis (CHI). In this sense, a review by Wong et al. 29 showed CHI was reported in 29.4% of SARS‐CoV‐2‐positive second‐ and third‐trimester placentas. Furthermore, other placental alterations observed in this study were also found in these 17 studies, such as infarcts (35.1%) and fibrin deposition (43.2%), being the most common features associated with SARS‐CoV‐2 infection. 29 So, this continues to support the inflammatory effect and maternal vascular malperfusion that viral infection can exert during the first weeks of gestation. Recently, Schwartz and cols. 15 findings underpin that the CHI could be a relevant mechanism for SARS‐CoV‐2 vertical transmission. Potentially, placental damage by necrosis of syncytiotrophoblasts may be associated with the viral cytotoxicity and ischemia produced by malperfusion from CHI. This effect in the placental monolayer of syncytiotrophoblast may serve as a potential pathway for viral access to the chorionic villi and finally reach fetal circulation. However, further studies to understand these mechanisms and events are required.
There is scarce data regarding COVID‐19 infection during the first trimester, and to date, no in utero infection has been documented earlier in pregnancy. However, neonatal COVID‐19 cases were reported worldwide during the pandemic. The results of a meta‐analysis showed that in 74 publications, 176 neonates tested positive for SARS‐CoV‐2, and a percentage of 5.7% was associated with congenital infection. It is possible that the fetal losses during the first trimesters in women infected with COVID‐19 could be considered a new factor in the epidemiology of the disease during pregnancy. 30 Shah et al. 23 have proposed a classification of maternal, fetal, and neonatal SARS‐CoV‐2 infections taking into account maternal/perinatal tests. According to this classification, a confirmed congenital infection with intrauterine fetal death/stillbirth is defined by virus detection by PCR or viral particle detection by electron microscopy from fetal or placental tissue and requires that maternal status is either definitive or probable. Therefore, our case complies with the criteria of transplacental transmission of SARS‐CoV‐2.
Although vertical transmission of coronavirus is a rare event, it was documented in the third trimester of pregnancy by (1) the presence of anti‐SARS‐CoV‐2 IgM antibodies in a neonate from a COVID‐19 pregnant woman, 6 (2) detection of the virus in the placenta, 10 , 11 , 12 , 13 , 14 , 15 (3) neonatal viremia associated with COVID‐19 and neurological symptoms, 9 (4) SARS‐CoV‐2 infection of the placenta and fetal intravascular monocytes, 14 and recently, (5) the detection of SARS‐CoV‐2 RNA in fetal lung samples obtained by needle puncture. 17 Together with previous reports, our findings support that in utero transmission of SARS‐CoV‐2, although a rare event, is actually possible in early pregnancy.
Vertical infection by viruses is a process that depends on several factors, such as cellular permissiveness of the virus to the components of the placenta. 31 A study based on single‐cell RNA‐seq analysis suggests that the receptor of SARS‐CoV‐2, ACE2, is highly expressed in trophoblast and syncytiotrophoblast, which could promote the entry of the virus in these cell types, and facilitate the dissemination into the fetal circulation. 32 However, our result shows that replication events of the viral genome take place in the cells of the placenta, as we detect the presence of the replicative intermediate (dsRNA) that only occurs when the viral genome is synthesized. Therefore, it is likely that placental components are a region during the first trimester of pregnancy that allows the establishment of tissue areas of viral production, as observed with viruses such aszika or cytomegalovirus. 33 However, another possible placental transfer mechanism that has been described with other pathogens such as in Cytomegalovirus maternal infections, could be through neonatal Fc receptor‐mediated transcytosis into syncytiotrophoblasts, cytotrophoblasts, and macrophages. 34 In our study, the most striking observation in the placenta was a prominent amount of macrophages (Hoffbauer cells). These cells are a normal component of the stroma of the chorionic villi of fetal origin, appearing in the chorionic villi at the 10th–18th days of gestation. An increased number of these cells is an abnormal condition that has been reported to occur as a result of a variety of pathological conditions associated with pregnancy, including ascending infections as well as maternal blood‐borne infections that cause villitis, like syphilis or cytomegalovirus infections. 35 It is possible that proliferation of these cells occurs during infection with SARS‐CoV‐2, as observed with the Zika virus. 36 We could also hypothesize that these cells can be susceptible to infection with SARS‐CoV‐2, possibly allowing them to be a virus‐transporting cell to regions of the placenta, and to the fetal capillaries for subsequent infection. 37 Evidence of macrophage responses activated in fetuses under stress (infections, pre‐eclampsia), induced by maternal cytokines release, could explain the massive inflammatory response we observed in our study. 38
5. CONCLUSIONS
The evidence presented in this case report shows fetal organs are targets for SARS‐CoV‐2 and comply with a congenital infection associated with fetal death in early pregnancy.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
María Y. Valdespino‐Vázquez, Cecilia A. Helguera‐Repetto, Moises León‐Juárez, Oscar Villavicencio‐Carrisoza, Arturo Flores‐Pliego, Elsa R. Moreno‐Verduzco, Diana L. Díaz‐Pérez, Isabel Villegas‐Mota, Elsa R. Moreno‐Verduzco, Arturo Cardona‐Pérez, and Claudine Irles had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: María Y. Valdespino‐Vázquez, Cecilia A. Helguera‐Repetto, Moises León‐Juárez, Oscar Villavicencio‐Carrisoza, Arturo Flores‐Pliego, Elsa R. Moreno‐Verduzco, Diana L. Díaz‐Pérez, Isabel Villegas‐Mota, Elsa R. Moreno‐Verduzco, Arturo Cardona‐Pérez, and Claudine Irles. Acquisition, analysis, or interpretation of data: María Y. Valdespino‐Vázquez, Cecilia A. Helguera‐Repetto, Moises León‐Juárez, Oscar Villavicencio‐Carrisoza, Arturo Flores‐Pliego, Elsa R. Moreno‐Verduzco, Diana L. Díaz‐Pérez, Isabel Villegas‐Mota, Elsa R. Moreno‐Verduzco, Elba Carrasco‐Ramírez, Irma E. López‐Martínez, David M. Giraldo‐Gómez, Rosalia Lira, Martha Yocupicio‐Monroy, Mario Rodríguez‐Bosch, Edgar E. Sevilla‐Reyes, Manuel Cortés‐Bonilla, Sandra Acevedo‐Gallegos, Horacio Merchant‐Larios, Arturo Cardona‐Pérez, and Claudine Irles. Supervision: María Y. Valdespino‐Vázquez, Cecilia A. Helguera‐Repetto, Moises León‐Juárez, Oscar Villavicencio‐Carrisoza, Arturo Flores‐Pliego, Elsa R. Moreno‐Verduzco, Diana L. Díaz‐Pérez, Isabel Villegas‐Mota, Elsa R. Moreno‐Verduzco, Arturo Cardona‐Pérez, and Claudine Irles. Drafting manuscript and critical revision of the manuscript for important intellectual content: all authors.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by the INPer under grant 2019‐1‐32. We thank Dr. Isabel Mora Mendoza for her assistance in the immunohistochemistry techniques. Funding: Instituto Nacional de Perinatología Isidro Espinosa de los Reyes.
Valdespino‐Vázquez MY, Helguera‐Repetto CA, León‐Juárez M, et al. Fetal and placental infection with SARS‐CoV‐2 in early pregnancy. J Med Virol. 2021;93:4480–4487. 10.1002/jmv.26965
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9(1):51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Peng H, Wang L, et al. Infants born to mothers with a new coronavirus (COVID‐19). Front Pediatr. 2020;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID‐19 infection. Prenat Diagn. 2020;40(13):1759‐1761. 10.1002/pd.5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323(18):1846‐1848. 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID‐19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu X, Gao J, Luo X, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID‐19) pneumonia. Obstet Gynecol. 2020;136(1):65‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivanti AJ, Vauloup‐Fellous C, Prevot S, et al. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun. 2020;11(1):1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of SARS‐CoV‐2 virus invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223(2):275‐278. 10.1016/j.ajog.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penfield CA, Brubaker SG, Limaye MA, et al. Detection of SARS‐COV‐2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020;2(3):100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of COVID‐19: SARS‐CoV‐2 RNA on the fetal side of the placenta in pregnancies with COVID‐19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2(3):100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS‐CoV‐2 infection in a neonate born to a woman with active SARS‐CoV‐2 infection. CMAJ. 2020;192(24):E647‐E650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Facchetti F, Bugatti M, Drera E, et al. SARS‐CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine. 2020;59:102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz DA, Baldewijns M, Benachi A, et al. Chronic histiocytic intervillositis with trophoblast necrosis are risk factors associated with placental infection from coronavirus disease 2019 (COVID‐19) and intrauterine maternal–fetal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmission in liveborn and stillborn infants. Arch Pathol Lab Med. Published online December 31, 2020. 10.5858/arpa.2020-0771-SA [DOI] [PubMed]
- 16. Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2021;224(1):35‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodrigues ML, Gasparinho G, Sepúlveda F, Matos T. Signs suggestive of congenital SARS‐CoV‐2 infection with intrauterine fetal death: a case report. Eur J Obstet Gynecol Reprod Biol. 2021;256:508‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Babka AM, Kearney BJ, Radoshitzky SR, Kuhn JH, Zeng X. Molecular detection of SARS‐CoV‐2 in formalin‐fixed, paraffin‐embedded specimens. JCI Insight. 2020;5(12):e139042. 10.1172/jci.insight.139042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golden JW, Cline CR, Zeng X, et al. Human angiotensin‐converting enzyme 2 transgenic mice infected with SARS‐CoV‐2 develop severe and fatal respiratory disease. JCI Insight. 2020;5(19):e142032. 10.1172/jci.insight.142032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schönborn J, Oberstrass J, Breyel E, Tittgen J, Schumacher J, Lukacs N. Monoclonal antibodies to double‐stranded RNA as probes of RNA structure in crude nucleic acid extracts. Nucleic Acids Res. 1991;19(11):2993‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Versteeg GA, Bredenbeek PJ, Van den Worm SHE, Spaan WJM. Group 2 coronaviruses prevent immediate early interferon induction by protection of viral RNA from host cell recognition. Virology. 2007;361(1):18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng B, Jiang T, Zhang L, et al. Clinical characteristics of pregnant women with coronavirus disease 2019 in Wuhan, China. Open Forum Infect Dis. 2020;7(8) ofaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS‐CoV‐2: a challenging task. Lancet. 2020;395(10238):e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yao X‐H, He Z‐C, Li T‐Y, et al. Pathological evidence for residual SARS‐CoV‐2 in pulmonary tissues of a ready‐for‐discharge patient. Cell Res. 2020;30(6):541‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopfer H, Herzig MC, Gosert R, et al. Hunting coronavirus by transmission electron microscopy—a guide to SARS‐CoV‐2‐associated ultrastructural pathology in COVID‐19 tissues. Histopathology. 2020;78(3):358‐370. 10.1111/his.14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwartz DA, Morotti D. Placental pathology of COVID‐19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS‐CoV‐2. Viruses. 2020;12(11):1308. 10.3390/v12111308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong YP, Khong TY, Tan GC. The effects of COVID‐19 on placenta and pregnancy: what do we know so far? Diagnostics (Basel). 2021;11(1):94. 10.3390/diagnostics11010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raschetti R, Vivanti AJ, Vauloup‐Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS‐CoV‐2 infections. Nat Commun. 2020;11(1):5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. León‐Juárez M, Martínez–Castillo M, González‐García LD, et al. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog Dis. 2017;75(7):ftx093. 10.1093/femspd/ftx093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li M, Chen L, Zhang J, Xiong C, Li X. The SARS‐CoV‐2 receptor ACE2 expression of maternal–fetal interface and fetal organs by single‐cell transcriptome study. PLOS One. 2020;15(4):e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petitt M, Tabata T, Puerta‐Guardo H, Harris E, Pereira L. Zika virus infection of first‐trimester human placentas: utility of an explant model of replication to evaluate correlates of immune protection ex vivo. Curr Opin Virol. 2017;27:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pereira L, Maidji E. Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol. 2008;325:383‐395. [DOI] [PubMed] [Google Scholar]
- 35. Hung T‐H, Chen S‐F, Hsu J‐J, Hsieh C‐C, Hsueh S, Hsieh T‐T. Tumour necrosis factor‐alpha converting enzyme in human gestational tissues from pregnancies complicated by chorioamnionitis. Placenta. 2006;27(9–10):996‐1006. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz DA. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch Gynecol Obstet. 2017;295(6):1361‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quicke KM, Bowen JR, Johnson EL, et al. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20(1):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang Z, Abrahams VM, Mor G, Guller S. Placental Hofbauer cells and complications of pregnancy. Ann N Y Acad Sci. 2011;1221:103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


